Abstract
Extracellular matrix and extracellular matrix-degrading matrix metalloproteinases play a key role in interactions between the epithelium and the mesenchyme during mammary gland development and disease. In patients with breast cancer, the mammary mesenchyme undergoes a stromal reaction, the etiology of which is unknown. We previously showed that targeting of an autoactivating mutant of the matrix metalloproteinase stromelysin-1 to mammary epithelia of transgenic mice resulted in reduced mammary function during pregnancy and development of preneoplastic and neoplastic lesions. Here we examine the cascade of alterations before breast tumor formation in the mammary gland stroma once the expression of the stromelysin-1 transgene commences. Beginning in postpubertal virgin animals, low levels of transgene expression in mammary epithelia led to increased expression of endogenous stromelysin-1 in stromal fibroblasts and up-regulation of other matrix metalloproteinases, without basement membrane disruption. These changes were accompanied by the progressive development of a compensatory reactive stroma, characterized by increased collagen content and vascularization in glands from virgin mice. This remodeling of the gland affected epithelial-mesenchymal communication as indicated by inappropriate expression of tenascin-C starting by day 6 of pregnancy. This, together with increased transgene expression, led to basement membrane disruption starting by day 15 of pregnancy. We propose that the highly reactive stroma provides a prelude to breast epithelial tumors observed in these animals.
Epithelial development depends on an exquisite series of inductive and instructive interactions between the differentiating epithelium and the mesenchymal (stromal) compartment. 1,2 The epithelium, which consists of luminal and myoepithelial cells, is separated from the stroma by a basement membrane (BM), which plays a central role in mammary gland homeostasis and gene expression. 3-5 In vivo, stromal cells produce fibronectin, collagens, proteoglycans, and some components of the BM, as well as a number of proteinases that can effectively degrade BM constituents. 6-9 Stromal and epithelial cells of the mammary gland interact to regulate BM synthesis and degradation and, thus, mammary function.
Matrix metalloproteinases (MMPs) are extracellular matrix (ECM)-degrading enzymes involved in mammary gland morphogenesis and involution. 10-13 During late pregnancy and lactation, when the gland becomes fully functional, the expression of MMPs is low; 11 however, during involution, when the gland loses function and is remodeled, synthesis of ECM-degrading proteinases increases dramatically. 11 Disturbance of the balance between MMPs and MMP inhibitors leads to either unscheduled involution or prolonged lactation. 9,12 Mammary glands of virgin mice expressing an autoactivating stromelysin-1 (SL-1) transgene display supernumerary branches and precocious alveolar development, accompanied by the synthesis of β-casein at levels found normally only during early pregnancy. 14,15 During late pregnancy, increased expression of the SL-1 transgene leads to a reduction in expression of pregnancy-specific genes. 14 Later in life, some SL-1 transgenic mice develop hyperplastic, dysplastic, and ductal carcinoma in situ-like lesions, as well as malignant tumors. 16
Little is known about the sequence of changes that occurs before formation of an overt reactive stroma in breast cancer. In the present study, we address the question of whether and how the stromal compartment is altered as a consequence of inappropriate SL-1 transgene expression in the epithelium.
Materials and Methods
Tissue Collection
Mammary tissue was obtained from normal CD-1 mice (Charles River, Wilmington, MA) and their transgenic counterparts. Transgenic mice expressing an autoactivating Val92-to-Gly92 mutation of the rat SL-1 gene under the control of a whey acidic protein promoter were generated previously. 14 Mammary glands from two different transgenic lines (M2-5 and M2-21) were used interchangeably. The inguinal and abdominal mammary glands were surgically excised from female mice at various stages of development and immediately frozen in liquid nitrogen for RNA extraction. A small portion of the gland was fixed in 4% paraformaldehyde for in situ hybridization analysis. All experiments were performed under protocols approved by the Animal Welfare and Research Committee, Lawrence Berkeley National Laboratory, and the Committee on Animal Research, University of California, San Francisco.
Northern Blot Analysis
RNA was prepared by the technique of Chomczynski and Sacchi. 17 Total RNA (15 μg) was separated on denaturing formaldehyde agarose gels, transferred to Hybond N+ membranes (Amersham, Arlington Heights, IL), and hybridized at high stringency with a riboprobe generated with T7 polymerase (New England Biolabs, Beverly, MA) from the mouse SL-1 cDNA pmTRM11 18 that was radiolabeled with 32P-UTP (Amersham) to a specific activity of 1 × 108 cpm/μg. Sequences corresponding to the 3′ untranslated region of the rat SL-1 cDNA (nucleotides 1514 to 1772) 19 were used to identify rat SL-1 transgene mRNA. Sequences corresponding to nucleotides 83 to 2069 of the full-length mouse tenascin-C cDNA 20 were used to identify tenascin-C mRNA. The cDNA probes for platelet endothelial cell adhesion molecule-1 (PECAM-1) 21 and tenascin-C were radiolabeled by random priming (Rediprime kit, Amersham) according to the manufacturer’s instructions. For normalization, blots were boiled in water and reprobed with a cDNA probe for ribosomal 28S RNA.
Reverse Transcription-Polymerase Chain Reaction and Southern Hybridization
Total RNA was resuspended in diethyl pyrocarbonate-pretreated water and reverse transcribed with 10 U/μl Moloney murine leukemia virus reverse transcriptase (Life Technologies, Gaithersburg, MD) in 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 3 mmol/L MgCl2, 10 mmol/L dithiothreitol, 0.5 mmol/L dATP, 0.5 mmol/L dCTP, 0.5 mmol/L dTTP, 0.5 mmol/L dGTP, and 12.5 mg/μl oligo(dT)12–18 (Life Technologies) for 30 minutes at 37°C. Polymerase chain reaction (PCR) amplification was performed with 2 ng/μl reverse-transcribed RNA, 0.025 U/μl Taq DNA polymerase (Life Technologies), 1 μmol/L 5′ primer, 1 μmol/L 3′ primer, 20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 2 mmol/L MgCl2, 0.2 mmol/L dATP, 0.2 mmol/L dCTP, 0.2 mmol/L dGTP, and 0.2 mmol/L dTTP with cycle numbers indicated in the figure legends. Each PCR cycle was performed at 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute. Reverse-transcribed RNA (100 ng) was amplified with the following primer pairs (all from Biosynthesis, Lewisville, TX): GCAGCCATTTCTTTAAAGGC as 5′ primer and CCACTTCAGTGCGCCAAGTT as 3′ primer for amplification of rat SL-1; CTATGCCTACTTCCTTCGTGGC as 5′ primer and ATCTCATTACCAACACCACTCC as 3′ primer for mouse stromelysin-3 (SL-3); TTGAGAAGGATGGCAAGTATGG as 5′ primer and ACACCTTGCCATCGTTGC as 3′ primer for mouse gelatinase A; TTGAAGGATGGCAAGTATGG as 5′ primer and CGAAGGCATGACCTAGAGTGT as 3′ primer for mouse matrilysin. PCR amplification products were resolved on 1.5% agarose gels. To verify the identity of the amplified sequences, Southern hybridizations were performed according to published procedures 22 with oligonucleotides complementary to the mRNA sequence of the gene examined. The following oligonucleotides were used: GAAACCCAAATGCTTCAAAGACAGCATCCA for rat SL-1, TATGGCTGGGTCTCTTACATGATCTAAG for SL-3, GTCCATCAGCATTGCCACCCATGGTAAACA for gelatinase A, and TGTCTCCATGATCTCTCCTTGCGAAGCCAA for matrilysin.
In Situ Hybridization
Deparaffinized sections (5 μm) were treated with proteinase K (5 μg/ml) and hybridized overnight with 35S-labeled transcripts from a mouse SL-1 cDNA 18 subcloned in Bluescript KS (+) (Strategene, La Jolla, CA). For antisense probe, pmTRM12 fragment (3115 to 4051) was used, and for sense probe pmTRM12 fragment (2205 to 2918) was used. After hybridization at 47°C, sections were treated with RNase A (20 μg/ml; Boehringer Mannheim, Indianapolis, IN) and RNase T1 (2 U/ml; Boehringer Mannheim) for 10 minutes at 37°C, followed by washes in 2× standard saline citrate, 50% formamide at 50°C for 2 hours and in 0.1× standard saline citrate at 25°C for 30 minutes, before autoradiography with NTB2 emulsion (Kodak, Rochester, NY). Slides were exposed for 10 days and counterstained with hematoxylin after developing.
Histochemistry
Paraffin-embedded tissue sections (5 μm) were deparaffinized in xylene, treated with ethanol, rinsed in phosphate-buffered saline, and incubated for 10 minutes at 37°C with 0.1% trypsin in 0.05 mol/L Tris-HCl and 0.1% CaCl2, pH 7.4. Sections were then incubated with antibodies against von Willebrand factor (vWF) (A 0082, 1:200 dilution; DAKO, Carpinteria, CA) in blocking buffer (phosphate-buffered saline with 2% bovine serum albumin) overnight at 4°C. After washing with phosphate-buffered saline, the specimens were incubated with biotinylated goat anti-F(ab′) mouse antibody (1:100 dilution; Amersham) for 30 minutes at ambient temperature. The specimens were then washed and incubated for 30 minutes at ambient temperature with Texas Red-conjugated streptavidin (1:100 dilution; Amersham). Nuclei were visualized by brief incubation of sections with 4′,6′-diamidino-2-phenyl indole (DAPI, 0.5 μg/ml) (Sigma Chemical Co., St. Louis, MO) before mounting with Vectashield (Vector Laboratories, Burlingame, CA).
For the detection of tenascin-C, frozen tissue sections (5 μm) were fixed in 2% paraformaldehyde for 20 minutes, washed with 0.1 mol/L glycine in phosphate-buffered saline for 15 minutes, and incubated with a rabbit polyclonal antiserum to mouse tenascin-C (1:500 dilution, pK7, gift from Dr. Melitta Schachner). Indirect immunofluorescence was performed as described previously. 23
Gomori’s trichrome staining was used to stain total collagens. 24
Results
Endogenous SL-1 Is Expressed in the Mammary Stroma and Is Up-Regulated in Transgenic Mice
In a previous study, we showed that the mammary glands of virgin SL-1 transgenic animals resembled those of glands from early pregnant normal mice and that during lactation the mammary glands of transgenic mice had smaller alveoli than wild-type mice and exhibited reduced milk expression. 13,14 Thus, the transition to loss of function in these animals must have begun during pregnancy or even earlier, rather than during lactation. To gain insight into the underlying mechanism of this phenomenon, we analyzed when and how expression of the SL-1 transgene led to alteration of gene expression in the developing mammary gland.
Endogenous SL-1 is the major MMP expressed in the stroma of mammary glands from virgin, early pregnant, and involuting mice. 9,12,15,25,26 We therefore examined expression of endogenous SL-1 as a function of the expression of the rat transgene. Reverse transcription-PCR amplification suggested that endogenous SL-1 mRNA was up-regulated as a consequence of SL-1 transgene expression (Figure 1A) ▶ . As a more quantitative measure of endogenous SL-1 mRNA, total RNA from mammary glands of normal and transgenic mice was analyzed by Northern hybridization. Endogenous SL-1 mRNA was up-regulated in mammary glands from 70-day virgin and 10- and 15-day pregnant transgenic mice, relative to their normal counterparts (Figure 1 ▶ , B and C). However, the difference in the expression of endogenous SL-1 in glands from transgenic and normal mice at 6 or 18 days of pregnancy was not statistically significant, and there was no difference during lactation and involution. Three other MMPs that have characteristic expression patterns during mammary gland development, matrilysin, gelatinase A, and SL-3, 9-15 were also examined by reverse transcription-PCR. The levels of these enzymes are too low to be detected by northern blots. Matrilysin, which is expressed only by epithelial cells, was expressed in glands from 70-day virgin transgenic mice, but was not found in glands from normal mice at the same developmental stage (Figure 1A) ▶ . SL-3, which is restricted to the mammary stroma, and gelatinase A were detected in glands from lactating transgenic, but not normal, mice (Figure 1A) ▶ .
Figure 1.

MMP expression during mammary gland development of normal and transgenic mice. A: Reverse transcription-PCR analysis of expression of the rat SL-1 (rSL-1) transgene, endogenous SL-1 (mSL-1), matrilysin (ML), gelatinase A (GA), and SL-3 (SL-3) in mammary glands in 70-day virgin (V 70), 10-day pregnant (P 10), 18-day pregnant (P 18), and 8-day lactating (L 8) normal (N) and transgenic (TG) mice. For each amplification product, the top row of each panel (a) shows negative images of ethidium bromide-stained PCR products after 40 cycles of amplification, and the bottom row of each panel (b) shows the Southern blot analysis with digoxygenin-labeled internal oliogodeoxynucleotides to confirm the identity of PCR products. The specificity of the primers for mouse and rat SL-1 was verified using vectors containing the appropriate mouse (mV) or rat (rV) cDNA. Note that rat SL-1 mRNA is expressed only in mammary glands of transgenic but not normal mice as expected, whereas endogenous mouse SL-1 mRNA is expressed in both normal and transgenic mice at all stages of development. B: Northern blot analysis of expression of endogenous SL-1 mRNA. Total RNA was extracted from two glands of 70-day virgin (V 70), 6-day pregnant (P 6), 10-day pregnant (P 10), 12-day pregnant (P 12), 15-day pregnant (P15), 18-day pregnant (P 18), 3-day lactating (L 3) and 8-day lactating (L 8) normal (N) and transgenic (TG) mice. Blots were hybridized with a cDNA probe specific for mouse SL-1 (mSL-1), stripped, and reprobed with a cDNA probe complementary to 28S ribosomal RNA (28S RNA). C: Northern hybridization signals were quantitated by scanning densitometry and normalized to signals obtained from blots reprobed with a cDNA complementary to 28S RNA. The value obtained at 8-day lactation for normal (open bars; N) and transgenic (solid bars; TG) mice was established as the baseline. For comparison of different blots, mRNA levels at various stages of development are expressed as a ratio relative to mRNA present at the normal glands of virgin animals, which was set to 1. Data are shown as means, with error bars representing SEM for four different sets of virgin, pregnant, and lactating glands. *Significant differences (P < 0.05) as determined by the Mann-Whitney U test.
SL-1 expression in glands from normal and transgenic animals was restricted to periductal and intralobular fibroblasts. However, in transgenic mice, the fibrous connective tissue was extended into the adipocyte compartment (Figure 2) ▶ . The pattern of endogenous SL-1 expression in transgenic animals during mid-pregnancy was reminiscent of the expression pattern of SL-1 observed in glands from normal involuting mice (Figure 2 ▶ , d and f). However, the pattern of expression in involution was similar in normal and transgenic mice (Figure 2 ▶ , f and h). In both normal and transgenic mice, endogenous SL-1 was expressed in fibroblasts surrounding epithelial cords during involution. In all cases, the endogenous SL-1 mRNA was undetectable in the epithelial compartment (Figure 2) ▶ .
Figure 2.
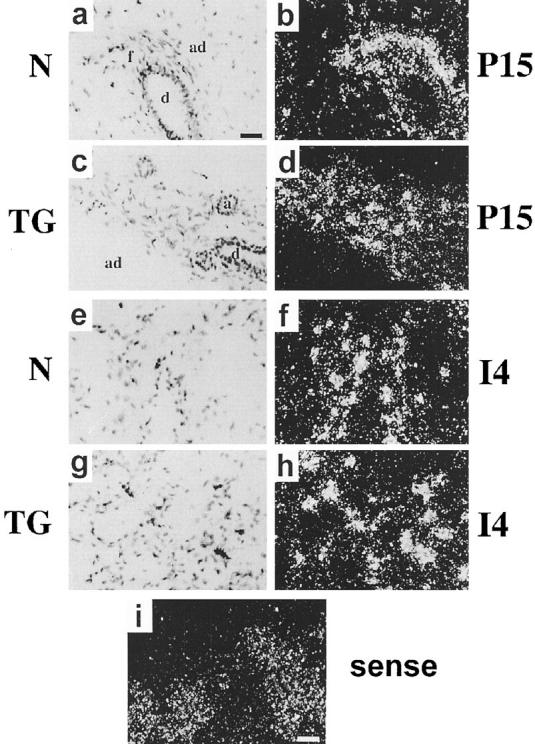
Localization of endogenous SL-1 mRNA in mid-pregnant and involuting glands. In situ hybridization was performed with an antisense (a to h) or sense (i) mouse SL-1 probes in 15-day pregnant (P15) (a to d and i) and 4-day involuting (I4) glands (e to h) from normal (N) (a, b, e, and f) and transgenic (TG) (c, d, g, h, and i) mice; bright-field (a, c, e, and g) and dark-field (b, d, f, h, and i) microscopy images. Bar in a indicates 100 μm for (a to h); bar in i indicates 200 μm. a, alveolus; ad, adipocyte; f, fibroblasts; d, duct.
Both Epithelial and Adipocyte BMs Are Degraded in Glands from Pregnant Transgenic Mice
During normal mammary gland involution, BM disruption is paralleled by increased expression of a number of MMPs in the stromal compartment. In our previous study, we documented loss of BM around epithelial components of the mammary glands from lactating transgenic mice. 14 When mammary glands from virgin (data not shown) and 6-day pregnant (Figure 3) ▶ transgenic animals were examined by immunostaining for laminin and type IV collagen, BMs appeared intact, despite the observed increase in endogenous SL-1 mRNA in the virgin animals. However, BM fragmentation was obvious in glands from 15-day pregnant mice (Figure 3) ▶ . Interestingly, not only the BMs around alveoli, but also those around adipocytes were affected (Figure 3 ▶ , d and f), whereas the BMs of blood vessels remained intact (Figure 3d) ▶ , indicating the significance of locally secreted SL-1, as opposed to systemic changes.
Figure 3.
Immunolocalization of type IV collagen and laminin in mammary glands. Type IV collagen (COL IV) (a to d) and laminin (LN) (e and f) were localized by indirect immunofluorescence staining in 6-day pregnant (P6) (a and b) and 15-day pregnant (P15) (c to f) glands from normal (N) (a, c, and e) and transgenic (TG) (b, d, and f) mice; g and h show 4′,6′-diamidino-2-phenyl indole staining for nuclei of the same samples as e and f, respectively. Note the reduction in type IV collagen and laminin in the BM surrounding alveoli (arrowheads) and adipocytes (open curved arrows) in glands from 15-day pregnant transgenic mice compared with normal mice (compare c with d and e with f). In contrast, the staining of the BM surrounding blood vessels in d (filled white arrows) is not altered. Bar in f, 50 μm for all panels.
We had proposed previously that loss of BM in culture leads to apoptosis of mammary epithelial cells. 27 It was also reported that apoptosis occurs in SL-1 transgenic animals during late pregnancy. 13 To determine when unscheduled apoptosis was initiated in the glands from pregnant transgenic animals, we examined apoptosis across all developmental stages. No difference in apoptosis between glands of wild-type and transgenic animals was seen at day 10 of pregnancy or earlier (not shown). However, by 15 days, when the glands of transgenic mice appeared morphologically underdeveloped, there were 37 cells, (±13% of the total number of cells) and a range of 2 to 12 (range per field) times the number of apoptotic cells per high-powered field in transgenic gland sections compared with the normal glands (Figure 4 ▶ ; Table 1 ▶ ). Therefore, the transgenic glands showed a peak increase of 7 to 10-fold times the apoptotic index (apoptotic cells/total cells) at 15 to 16 days of pregnancy, compared with normal glands. The enhanced apoptosis in glands from transgenic animals was suppressed during lactation (not shown). These results indicate that the transgenic phenotype of smaller alveoli and lower β-casein expression during the later stages of pregnancy can be attributed to unscheduled apoptosis. Our data also suggest that the lactational phenotype is strongly dominant, because it overrides the aberrant consequences of SL-1 transgene expression during pregnancy.
Figure 4.
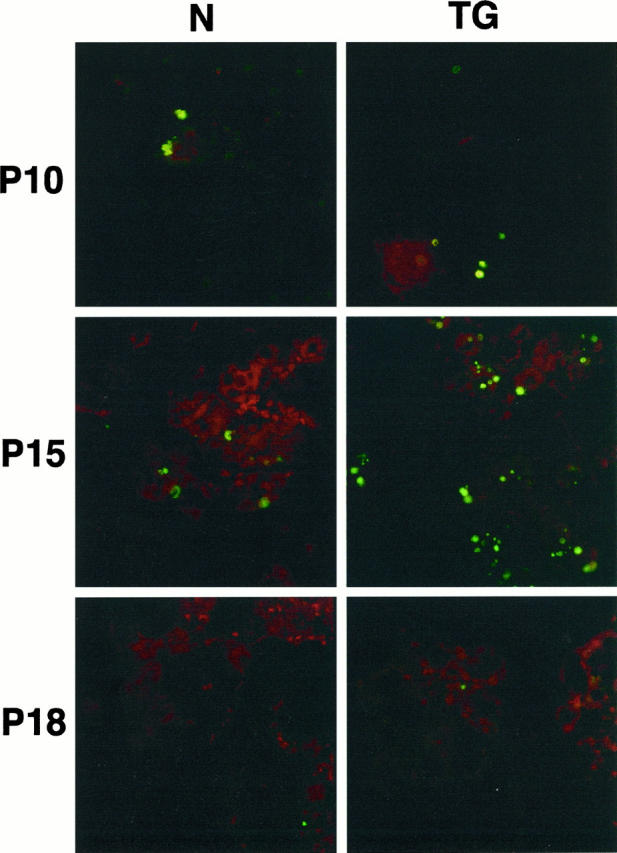
Apoptosis in normal and transgenic mammary glands. In situ detection of fragmented DNA detected by fluorescein isothiocyanate-digoxigenin nucleotide labeling of 3′-OH end (Apotag, Oncor) in 10-day (P10), 15-day (P15), and 18-day (P18) pregnant glands from normal (N) and transgenic (TG) mice. Note the increase of epithelial cells undergoing apoptosis in the 15-day pregnant gland from transgenic mice. The total number of cells per field was also greatly decreased in glands from late pregnant transgenic mice.
Table 1.
Apoptosis in Pregnant Mammary Glands
| Days of pregnancy | Apoptotic cells | |
|---|---|---|
| Normal | Transgenic | |
| 10 | 3.6 ± 1.3 (n = 5) | 3.0 ± 1.7 (n = 6) |
| 15 | 8.0 ± 4.0 (n = 6) | 21.5 ± 8.0 (n = 6) |
| 18 | 3.0 ± 1.5 (n = 5) | 2.4 ± 1.1 (n = 6) |
Paraffin sections were stained with Apotag fluorescein kit and number of apoptotic cells per random ×10 field counted. Results are given as mean ± standard error.
Tenascin-C Is Expressed Inappropriately in Glands from Pregnant and Lactating Transgenic Mice
Tenascin-C is up-regulated in the mammary gland when epithelial-mesenchymal communications are altered, such as in involution or in the stromal reaction in response to breast cancer. 23,28,29 Thus, we would expect inappropriate tenascin-C expression if the microenvironment of the mammary stroma were altered to resemble the involuting and/or the tumor stroma. Tenascin-C mRNA was not expressed in glands from virgin, pregnant, or lactating normal mice, but was abundant during involution (Figure 5A) ▶ . There was no tenascin-C expression in glands from virgin transgenic mice, consistent with the observation that BM was not degraded. During pregnancy and lactation, however, tenascin-C was expressed in transgenic mammary tissue (Figure 5A) ▶ . Interestingly, two tenascin-C mRNA species were found, with the larger transcript predominating in pregnancy and involution. In contrast, only the smaller mRNA was present during lactation in transgenic mice (Figure 5A) ▶ . Furthermore, the amount was very small, underscoring again the dominance of the lactational phenotype mentioned above. Mammary glands from normal mice expressed both tenascin-C transcripts at similar levels only during involution, although in contrast to transgenic animals, expression was much lower at day 2 than at day 4 of involution. Tenascin-C protein was localized to the proximity of the BM surrounding the alveoli and ducts in glands from lactating transgenic mice (Figure 5B) ▶ .
Figure 5.
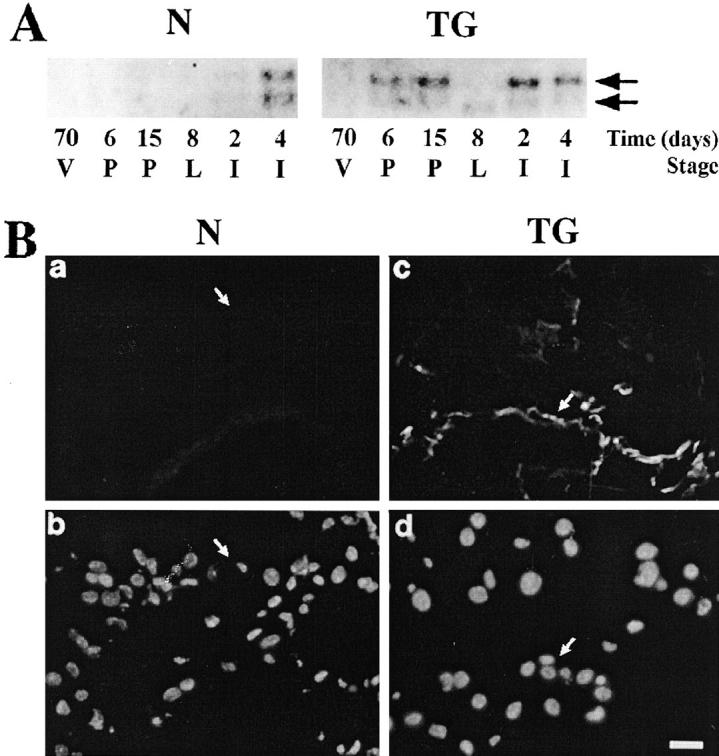
Expression of tenascin-C in mammary glands of normal and transgenic mice. A: Northern blot analysis for tenascin-C mRNA. Total RNA was extracted from mammary glands of 70-day virgin (70 V), 6-day pregnant (6 P), 15-day pregnant (15 P), 8-day lactating (8 L), 2-day involuting (2 I), and 4-day involuting (4 I) normal (N) and transgenic (TG) mice. Blots were hybridized with a cDNA probe for tenascin-C. Arrows indicate positions of the large and small tenascin-C transcripts. B: Immunolocalization of tenascin-C in mammary glands from 8-day lactating mice. Frozen tissue sections from mammary glands of normal (N) (a and b) and transgenic (TG) (c and d) mice were stained with a polyclonal antiserum to tenascin-C followed by a fluorescein-labeled conjugate (a and c) and counterstained with 4′,6′-diamidino-2-phenyl indole to visualize nuclei (b and d). Arrows indicate borders of duct. Bar in d, 25 μm for a to d. Note that tenascin-C was not detected in normal lactating mice (a) but was found in the proximity of the BM surrounding alveoli and ducts (arrow) in glands from transgenic mice.
Collagen and Vascularization Are Increased in Glands from Transgenic Mice
The results described above suggest that in glands from pregnant transgenic mice, both stromal and epithelial compartments express characteristics usually found only during involution in normal mice, and in the stroma from breast cancer patients. 9,28 To further explore this possibility, we stained for collagen content throughout mammary gland development using Gomori’s trichrome method. There was a considerable increase in collagen deposition around the ducts and alveoli as well as in the adipose compartment, in both virgin and pregnant transgenic animals (Figure 6) ▶ . Collagen deposition increased approximately 8-fold in virgin and 2-fold in 6-day pregnant glands from transgenic mice compared with normal mice (Table 2) ▶ . At day 15 of pregnancy and during lactation, the number of ducts and alveoli were no longer significantly different in normal and transgenic mice; however, there was still a statistically significant increase in collagen content at day 15 of pregnancy, because of increased deposition around the ducts.
Figure 6.
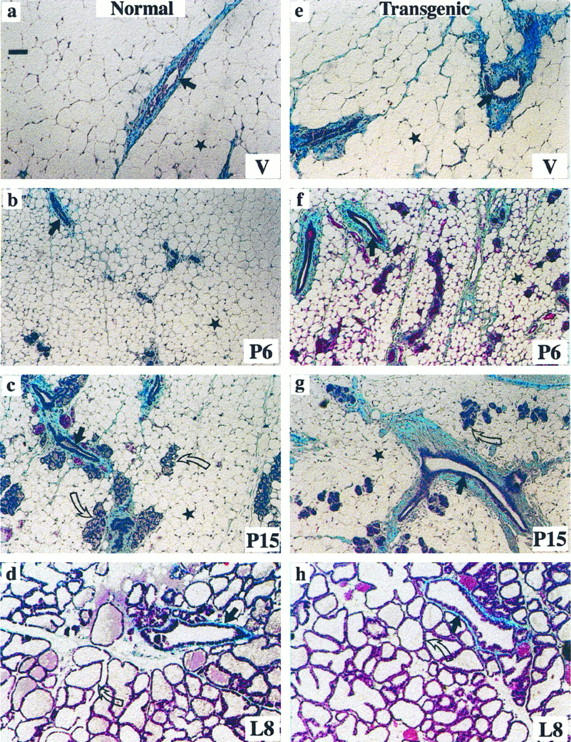
Accumulation of collagen in mammary glands of transgenic mice. Gomori’s trichrome staining for total collagen (blue) in 70-day virgin (V) (a and e), 6-day pregnant (P6) (b and f), 15-day pregnant (P15) (c and g) and 8-day lactating (L8) (d and h) glands from normal (a to d) and transgenic (e to h) mice. Straight arrows indicate mammary ducts, curved arrows indicate alveolar structures; stars indicate adipocytes. Bar in a, 50 μm for a to h. Note that collagen deposition around mammary ducts is much more prominent in transgenic compared with normal mice (see also Table 1 ▶ ).
Table 2.
Morphometric Analysis of Collagen Content in Mammary Glands of Normal and Transgenic Mice
| Stage | Mice | mm2 collagen/mm2 gland | P | mm2 collagen/mm2 of duct | P | Number of ductal and alveolar profiles/mm2 gland | P |
|---|---|---|---|---|---|---|---|
| 70-day virgin | Normal | 0.026 ± 0.004 | <0.01 | ND | ND | ||
| Transgenic | 0.210 ± 0.011 | ||||||
| 6-day pregnant | Normal | 0.117 ± 0.003 | <0.05 | ND | ND | ||
| Transgenic | 0.261 ± 0.006 | ||||||
| 15-day pregnant | Normal | 0.213 ± 0.006 | <0.05 | 0.027 ± 0.003 | <0.001 | 39.78 ± 1.31 | <0.77 |
| Transgenic | 0.257 ± 0.005 | 0.043 ± 0.002 | 40.91 ± 2.17 |
The morphometric analysis was performed using a color image processing workstation (Visiolab, Biocom, Les Ulis, France). For each determination, areas of collagen were stained using the Gomori trichrome method (5-mm2 areas of glands and 10-mm2 areas of ducts from two mice). Values are given as means ± SEM. Significance of the data (P) was determined by Mann-Whitney test. ND, not determined.
Reactive stroma is characterized by increased vascularization. We noted that the number of blood vessels in transgenic glands appeared more numerous than in normal glands in Gomori’s trichrome-stained sections. This observation was confirmed when we immunostained sections with antibodies against von Willebrand factor (vWF), a specific marker of endothelial cells (Figure 7A) ▶ . To quantify the blood vessels, we then immunoblotted gland extracts and found a 2-fold increase in vWF per unit weight of mammary gland (not shown). The increase in vascularization was observable also by measuring PECAM-1 mRNA. At all developmental stages examined, we found an increase in mRNA for the adhesion molecule PECAM-1, a constitutive marker of endothelial cells (Figure 7 ▶ , B and C). 21
Figure 7.

Vascularization of mammary glands in transgenic mice. A: Immunolocalization of vWF in blood vessels localized in alveoli (arrows) and in periductal stroma (arrowhead) of virgin (V) (a and c) and mid-pregnant (P) (b and d) mammary glands from normal (N) (a and b) and transgenic (TG) (c and d) mice. Note the increase in staining of vWF in glands from virgin and pregnant transgenic mice. B: Expression of the endothelial cell marker PECAM-1 in the glands of 70-day virgin (70 V), 15-day pregnant (15 P), and 8-day lactating (8 L) normal (N) and transgenic (TG) mice. Total RNA was analyzed by Northern blot analysis with a cDNA specific for PECAM-1 and a cDNA complementary to 28S ribosomal RNA (28S RNA). C: Quantification of PECAM-1 mRNA levels in mammary glands of 70-day virgin (70 V), 6-day pregnant (6 P), 10-day pregnant (10 P), 15-day pregnant (15 P), 8-day lactating (8 L), 2-day involuting (2 I), and 4-day involuting (4 I) normal (open bars; N) and transgenic (filled bars; TG) mice by scanning densitometry. The PECAM-1 levels were normalized by hybridization with a 28S RNA probe. For comparison of different blots, mRNA levels at various stages of development are expressed as a ratio relative to mRNA present at the normal virgin stage, which was set to 1. Error bars represent standard deviations from four independent experiments using glands from virgin 15-day pregnant and 8-day lactating mice. Values for 6 P, 10 P, 2 I, and 4 I represent the mean for two independent experiments.
Discussion
The goal of the present study was to determine whether stromal-epithelial interactions were altered before tumor formation in SL-1 transgenic mice. In the mammary glands from virgin and mid-pregnant normal and transgenic mice, endogenous SL-1 mRNA was localized to stromal fibroblasts surrounding ducts and in the intralobular units. Witty et al 15 have shown a similar localization of SL-1 mRNA in virgin mice. Previous studies using antibodies to SL-1 revealed a myoepithelial localization of SL-1 protein in rodent mammary glands during involution, 26,30 a finding that we have confirmed in both normal and transgenic mice (not shown). Thus, it appears likely that, during all stages of development, SL-1 protein is synthesized and secreted by fibroblasts and subsequently sequestered by myoepithelial cells.
SL-1 degrades many ECM substrates, including fibronectin, types III, IV and V collagen, laminin, and proteoglycans. 13,31 Thus, the BM and other ECM constituents of the mammary ducts 32 could be targets for proteolytic activity of SL-1. This suggests that the activity of endogenous MMPs was highly regulated during normal mammary gland development, as well as in the precocious development of the glands from virgin transgenic mice. Although endogenous SL-1 and matrilysin were increased in addition to SL-1 transgene expression, laminin and type IV collagen-containing BM appeared intact in virgin and 6-day pregnant transgenic mice. Despite the apparent continuity of BM, the epithelium of the glands of virgin and early pregnant transgenic mice resembled the early pregnant state, both morphologically and functionally. Fibroblasts may participate in mammary gland branching morphogenesis and lobuloalveolar development by focal activation of MMPs, or by simply altering the proteinase-to-inhibitor ratio in favor of MMPs, as was suggested for collagenase in salivary gland development. 33 The stroma in the glands of transgenic animals had already acquired a highly reactive phenotype, even before pregnancy, as indicated by an increase in collagen content, vascularization, and augmented levels of stromal cell-derived endogenous SL-1. The altered stroma can progressively modify epithelial-mesenchymal interactions as evidenced by expression of tenascin-C at 6 days of pregnancy in transgenic mice. During embryogenesis and mammary gland involution, tenascin-C is induced in the stroma when the epithelia and the surrounding mesenchymal tissues are in contact, but it is absent from the glands of postnatal virgin, pregnant, and lactating mice. 34,35 Thus, tenascin-C expression in pregnant transgenic mice may be a direct consequence of BM disruption and increased physical contact between epithelium and mesenchyme. 28,35 Significantly, tenascin-C has been shown to inhibit the functional differentiation of mammary cells 23,36 and may thus contribute to the loss of function phenotype observed in pregnant transgenic mice. The observation that the larger tenascin-C transcript was more highly expressed in transgenic glands may be significant: in other systems, the larger mRNA species encodes the 220-kd tenascin-C isoform made only by proliferating cells 34 and in neoplastic human breast tissues. 37 The progressively altered stroma in transgenic mice appears to trigger a maximum of discordance in epithelial-mesenchymal communications with disruption of BM and apoptosis by day 15 of pregnancy. Apoptosis in vivo was directly correlated with the disruption of BM as Boudreau et al 27 have shown for cultured mammary cells. These observations indicate that inappropriate reciprocal communication took place between the two compartments in virgin and early pregnant transgenic glands.
Whereas the BM in glands of virgin mice were intact, the glands of mid-pregnant transgenic mice displayed dramatically reduced levels of type IV collagen and laminin around alveoli compared with normal glands. This diminution could be the direct effect of SL-1 transgene activity and/or a consequence of the induced expression of endogenous MMPs.
Stromal ECM is dramatically altered not only during involution, but also in mammary tumors. 12,28 In tumors, this fibrosis (desmoplasia) is characterized by both qualitative and quantitative changes in collagen production. 1,38 Barsky et al 39 have proposed that tumor cells induce the stromal fibroblasts to synthesize the surplus collagen. In the present study, the collagen content in virgin glands from transgenic animals was increased very early and persisted through all developmental stages long before any tumors were detected. Physical constraints imposed on cells by the surrounding ECM have been shown to be powerful modulators of cell function. 40-42 Thus, release from this organization/orientation by an increase in collagen content may be sufficient to stimulate endothelial cell migration and may account for the increased vascularization observed in virgin glands from transgenic mice. The dramatic disruption of BM observed in glands from mid-pregnant and lactating transgenic mice could also facilitate neovascularization by liberating ECM-bound angiogenic factors such as basic fibroblast growth factor or vascular endothelial growth factor. 43 Focal dissolution of ECM proteins such as laminin and type IV collagen may also promote endothelial cell attachment, migration, proliferation, and/or organization. 44-46 Similar factors may contribute to the formation of a reactive stroma and increased angiogenesis characteristic of breast cancer. 47,48
Our study shows that the expression of an autoactivating rat SL-1 transgene in epithelial cells triggers dynamic and progressive stromal modifications during development of the mammary glands of transgenic mice, beginning with an increase of endogenous SL-1 expression in glands from virgin mice. At this stage of development, increased SL-1 expression leads to a gain of function not only for epithelial branching and alveolar development, 14 but also for endothelial morphogenesis. This, in turn, disrupts the normal stromal-epithelial homeostasis, promoting a reactive stroma normally found only during involution and in mammary gland tumors. Disturbances in the microenvironment and communication between epithelial and mesenchymal cells leads to altered functional differentiation and onset of inappropriate apoptosis. These changes may then become the basis for the prevalence of mammary gland pathologies, including hyperplasia and neoplasia, subsequently found in these animals as they age (manuscript in preparation). SL-1 displays increased expression in breast tumors and has been cloned a number of times as a metastasis-specific gene. 49 Ectopic expression of an autoactivating rat SL-1 transgene in the mammary gland of control mice leads to formation of preneoplastic lesions and invasive breast tumors later in the life of the animal. 16 Inhibition of SL-1 prevents ECM invasion of mammary tumor cells, 22 and its overexpression in functionally normal mammary epithelial cells in culture causes a premalignant phenotype. 50 The details of molecular mechanism(s) by which the SL-1 transgene induces such dramatic changes in stromal-epithelial interactions, and the implications that this may have for tumor progression, remain to be determined.
Acknowledgments
We are indebted to Dr. M. Schachner for providing anti-tenascin-C antiserum and to Drs. Y. Eeckhout, S. Baldwin, and P. Ekblom for the gift of plasmids for mouse SL-1, PECAM-1, and tenascin-C, respectively. We also thank Drs. C. Alexander, N. Boudreau, P-Y. Desprez, C. Roskelley, and M. Sternlicht for helpful discussions and advice; N. Bailey for technical assistance; M. McKenney and B. Johansen for help with editing the manuscript; and A. Culcleasure and M. Rose for secretarial assistance.
Footnotes
Address reprint requests to Dr. Mina J. Bissell, Life Sciences Division, Ernest Orlando Lawrence Berkeley National Laboratory, 1 Cyclotron Road, MS 83-101, Berkeley, CA 94720. E-mail: mjbissell@lbl.gov.
Supported by the Office of Health and Environmental Research, U.S. Department of Energy (under contracts DE-AC03-76-SF00098 and DE-AC03-76-SF01012); by funds from the National Cancer Institute (CA 57621 and CA 64786), the Danish Cancer Society, and the Institut National de la Santé et de la Recherche Médicale; and by fellowships from NATO (to NT), the European Molecular Biology Organisation (to AL), and the California Breast Cancer Research Program (to AL).
Nicole Thomasset’s present address is INSERM U 433, Faculté Laennec, Lyon, France.
André Lochter’s present address is Center for Clinical and Basic Research, Ballerup, Denmark.
Carolyn J. Sympson’s present address is Immunology Department, Searle Research and Development, Monsanto Company, Chesterfield, MO.
Leif R. Lund’s present address is Finsen Laboratory, Rigshospitalet, Copenhagen, Denmark.
References
- 1.Van den Hooff A: Stromal involvement in malignant growth. Adv Cancer Res 1988, 50:159-196 [DOI] [PubMed] [Google Scholar]
- 2.Sakakura T: New aspects of stroma-parenchyma relations in mammary gland differentiation. Int Rev Cytol 1991, 125:165-202 [DOI] [PubMed] [Google Scholar]
- 3.Bissell MJ, Hall HG: Form and function in the mammary gland: the role of extracellular matrix. Neville M Daniel C eds. The Mammary Gland Development, Regulation and Function. 1987, :pp 97-146 Plenum Publishing Corp, New York [Google Scholar]
- 4.Adams JC, Watt FM: Regulation of development and differentiation by the extracellular matrix. Development 1993, 117:1183-1198 [DOI] [PubMed] [Google Scholar]
- 5.Roskelley CD, Srebrow A, Bissell MJ: A hierarchy of ECM-mediated signaling regulates tissue-specific gene expression. Curr Opin Cell Biol 1995, 7:736-747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warburton MJ, Kimbell R, Ferns SA, Hayman AR, Perusinghe N, Monaghan P: Characterization of chondroitin/dermatan sulphate proteoglycans synthesized by rat mammary myoepithelial and fibroblastic cell lines. Biochim Biophys Acta 1992, 1117:291-300 [DOI] [PubMed] [Google Scholar]
- 7.Busso N, Huarte J, Vassalli JD, Sappino A-P, Belin D: Plasminogen activators in the mouse mammary gland: decreased expression during lactation. J Biol Chem 1989, 264:7455-7457 [PubMed] [Google Scholar]
- 8.Lefebvre O, Wolf C, Limacher J-M, Hutin P, Wendling C, LeMeur M, Basset P, Rio MC: The breast cancer-associated SL-3 gene is expressed during mouse mammary gland apoptosis. J Cell Biol 1992, 119:997-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Werb Z: Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development 1996, 122:181-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wicha MS, Liotta LA, Vonderhaar BK, Kidwell WR: Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev Biol 1980, 80:253-256 [DOI] [PubMed] [Google Scholar]
- 11.Talhouk RS, Chin JR, Unemori EN, Werb Z, Bissell MJ: Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development 1991, 112:439-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talhouk RS, Bissell MJ, Werb Z: Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol 1992, 118:1271-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander CM, Howard EW, Bissell MJ, Werb Z: Rescue of epithelial cell apoptosis and entactin degradation by a TIMP-1 transgene. J Cell Biol 1996, 135:1669-1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z: Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol 1994, 125:681-693(published erratum J Cell Biol 1996, 132:753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witty JP, Wright JH, Matrisian LM: Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell 1995, 6:1287-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sympson CJ, Bissell MJ, Werb Z: Mammary gland tumor formation in transgenic mice overexpressing stromelysis-1. Semin Cancer Biol 1995, 6:159-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 18.Hammani K, Henriet P, Eeckhout Y: Cloning and sequencing of a cDNA encoding mouse stromelysin-1. Gene 1992, 120:321-322 [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Lopez R, Nicholson R, Gesnel MC, Matrisian LM, Breathnach R: Structure-function relationships in the collagenase family member transin. J Biol Chem 1988, 263:11892-11899 [PubMed] [Google Scholar]
- 20.Weller A, Beck S, Ekblom P: Amino acid sequence of mouse tenascin-C and differential expression of two tenascin-C isoforms during embryogenesis. J Cell Biol 1991, 112:355-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, Buck CA: Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development 1994, 120:2539-2553 [DOI] [PubMed] [Google Scholar]
- 22.Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ: Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem 1997, 272:5007-5015 [DOI] [PubMed] [Google Scholar]
- 23.Jones LP, Boudreau N, Myers CA, Erickson HP, Bissell MJ: Tenascin-C inhibits extracellular matrix-dependent gene expression in mammary epithelial cells: localization of active regions using recombinant tenascin-C fragments. J Cell Sci 1995, 108:519-527 [DOI] [PubMed] [Google Scholar]
- 24.Barr RJ, Stegman SJ: Delayed skin test reaction to injectable collagen implant (Zyderm): the histopathologic comparative study. J Am Acad Dermatol 1984, 10:652-658 [DOI] [PubMed] [Google Scholar]
- 25.Strange R, Li F, Saurer S, Burkhardt A, Friis RR: Apoptotic cell death and tissue remodeling during mouse mammary gland involution. Development 1992, 115:49-58 [DOI] [PubMed] [Google Scholar]
- 26.Dickson SR, Warburton MJ: Enhanced synthesis of gelatinase and stromelysin by myoepithelial cells during involution of the rat mammary gland. J Histochem Cytochem 1992, 40:697-703 [DOI] [PubMed] [Google Scholar]
- 27.Boudreau N, Sympson CJ, Werb Z, Bissell MJ: Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995, 267:891-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rønnov-Jessen L, Petersen OW, Bissell MJ: Cellular changes involved in conversion of normal to malignant breast: the importance of the stromal reaction. Physiol Rev 1995, 76:69-125 [DOI] [PubMed] [Google Scholar]
- 29.Lochter A, Bissell MJ: Involvement of extracellular matrix constituents in breast cancer. Semin Cancer Biol 1995, 6:1631-1673 [DOI] [PubMed] [Google Scholar]
- 30.Li F, Strange R, Friis RR, Djonov V, Altermatt H-J, Saurer S, Niemann H, Andres AC: Expression of stromelysin-1 and TIMP-1 in the involuting mammary gland and in early invasive tumors of the mouse. Int J Cancer 1994, 59:560-568 [DOI] [PubMed] [Google Scholar]
- 31.Chin JR, Murphy G, Werb Z: Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase: biosynthesis, isolation, characterization, and substrates. J Biol Chem 1985, 260:12367-12376 [PubMed] [Google Scholar]
- 32.Silberstein GB, Daniel CW: Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol 1982, 90:215-222 [DOI] [PubMed] [Google Scholar]
- 33.Fukuda Y, Masuda Y, Kishi J-I, Hashimoto Y, Hayakawa T, Nogawa H, Nakanishi Y: The role of interstitial collagens in cleft formation of mouse embryonic submandibular gland during initial branching. Development 1988, 103:259-267 [DOI] [PubMed] [Google Scholar]
- 34.Kalembey I, Yoshida T, Iriyama K, Sakakura: Analysis of tenascin mRNA in the murine mammary gland from embryogenesis to carcinogenesis: an in situ hybridization study. Int J Dev Biol 1997, 41:131-139 [PubMed] [Google Scholar]
- 35.Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T: Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 1986, 47:131-139 [DOI] [PubMed] [Google Scholar]
- 36.Chammas R, Taverna D, Cella N, Santos C, Hynes NE: Laminin and tenascin assembly and expression regulate HC11 mouse mammary cell differentiation. J Cell Sci 1994, 107:1031-1040 [DOI] [PubMed] [Google Scholar]
- 37.Borsi L, Carnemolla B, Nicol G, Spina B, Tanara G, Zardi L: Expression of different tenascin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int J Cancer 1992, 52:688-692 [DOI] [PubMed] [Google Scholar]
- 38.Sieweke MH, Bissell MJ: The tumor-promoting effect of wounding: a possible role for TGF-β induced stromal alterations. Crit Rev Oncogenesis 1994, 5:297-311 [DOI] [PubMed] [Google Scholar]
- 39.Barsky SH, Rao CN, Grotendorst GR, Liotta LA: Increased content of type V collagen in desmoplasia of human breast carcinoma. Am J Pathol 1982, 108:276-283 [PMC free article] [PubMed] [Google Scholar]
- 40.Emerman JT, Burwen SJ, Pitelka DR: Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell 1979, 11:109-119 [DOI] [PubMed] [Google Scholar]
- 41.Lee EY-H, Parry G, Bissell MJ: Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol 1984, 98:146-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingber DE, Jamieson JD: Cells as tensegrity structures: architectural regulation of histiodifferentiation by physical forces transduced over basement membrane. Anderson LC Gahmberg CG Ekblom P eds. Gene Expression during Normal and Malignant Differentiation. 1985, :pp 13-32 Academic Press, Orlando [Google Scholar]
- 43.Klagsbrun M: Mediators of angiogenesis: the biological significance of basic fibroblast growth factor (bFGF)-heparin and heparan sulfate interactions. Semin Cancer Biol 1992, 3:81-87 [PubMed] [Google Scholar]
- 44.Madri JA, Williams SK: Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol 1983, 97:153-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ingber DE, Madri JA, Folkman J: Endothelial growth factors and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell Dev Biol 1987, 23:387-394 [DOI] [PubMed] [Google Scholar]
- 46.Herbst TJ, McCarthy JB, Tsilibary EC, Furcht LT: Differential effects of laminin, intact type IV collagen, and specific domains of type IV collagen on endothelial cell adhesion and migration. J Cell Biol 1988, 106:1365-1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weidner N, Semple JP, Welch WR, Folkman J: Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. New Engl J Med 1991, 324:1-8 [DOI] [PubMed] [Google Scholar]
- 48.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G: Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1992, 84:1875-1887 [DOI] [PubMed] [Google Scholar]
- 49.MacDougall JR, Matrisian LM: Contribution of tumor and stromal matrix metalloproteinases in tumor progression, invasion and metastasis. Cancer Metastasis Rev 1995, 14:351-362 [DOI] [PubMed] [Google Scholar]
- 50.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ: Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and premalignant phenotype in mammary epithelial cells. J Cell Biol 1997, 139:1861-1872 [DOI] [PMC free article] [PubMed] [Google Scholar]



