Abstract
In earlier experiments, exogenous administration of secretory leukocyte protease inhibitor (SLPI) suppressed acute lung injury induced by deposition of IgG immune complexes. In the current studies we examined the mechanism of the protective effects of SLPI in this model. The presence of SLPI in the IgG immune complex-model of lung injury reduced the increase in extravascular leakage of 125I-albumin, the intensity of up-regulation of lung vascular intercellular adhesion molecule-1, and the numbers of neutrophils accumulating in the lung. The presence of SLPI caused greatly reduced activation (ie, nuclear translocation) of the transcription nuclear factor-κB (NF-κB) in lung cells but did not suppress activation of lung mitogen-activated protein kinase. SLPI did not alter NF-κB activation in alveolar macrophages harvested 30 minutes after initiation of lung inflammation. In the presence of SLPI, content of tumor necrosis factor-α, CXC chemokines, and C5a in bronchoalveolar fluids was unaffected. In the inflamed lungs, inhibition of NF-κB activation by SLPI was associated with elevated levels of lung IκBβ (but not IκBα) protein in the absence of elevated mRNA for IκBβ. When instilled into normal lung, SLPI also caused similar changes (increases) in lung IκBβ. Finally, in the lung inflammatory model used, the presence of anti-SLPI caused accentuated activation of NF-κB. These data confirm the anti-inflammatory effect of SLPI in lung and point to a mechanism of anti-inflammatory effects of SLPI. SLPI appears to function as an endogenous regulator of lung inflammation.
During acute inflammatory injury of lung, neutrophils are recruited from the vascular space into interstitial and distal airway compartments. Secretory products of activated neutrophils, including oxidants and proteolytic enzymes, mediate lung parenchymal injury. Under normal circumstances, lung cells are protected from oxidant- and protease-mediated damage by a number of endogenous factors. Secretory leukocyte protease inhibitor (SLPI) represents an important component of the pulmonary anti-protease defense system. SLPI, a 12-kd protein that potently inhibits enzymes with serine protease activity, is constitutively expressed by a number of lung cell types including Clara cells of the bronchial epithelium and type II pneumocytes. 1-3 Recently, we have also shown that SLPI is expressed by pulmonary vascular endothelial cells in the inflamed lung. 1 It is known that inflammatory mediators induce SLPI expression in lung cells as well as in macrophages and neutrophils. 4-6 The protective properties of SLPI were originally thought to be due solely to its inhibitory effects on serine proteases because administration of SLPI reduced neutrophil elastase-induced emphysema. 7,8 However, other studies have suggested that SLPI increases the level of pulmonary glutathione, which may serve to reduce the extent of oxidant-mediated tissue injury. 9,10 SLPI also interferes with HIV entry into CD4+ cells, 11 suggesting that its biological effects may extend beyond inhibition of serine proteases.
Acute lung injury induced by intrapulmonary deposition of IgG immune complexes in rats exhibits a pathophysiology similar to that observed during sepsis, ischemia/reperfusion, or trauma. 12-15 In this model, enhanced production of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) by activated lung macrophages causes up-regulation of the adhesion molecules, intercellular adhesion molecule-1 (ICAM-1), and E-selectin on the pulmonary endothelium. 16,17 Interactions of these vascular adhesion molecules with their respective ligands on blood neutrophils cause leukocyte adhesion to the endothelium and recruitment of neutrophils into the alveolar compartment. The ensuing lung injury is mediated by oxidants and proteases released by neutrophils and lung macrophages and is characterized by increased vascular permeability and alveolar hemorrhage. 18,19 During this inflammatory response, activation of the transcription factor, nuclear factor-κB (NF-κB), occurs in a time course similar to that for the production of TNF-α, IL-1β, and the expression of adhesion molecules in the pulmonary vasculature. 20
Administration of SLPI attenuates pulmonary recruitment of neutrophils and decreases lung injury induced by intrapulmonary deposition of IgG immune complexes. 21 Blockade of endogenous SLPI results in an augmented inflammatory response with increased recruitment of neutrophils and lung injury (Gipson TS, Bless NM, Crouch LD, Shanley TP, Bleavins MR, Tefera W, McConnell PC, Mueller WT, Johnson KJ, Ward PA: Role of endogenous protease inhibitors in regulation of acute lung inflammatory injury. Submitted). However, the exact mechanisms by which SLPI exerts its anti-inflammatory effects in lung are unclear. Although SLPI inhibits serine proteases released by activated phagocytes, more recent studies have shown that SLPI interferes with the signal transduction pathway involved in the generation of matrix metalloproteinases. 22 In the current studies, we sought to determine whether the protective effects of SLPI during lung inflammation might be caused by effects on other signal transduction mechanisms including NF-κB and mitogen-activated protein kinase (MAPK).
Materials and Methods
Reagents
Recombinant human SLPI and tissue inhibitor of metalloproteinases-2 (TIMP-2) were kindly provided by Dr. Thomas R. Ulich, (Amgen Inc., Thousand Oaks, CA). Rabbit polyclonal antibody to rat SLPI was prepared as described elsewhere. 1 New Zealand White rabbits were immunized with rat SLPI in incomplete Freund’s adjuvant. Immune serum (10−6 titer) was affinity purified, and the IgG administered intratracheally at a dose of 300 μg.
IgG Immune Complex-Induced Alveolitis
Pathogen-free male Long-Evans rats (275 to 300 g; Harlan Sprague-Dawley, Indianapolis, IN) were anesthetized with Ketamine HCl (150 mg/kg, intraperitoneally). A total of 1.5 mg of rabbit polyclonal IgG anti-bovine serum albumin (BSA) (ICN Biomedicals, Inc., Costa Mesa, CA) in a volume of 0.3 ml of phosphate-buffered saline (PBS) was instilled via an intratracheal catheter during inspiration. Immediately thereafter, 10 mg of BSA in 0.5 ml of PBS were injected intravenously. Negative control rats received PBS intratracheally. For analysis of pulmonary vascular permeability, trace amounts of 125I-labeled BSA were injected intravenously. Four hours after IgG immune complex deposition, rats were exsanguinated, the pulmonary circulation was flushed with 10 ml of PBS by pulmonary artery injection and the lungs were surgically dissected. The extent of lung injury was quantified by calculating the lung permeability index (dividing the amount of radioactivity (125I-labeled BSA) in the perfused lungs by the amount of radioactivity in 1.0 ml of blood obtained at the time of death). For analysis of NF-κB and MAPK activation, lungs were immediately frozen in liquid nitrogen after vascular perfusion with PBS.
NF-κB Activation
Nuclear extracts of whole lung tissues and alveolar macrophages were prepared as previously described 20 and analyzed by electrophoretic mobility shift assay (EMSA). Briefly, double-stranded NF-κB consensus oligonucleotide (5′-GTGAGGGGACTTTCCCAGGC-3′; Promega, Madison, WI) was end-labeled with γ[32P]ATP (3000 Ci/mmol at 10 mCi/ml, Amersham, Arlington Heights, IL). Binding reactions containing equal amounts of nuclear protein extract (10 μg) and 35 fmols (∼50,000 cpm, Cherenkov counting) of oligonucleotide were performed for 30 minutes in binding buffer (4% glycerol, 1 mmol/L MgCl2, 0.5 mmol/L EDTA, pH 8.0, 0.5 mmol/L dithiothreitol, 50 mmol/L NaCl, 10 mmol/L Tris, pH 7.6, 50 μg/ml poly(dI-dC); Pharmacia, Piscataway, NJ). Reaction volumes were held constant to 15 μl. Reaction products were separated in a 4% polyacrylamide gel in 0.25 × TBE buffer (25 mmol/L Tris, 22.5 mmol/L boric acid, 0.25 mmol/L EDTA) and analyzed by autoradiography. NF-κB activation was quantitated from digitized autoradiography films using image analysis software (Adobe Systems, San Jose, CA).
MAPK Activation and Western Blot Analysis
Whole lung tissue or alveolar macrophages obtained by BAL (bronchoalveolar lavage) were homogenized in lysis buffer (10 mmol/L HEPES, pH 7.9, 150 mmol/L NaCl, 1 mmol/L EDTA, 0.6% Nonidet P-40, 0.5 mmol/L phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 10 μg/ml soybean trypsin inhibitor, 1 μg/ml pepstatin) on ice. Homogenates were sonicated and centrifuged at 14,000 rpm to remove cellular debris. Interfering IgG anti-BSA in homogenates was removed with GammaBind G sepharose (Pharmacia). Protein concentrations were determined as described for nuclear extracts. Samples (10 μg for MAPK analysis, 100 μg for IκB proteins) were separated in a denaturing 12.5% polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with Tris-buffered saline containing Tween 20 (40 mmol/L Tris, pH 7.6, 300 mmol/L NaCl, 0.1% Tween 20) containing 5% nonfat dry milk for 12 hours at 4°C. For analysis of MAPK proteins, membranes were incubated in a 1:1000 dilution of rabbit polyclonal anti-phospho p42/p44 or anti-nonphosphorlyated p42/p44 (New England Biolabs, Inc., Beverly, MA). For analysis of IκB proteins, membranes were incubated in a 1:1000 dilution of rabbit polyclonal anti-IκBα or anti-IκBβ (Santa Cruz Biotechnology, Santa Cruz, CA) in TBST. After three washes in TBST, membranes were incubated in a 1:50,000 dilution of horseradish peroxidase-conjugated donkey anti-rabbit IgG (Amersham Corp., Arlington Heights, IL). Immunoreactive proteins were detected by enhanced chemiluminescence.
Northern Blot Analysis
Total RNA from whole lung tissue was extracted using a guanidinium-isothiocyanate method as described previously. 23 Samples (20 μg of RNA) were fractionated electrophoretically in a 1% formaldehyde gel and transferred to a nylon membrane (MSI, Westboro, MA). cDNA for rat IκBβ was generated by reverse transcriptase-polymerase chain reaction using the following oligonucleotide primers based on the murine sequence: 24 5′ primer: 5′-CATGTAGCTGTCATCCACA-3′ and 3′ primer: 5′-TGTGCACGGAGGAGGCG-3′. The polymerase chain reaction product was sequenced for verification, and the rat IκBβ cDNA probe was end labeled with [32P]dCTP (NEN-DuPont, Boston, MA) using Random Prime (Amersham Corp.). Northern blots were hybridized with the cDNA probe in QuikHyb® hybridization solution (Stratagene Cloning Systems, La Jolla, CA) at 68°C for 4 hours and an autoradiogram developed on Kodak BioMax-MR film (Rochester, NY). Equal loading of RNA samples was confirmed by probing Northern blots with cDNA to β-actin (Stratagene Cloning Systems) end-labeled with [32P]dCTP.
BAL Fluid Analyses
BAL fluids were collected by instilling and withdrawing 5 ml of sterile PBS three times from the lungs via an intratracheal cannula. BAL content of TNF-α was measured using a standard WEHI cell cytotoxicity assay as previously reported. 25 Measurement of macrophage inflammatory protein-2 (MIP-2), cytokine-induced neutrophil chemoattractant (CINC), and C5a in BAL fluids were by enzyme-linked immunosorbent assay as described elsewhere. 26,27
Lung Vascular Expression of ICAM-1
Rats were injected intravenously with 1.5 μCi of 125I-labeled anti-ICAM-1 (clone 1A29; PharMingen, Inc. San Diego, CA) 3.75 hours after induction of lung injury. The specific activity of the anti-ICAM-1 was 11.3 μCi/μg. Fifteen minutes later (4 hours after induction of lung injury), rats were sacrificed, and the lung vasculature was flushed with 10 ml of PBS. Lung vascular ICAM-1 expression (binding index) was calculated by dividing the amount of radioactivity (125I-labeled antibody) in lungs by the amount of radioactivity in 1.0 ml of blood. To control for nonspecific binding and potential accumulation of anti-ICAM-1 antibody in lung parenchyma caused by injury, 1.5 μCi of 125I-labeled nonspecific isotype-matched mouse IgG (clone MOPC-21, ICN Biomedicals, Inc.) were administered in a separate set of rats. The specific activity of the irrelevant IgG antibody was 13.0 μCi/μg.
Lung Myeloperoxidase (MPO) Content
Whole lung MPO activity was quantitated as described previously. 28 Briefly, whole lungs homogenates were diluted in 50 mmol/L potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide, pH 6.0. After sonication and two freeze-thaw cycles, samples were centrifuged at 4000 × g for 30 minutes. The supernatants were reacted with H2O2 (0.3 mmol/L) in the presence of tetramethylbenzidine (1.6 mmol/L). MPO activity was assessed by measuring the change in absorbance at 655 nm.
Statistical Analyses
All values are expressed as mean ± SEM. Data were analyzed with a one-way analysis of variance and individual group means were then compared with a Student-Newman-Keuls test. Differences were considered significant when P < 0.05. For calculations of percent change, negative control values were subtracted from positive control and treatment group values.
Results
Effects of SLPI on IgG Immune Complex-Induced Lung Injury
Previous studies demonstrated that when administered intratracheally, 1 mg of SLPI suppressed IgG immune complex-induced lung injury. 21 To determine the lowest effective dose of intratracheally administered SLPI, dose response experiments were performed with the endpoint of extravascular leakage of 125I-albumin. As shown in Figure 1 ▶ , neither 5 nor 25 μg of SLPI (given intratracheally along with the anti-BSA) caused any significant reduction in IgG immune complex-induced lung vascular permeability. However, 100 μg of SLPI reduced lung vascular permeability by 45% (P = 0.006). TIMP-2 had similar effects, reducing albumin leak by 40% (Figure 1) ▶ . The effects of 100 μg SLPI on IgG immune complex-induced increases in proinflammatory mediators in BAL fluids were assessed. As anticipated, intrapulmonary deposition of IgG immune complexes caused significant increases in BAL content of TNF-α, MIP-2, CINC, and C5a (Table 1) ▶ . Co-treatment with 100 μg of SLPI did not significantly alter BAL levels of any of these proinflammatory mediators.
Figure 1.
Effects of SLPI and TIMP-2 on IgG immune complex-induced lung vascular permeability. The permeability index was calculated 4 hours after intratracheal administration of 1.5 mg of anti-BSA followed by intravenous infusion of 10 mg of BSA. SLPI and TIMP-2 were administered intratracheally with the IgG anti-BSA. Values represent mean ± SEM, with n = 4 to 6 for each group. *P < 0.05, compared with absence of SLPI or TIMP-2.
Table 1.
Effects of SLPI on Proinflammatory Mediators in BAL Fluids
| Group | TNF-α (ng/ml) | MIP-2 (ng/ml) | CINC (μg/ml) | C5a (ng/ml) |
|---|---|---|---|---|
| IgG-IC | 536.7 ± 20.1 | 440.7 ± 75.1 | 4.30 ± 0.54 | 14.5 ± 3.6 |
| IgG-IC+ SLPI | 494.7 ± 54.3 | 505.6 ± 74.9 | 3.74 ± 0.61 | 14.8 ± 5.3 |
Values represent mean ± SEM with n = 4 for each group. SLPI (100 μg) was administered intratracheally with the anti-BSA.
Because of a key role for vascular ICAM-1 in this lung inflammatory model, experiments were designed to determine whether SLPI had any effect on the expression of ICAM-1 in the pulmonary vasculature. Intrapulmonary deposition of IgG immune complexes caused the expected increase in expression of lung vascular ICAM-1 (Figure 2) ▶ . The increase in binding index for the anti-ICAM-1 antibody could not be attributed to nonspecific binding or sequestration from lung injury as the binding index for the irrelevant IgG control antibody was only 0.091 ± 0.011 in inflamed lungs (versus 0.065 ± 0.015 in noninflamed lungs). In the presence of 100 μg of SLPI, there was a 67% reduction (P = 0.041) in ICAM-1 expression in the pulmonary vasculature. Because SLPI caused a substantial decrease in lung vascular ICAM-1 expression, we examined whether this effect was associated with decreased lung recruitment of neutrophils. Pulmonary accumulation of neutrophils was quantitated by lung content of MPO. As expected, intrapulmonary deposition of IgG immune complexes caused a greater than sevenfold increase in lung MPO content (Figure 3) ▶ . Co-treatment with SLPI reduced IgG immune complex-induced lung MPO content by 39% (P < 0.001).
Figure 2.
Effects of SLPI on IgG immune complex-induced lung vascular ICAM-1 expression. Lung vascular expression of ICAM-1 was determined 4 hours after intratracheal administration of PBS or 1.5 mg of anti-BSA (IgG-IC) followed by intravenous infusion of 10 mg of BSA. SLPI (100 μg) was administered intratracheally with the IgG anti-BSA. Values represent mean ± SEM, with n = 3 for each group.
Figure 3.
Effects of SLPI on lung neutrophil accumulation following intrapulmonary deposition of IgG immune complexes. Lung MPO content was determined 4 hours after intratracheal administration of PBS or 1.5 mg of anti-BSA (IgG-IC) followed by intravenous infusion of 10 mg of BSA. SLPI (100 μg) was administered intratracheally with the IgG anti-BSA. Values represent mean ± SEM with n = 5 for each group.
Effects of SLPI on Intrapulmonary Activation of NF-κB and MAPK
To investigate whether the protective effects of SLPI might be related to inhibition of pulmonary NF-κB activation (defined as nuclear translocation), nuclear extracts from whole lungs harvested 4 hours after IgG immune complex deposition were analyzed by EMSA. As expected, little NF-κB was present in lung nuclei from rats treated intratracheally with PBS (Figure 4) ▶ . Treatment with IgG immune complexes resulted in a significant increase in nuclear translocation of NF-κB. In the presence of 100 μg of SLPI, NF-κB activation was greatly reduced. Image analysis of digitized EMSA blots demonstrated that the presence of SLPI reduced nuclear localization of lung NF-κB by 64% (P = 0.005). Interestingly, 100 μg of TIMP-2 had no effect on lung NF-κB activation.
Figure 4.
Effects of SLPI on IgG immune complex-induced lung activation of NF-κB. A: NF-κB activation in whole lung tissues harvested 4 hours after intratracheal administration of PBS or 1.5 mg of anti-BSA (IgG-IC) followed by intravenous infusion of 10 mg of BSA. SLPI and TIMP-2 (100 μg) were administered intratracheally with the IgG anti-BSA. B: Quantitation of EMSA blots by image analysis. Values represent mean ± SEM, with n = 5 for each group.
Effects of SLPI on lung MAPK activation in the lung injury model were assessed by Western blot analysis of lung homogenates (Figure 5) ▶ . Four hours after intrapulmonary deposition of IgG immune complexes, phosphorylation (a measure of activation) of MAPK was evident. No phosphorylation of p42 or p44 was found in extracts from lungs instilled with PBS, in striking contrast to phosphorylation of p42 and p44 in inflamed lungs (Figure 5 ▶ , upper frame). Phosphorylation was primarily associated with the p44 form of MAPK (slower migrating band) as contrasted to the p42 form. It also seems likely that in this model of inflammation there is induction of p44 because the Western blot bands were more intense in lungs receiving IgG immune complexes (Figure 5 ▶ , lower frame, second lane versus third and fourth lanes). Quite clearly, co-treatment with SLPI did not seem to interfere with MAPK activation in lung.
Figure 5.
Effects of SLPI on IgG immune complex-induced MAPK activation. MAPK activation was determined by Western blot of whole lung tissues harvested 4 hours after intratracheal administration of PBS or 1.5 mg of anti-BSA (IgG-IC) followed by intravenous infusion of 10 mg of BSA. SLPI (100 μg) was administered intratracheally with the IgG anti-BSA. Activation of MAPK was assessed by the extent of phosphorylation of p42/p44 proteins (upper panel), compared with unphosphorylated p42/p44 proteins (lower panel). Standard controls (std) for p42/p44 proteins verified the location of the phosphorylated and nonphosphorylated MAPK proteins. Results are representative of 5 independent experiments.
We have previously shown that NF-κB activation in alveolar macrophages in vivo is increased as early as 30 minutes after deposition of IgG immune complexes. 29 Furthermore, in a macrophage cell line transgenically over-expressing SLPI, LPS-induced NF-κB activation was attenuated. 5 Therefore, we assessed whether 100 μg of SLPI administered intratracheally reduced NF-κB activation in alveolar macrophages following intrapulmonary deposition of IgG immune complexes. Treatment with SLPI had no effect on NF-κB activation in alveolar macrophages retrieved by BAL 30 minutes after initiation of IgG immune complex deposition (Figure 6) ▶ . Similarly, NF-κB activation in alveolar macrophages stimulated in vitro with 100 μg/ml IgG-BSA immune complexes or LPS (50 ng/ml) was unaffected by co-treatment with 10 μg/ml SLPI (data not shown). Intrapulmonary instillation of SLPI failed to inhibit MAPK activation in alveolar macrophages retrieved by BAL 30 minutes after IgG immune complex deposition (data not shown). Thus, it would appear that the effects of SLPI in lung are not directed toward alveolar macrophages.
Figure 6.

Effects of SLPI on NF-κB activation in alveolar macrophages. NF-κB activation in alveolar macrophages harvested by BAL 30 minutes after intratracheal administration of PBS or 1.5 mg of anti-BSA (IgG-IC) followed by intravenous infusion of 10 mg of BSA. SLPI (100 μg) was administered intratracheally with the anti-BSA.
Endogenous SLPI Regulates Lung NF-κB Activation
Because treatment with SLPI almost completely ameliorated IgG immune complex-induced NF-κB activation in whole lung tissues, we investigated whether endogenous SLPI regulated activation of lung NF-κB. For these experiments, a reduced intratracheal dose of anti-BSA (250 μg) was used to produce minimal activation of NF-κB. Endogenous SLPI was blocked by intratracheal administration of 300 μg of anti-rat SLPI together with the anti-BSA. This dose of anti-SLPI was shown to effectively neutralize endogenous SLPI in this model of lung inflammation. 1 Positive controls received 300 μg of preimmune rabbit IgG. Four hours after intrapulmonary deposition of IgG immune complexes there was a detectable increase in lung NF-κB activation (Figure 7) ▶ . In the presence of anti-SLPI there was significantly increased NF-κB activation in lung. Image analysis of digitized EMSA blots dem- onstrated that anti-SLPI increased nuclear localization of lung NF-κB by 141% (P = 0.006). Blockade of endogenous SLPI had no effect on lung MAPK activation (data not shown).
Figure 7.

Effects of anti-SLPI on IgG immune complex-induced lung activation of NF-κB. A: NF-κB activation in whole lung tissues harvested 4 hours after intratracheal administration of PBS or 0.25 mg of anti-BSA (IgG-IC) followed by intravenous infusion of 10 mg of BSA. Positive controls received preimmune rabbit IgG (300 μg) intratracheally with the IgG anti-BSA. Blockade of endogenous SLPI was achieved by intratracheal administration of 300 μg of rabbit anti-rat SLPI with the IgG anti-BSA. B: Quantitation of EMSA blots by image analysis. Values represent mean ± SEM, with n = 3 for each group.
Effects of SLPI on IκBα and IκBβ
As SLPI inhibited NF-κB activation in whole lungs (Figure 4) ▶ , we designed experiments to determine whether the inhibitory effects of SLPI during lung inflammation might be related to effects on the cytoplasmic NF-κB regulatory proteins, IκBα and IκBβ. Western blot analysis of whole lung homogenates obtained 4 hours after initiation of lung injury confirmed decreases in the amounts of detectable IκBα protein after intrapulmonary deposition of IgG immune complexes as compared with intrapulmonary instillation of PBS (Figure 8A ▶ , upper panel). Neither co-treatment with SLPI nor TIMP-2 had any measurable effect on decreases in IκBα protein in the inflamed lung. In striking contrast, IgG immune complex deposition appeared to cause an increase in the amount of detectable IκBβ protein compared with treatment with PBS (Figure 8A ▶ , lower panel). Image analysis of digitized Western blots is shown in Figure 8B ▶ . Western blots of samples from lungs treated with IgG immune complexes showed a second, faint, and more rapidly migrating IκBβ band. Other studies have identified this band to be a dephosphorylated form of IκBβ, which is an intermediate during IκBβ degradation. 30 Co-treatment with SLPI resulted in a marked increase in the slower migrating, presumably hyperphosphorylated, form of IκBβ when compared with treatment with IgG immune complexes. Co-treatment with TIMP-2 had no effect on IgG immune complex-induced changes in IκBβ protein (Figure 8A ▶ , lower panel).
Figure 8.
Effects of SLPI and TIMP-2 on pulmonary IκBα and IκBβ protein expression during IgG immune complex-induced lung inflammation. A: Western blot analysis of whole lung homogenates was performed 4 hours after intratracheal administration of PBS or 1.5 mg of anti-BSA (IgG-IC) followed by intravenous infusion of 10 mg of BSA. SLPI and TIMP-2 (100 μg) were administered intratracheally with the IgG anti-BSA. B: Image analysis of the Western blot.
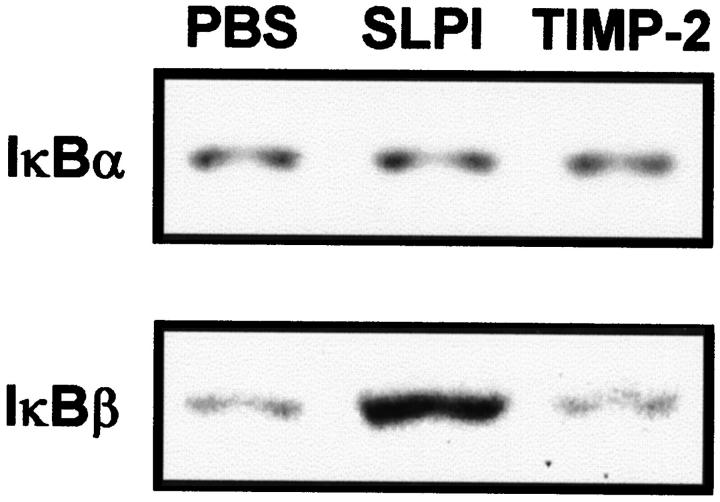
To assess whether SLPI might be directly augmenting the production of IκBβ and/or suppressing IκBβ degradation, rats received lung instillation of PBS, SLPI, or TIMP-2 (each at 100 μg) in the absence of IgG immune complexes. Neither SLPI nor TIMP-2 had any detectable effect on IκBα protein expression compared with treatment with PBS (Figure 9 ▶ , upper panel). However, treatment with SLPI, but not with TIMP-2, resulted in a marked increase in IκBβ protein expression compared with treatment with PBS (Figure 9 ▶ , lower panel). In these experiments only the slower migrating IκBβ band was observed, suggesting that under these conditions degraded IκBβ could not be detected.
Figure 9.
Effects of SLPI and TIMP-2 on IκBα and IκBβ protein expression in lung. Western blot analysis of whole lung homogenates was performed 4 hours after intratracheal administration of PBS, 100 μg of SLPI, or 100 μg of TIMP-2. Results are representative of 3 independent experiments.
To determine whether SLPI-induced increases in IκBβ protein expression were related to enhanced IκBβ gene activation, Northern blot analysis of IκBβ mRNA in whole lung extracts was performed. Lung extracts from rats undergoing 4 hours of injury were analyzed. As shown in Figure 10 ▶ , intrapulmonary deposition of IgG immune complexes caused a marked increase in IκBβ mRNA expression compared with PBS controls. Co-treatment with SLPI failed to provide any clear evidence for IgG immune complex-induced increases in IκBβ mRNA expression. Finally, lung instillation of SLPI in otherwise unmanipulated rats did not induce expression of mRNA for IκBβ (Figure 10) ▶ .
Figure 10.
Effects of SLPI on IgG immune complex-induced pulmonary expression of IκBβ mRNA. Northern blot analysis was performed on RNA extracts from whole lung tissues obtained 4 hours after intratracheal administration of PBS or 1.5 mg of anti-BSA (IgG-IC) followed by intravenous infusion of 10 mg of BSA. SLPI (100 μg) was administered intratracheally with the IgG anti-BSA (IgG-IC) or in the absence of IgG immune complexes (SLPI). Equal loading of RNA was confirmed by probing the Northern blot for β-actin mRNA. Results are representative of two separate experiments.
Discussion
SLPI is a central component of pulmonary defenses against protease-mediated lung injury. 6-8 There is also evidence that SLPI augments glutathione production in lung. 9 Thus, SLPI may also serve as an important defense against oxidant-mediated lung injury. More recent studies have demonstrated that SLPI interferes with signal transduction pathways in monocytes, reducing the production of matrix metalloproteinases. 22 SLPI also blocks entry of HIV into CD4+ lympocytes. 11 These studies suggest that SLPI has biological activities unrelated to protease inhibition. The present report expands the current knowledge of the cellular and molecular effects of SLPI. The data demonstrate that SLPI regulates the activation of NF-κB (but not MAPK) during lung inflammatory injury and that the inhibitory effects on NF-κB seem linked to preserved expression of IκBβ but not IκBα.
The administration of exogenous SLPI at the onset of IgG immune complex-induced lung injury caused a dramatic reduction in the nuclear translocation (activation) of NF-κB in lung tissues. In parallel, blockade of endogenous SLPI using polyclonal antibody resulted in significant enhancement of NF-κB activation. The activation of NF-κB is regulated by cytoplasmic proteins of the IκB family. Two of these proteins, IκBα and IκBβ, are known to be largely responsible for the control of NF-κB activation. 24,31 We found that exogenously administered SLPI had no measurable effect on IκBα protein expression in lung but clearly caused increased levels of IκBβ protein (as determined by Western blot technique) in otherwise normal lungs and in lungs undergoing IgG immune complex-induced inflammation. SLPI had no measurable effects on IκBβ mRNA expression, suggesting that SLPI may operate by preventing degradation of IκBβ protein.
The inhibitory effects of SLPI on lung NF-κB activation was not a generalized effect of protease inhibitors. Treatment with TIMP-2, a potent inhibitor of matrix metalloproteinases, had no effect on NF-κB activation induced by intrapulmonary deposition of IgG immune complexes, even though it is known that TIMP-2 is protective in this model of lung injury. 21 Furthermore, in contrast to SLPI, lung instillation of TIMP-2 into otherwise normal lungs had no effect on the protein expression of either IκBα or IκBβ. These data suggest that the inhibitory effects of SLPI are selective for the signal transduction pathway leading to NF-κB activation. IgG immune complex-induced activation of MAPK in alveolar macrophages and lung tissues was not reduced in the presence of SLPI.
Endogenous SLPI has been shown to regulate the lung inflammatory response. Studies of the IgG immune complex model of lung injury have demonstrated that blockade of endogenous SLPI with neutralizing antibody causes enhanced pulmonary accumulation of neutrophils and increased lung vascular permeability. 1 The data presented here suggest that the deleterious effects of SLPI blockade are associated with greatly enhanced (by 141%) intrapulmonary activation of NF-κB. Interestingly, these effects cannot be attributed to augmented production of TNF-α as SLPI blockade does not result in increased BAL levels of TNF-α. 1 The lack of an effect of SLPI blockade on lung TNF-α is consistent with the current data demonstrating an inability of exogenous SLPI to reduce BAL levels of TNF-α. Thus, it appears that endogenous SLPI regulates lung inflammatory reactions, at least in part, through effects on lung NF-κB. However, alveolar macrophages, which are the primary source of lung TNF-α, seem not to be a target of the suppressive effects of SLPI. Rather, vascular endothelial cells seem to be the target for SLPI-induced inhibition of NF-κB activation. This would be consistent with the findings that SLPI does not interfere with levels of TNF-α but nevertheless reduces up-regulation of lung vascular ICAM-1.
Alveolar macrophage activation is an initial event in the genesis of lung inflammatory reactions. In lung inflammation induced by intrapulmonary deposition of IgG immune complexes, we have shown that early activation (within 30 minutes) of alveolar macrophages occurs in an NF-κB-dependent manner. 29 Furthermore, NF-κB activation in alveolar macrophages in vivo occurs prior to NF-κB activation in whole lung tissues, suggesting that products of activated alveolar macrophages are required to stimulate nuclear translocation of NF-κB in other lung cell types. Indeed, TNF-α and IL-1β, which are required for the full induction of lung injury in this model, 32,33 seem to operate as autocrine/paracrine stimulators of alveolar macrophages. 20 Administration of the anti-inflammatory cytokines IL-10 or IL-13 greatly suppresses NF-κB activation in alveolar macrophages in association with preserved levels of IκBα and decreased lung production of TNF-α. 29,34 In the current studies, administration of SLPI neither suppressed IgG immune complex-induced increases in alveolar macrophage NF-κB activation nor reduced BAL levels of TNF-α, MIP-2, CINC, or C5a. These data suggest that SLPI does not affect the activation of alveolar macrophages but inhibits the subsequent activation of NF-κB in nonmacrophage lung cells and reduces the development of lung injury.
Our data suggest that one such candidate for the effects of SLPI may be the pulmonary vascular endothelial cell. SLPI greatly reduced IgG immune complex-induced expression of ICAM-1 in the lung vasculature. Although the expression of lung vascular ICAM-1 is known to be regulated primarily by TNF-α, 16 treatment with SLPI in this lung inflammation model did not reduce levels of TNF-α in BAL fluids. One possible explanation for this phenomenon is that SLPI directly reduces ICAM-1 expression through suppression of endothelial NF-κB activation. It is known that NF-κB regulates the transcriptional activation of genes for ICAM-1 and other vascular adhesion molecules. 35 Treatment with SLPI also reduced IgG immune complex-induced lung neutrophil accumulation. Because SLPI did not reduce BAL levels of the CXC chemokines MIP-2 or CINC, it would appear that SLPI reduced pulmonary neutrophil recruitment by decreasing lung vascular ICAM-1 expression, thus interfering with adhesion events required for recruitment of neutrophils.
This study identifies a novel function for SLPI, namely suppression of NF-κB activation during lung inflammation. The data suggest that SLPI suppresses NF-κB in cell types other than lung macrophages, as these effects are associated with augmented protein expression of IκBβ. As indicated above, it seems possible that SLPI directly blocks endothelial cell activation of NF-κB, preventing full expression of ICAM-1. Although it is not known if receptors for SLPI exist on endothelial cells, high affinity binding to monocytes has been reported. 36 These studies also indicate that endogenous SLPI may regulate the extent of the lung inflammatory response by suppressing pulmonary NF-κB activation.
Footnotes
Address reprint requests to Dr. Peter A. Ward, Department of Pathology, University of Michigan Medical School, 1301 Catherine Road, Ann Arbor, Michigan 48109-0602. E-mail: pward@umich.edu.
Supported in part by the National Institutes of Health Grants GM-29587 and HL-31963 (P. A. Ward) and a grant from the American Lung Association (V. Sarma).
References
- 1.Van Seuningen I, Audie JP, Gosselin B, Lafitte JJ, Davril M: Expression of human mucous proteinase inhibitor in respiratory tract: a study by in situ hybridization. J Histochem Cytochem 1995, 43:645-648 [DOI] [PubMed] [Google Scholar]
- 2.De Water R, Willems LN, Van Muijen GN, Franken C, Fransen JA, Dijkman JH, Kramps JA: Ultrastructural localization of bronchial antileukoprotease in central and peripheral human airways by a gold-labeling technique using monoclonal antibodies. Am Rev Respir Dis 1986, 133:882-890 [PubMed] [Google Scholar]
- 3.Sallenave JM, Silva A, Marsden ME, Ryle AP: Secretion of mucus proteinase inhibitor and elafin by Clara cell and type II pneumocyte cell lines. Am J Respir Cell Mol Biol 1993, 8:126-133 [DOI] [PubMed] [Google Scholar]
- 4.Sallenave JM, Shulmann J, Crossley J, Jordana M, Gauldie J: Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. Am J Respir Cell Mol Biol 1994, 11:733-741 [DOI] [PubMed] [Google Scholar]
- 5.Jin F, Nathan C, Radzioch D, Ding A: Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell 1997, 88:417-426 [DOI] [PubMed] [Google Scholar]
- 6.Sallenave JM, Si-Tahar M, Cox G, Chignard M, Gauldie J: Secretory leukocyte proteinase inhibitor is a major leukocyte elastase inhibitor in human neutrophils. J Leuk Biol 1997, 61:695-702 [DOI] [PubMed] [Google Scholar]
- 7.Lucey EC, Stone PJ, Ciccolella DE, Breuer R, Christensen TG, Thompson RC, Snider GL: Recombinant human secretory leukocyte-protease inhibitor: in vitro properties, and amelioration of human neutrophil elastase-induced emphysema and secretory cell metaplasia in the hamster. J Lab Clin Med 1990, 115:224-232 [PubMed] [Google Scholar]
- 8.Rudolphus A, Kramps JA, Dijkman JH: Effect of human antileucoprotease on experimental emphysema. Eur Resp J 1991, 4:31-39 [PubMed] [Google Scholar]
- 9.Gillissen A, Birrer P, McElvaney NG, Buhl R, Vogelmeier C, Hoyt RF, Jr, Hubbard RC, Crystal RG: Recombinant secretory leukoprotease inhibitor augments glutathione levels in lung epithelial lining fluid. J Appl Physiol 1993, 75:825-832 [DOI] [PubMed] [Google Scholar]
- 10.Linden M, Hakansson L, Ohlsson K, Sjodin K, Tegner H, Tunek A, Venge P: Glutathione in bronchoalveolar lavage fluid from smokers is related to humoral markers of inflammatory cell activity. Inflammation 1989, 13:651-658 [DOI] [PubMed] [Google Scholar]
- 11.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM: Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest 1995, 96:456-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KJ, Ward PA: Acute immunologic pulmonary alveolitis. J Clin Invest 1974, 54:349-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welbourn CR, Young Y: Endotoxin, septic shock, and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br J Surg 1992, 79:998-1003 [DOI] [PubMed] [Google Scholar]
- 14.Turnage RH, Guice KS, Oldham KT: Pulmonary microvascular injury following intestinal reperfusion. New Horiz 1994, 2:463-475 [PubMed] [Google Scholar]
- 15.Ratliff NB, Wilson JW, Mikat E, Hackel DB, Graham TC: The lung in hemorrhageic shock: IV. The role of neutrophilic polymorphonuclear leukocytes. Am J Pathol 1971, 65:325-334 [PMC free article] [PubMed] [Google Scholar]
- 16.Mulligan MS, Vaporciyan AA, Miyasaka M, Tamatani T, Ward PA: Tumor necrosis factor α regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol 1993, 142:1739-1749 [PMC free article] [PubMed] [Google Scholar]
- 17.Mulligan MS, Varani J, Dame MK, Lane CL, Smith CW, Anderson DC, Ward PA: Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest 1991, 88:1396-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KJ, Ward PA: Role of oxygen metabolites in immune complex injury of lung. J Immunol 1981, 126:2365-2369 [PubMed] [Google Scholar]
- 19.Bless NM, Smith D, Charlton J, Czermak BJ, Schmal H, Friedl HP, Ward PA: Protective effects of an aptamer inhibitor of neutrophil elastase in lung inflammatory injury. Curr Biol 1997, 7:877-880 [DOI] [PubMed] [Google Scholar]
- 20.Lentsch AB, Czermak BJ, Bless NM, Ward PA: NF-κB activation during IgG immune complex-induced lung injury: requirements for TNF-α and IL-1β but not complement. Am J Pathol 1998, 152:1327-1336 [PMC free article] [PubMed] [Google Scholar]
- 21.Mulligan MS, Desrochers PE, Chinnaiyan AM, Gibbs DF, Varani J, Johnson KJ, Weiss SJ: In vivo suppression of immune complex-induced alveolitis by secretory leukoproteinase inhibitor, and tissue inhibitor of metalloproteinases 2. Proc Natl Acad Sci USA 1993, 90:11523-11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, DeWitt DL, McNeely TB, Wahl SM, Wahl LM: Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2, and matrix metalloproteinases. J Clin Invest 1997, 99:894-900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 24.Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S: I kappa B-β regulates the persistent response in a biphasic activation of NF-kappa B. Cell 1995, 80:573-582 [DOI] [PubMed] [Google Scholar]
- 25.Espevik T, Nissen-Meyer J: A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods 1986, 95:99-105 [DOI] [PubMed] [Google Scholar]
- 26.Shanley TP, Schmal H, Warner RL, Schmid E, Friedl HP, Ward PA: Requirement for C-X-C chemokines (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J Immunol 1997, 158:3439-3448 [PubMed] [Google Scholar]
- 27.Schmid E, Piccolo MT, Friedl HP, Warner RL, Mulligan MS, Hugli TE, Till GO, Ward PA: Requirement for C5a in lung vascular injury following thermal trauma to rat skin. Shock 1997, 8:119-124 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Sasagawa OH, Sakatani S, Fujikura T: Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 1983, 132:345-352 [DOI] [PubMed] [Google Scholar]
- 29.Lentsch AB, Shanley TP, Sarma V, Ward PA: In vivo suppression of NF-kappa B, and preservation of I kappa B α by interleukin-10, and interleukin-13. J Clin Invest 1997, 100:2443-2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weil R, Laurent-Winter C, Israel A: Regulation of IκBβ degradation: similarities to and differences from IκBα. J Biol Chem 1997, 272:9942-9949 [DOI] [PubMed] [Google Scholar]
- 31.Baldwin AS: The NF-kappa B, and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 1996, 14:649-683 [DOI] [PubMed] [Google Scholar]
- 32.Warren JS, Yabroff KR, Remick DG, Kunkel SL, Chensue SW, Kunkel RG, Johnson KJ, Ward PA: Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest 1989, 84:1873-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren JS: Intrapulmonary interleukin 1 mediates acute immune complex alveolitis in the rat. Biochem Biophys Res Commun 1991, 175:604-610 [DOI] [PubMed] [Google Scholar]
- 34.Mulligan MS, Warner RL, Foreback JL, Shanley TP, Ward PA: Protective effects of IL-4, IL-10, IL-12, and IL-13 in IgG immune complex-induced lung injury: role of endogenous IL-12. J Immunol 1997, 159:3483-3489 [PubMed] [Google Scholar]
- 35.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T: Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J 1995, 9:899-909 [PubMed] [Google Scholar]
- 36.McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM: Inhibition of human immunodeficiency virus type I infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood 1997, 90:1141-1149 [PubMed] [Google Scholar]