Abstract
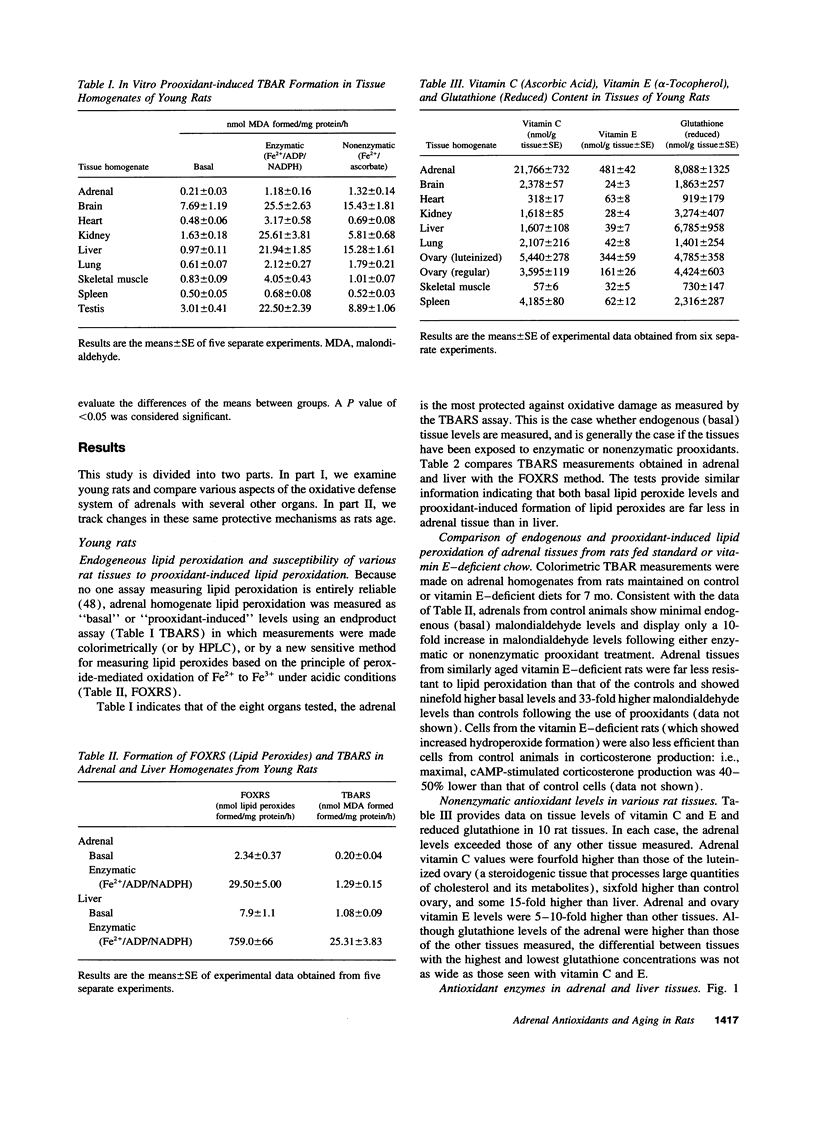
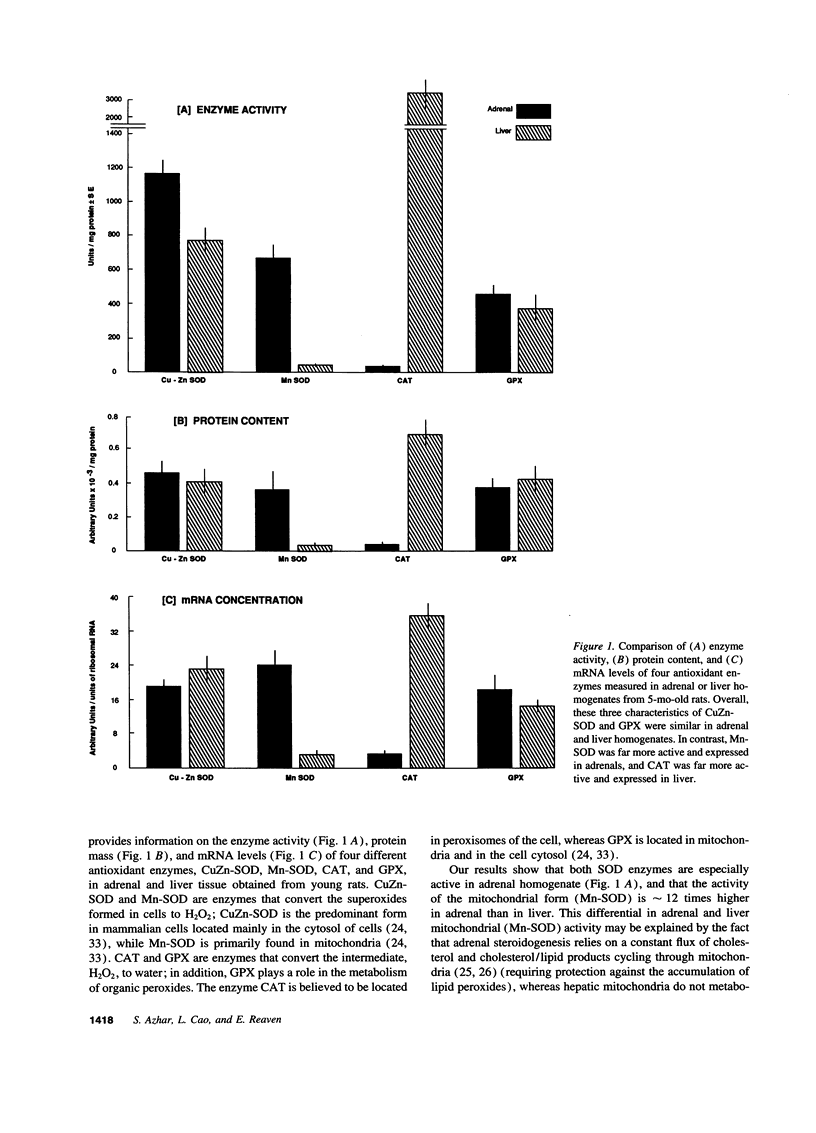
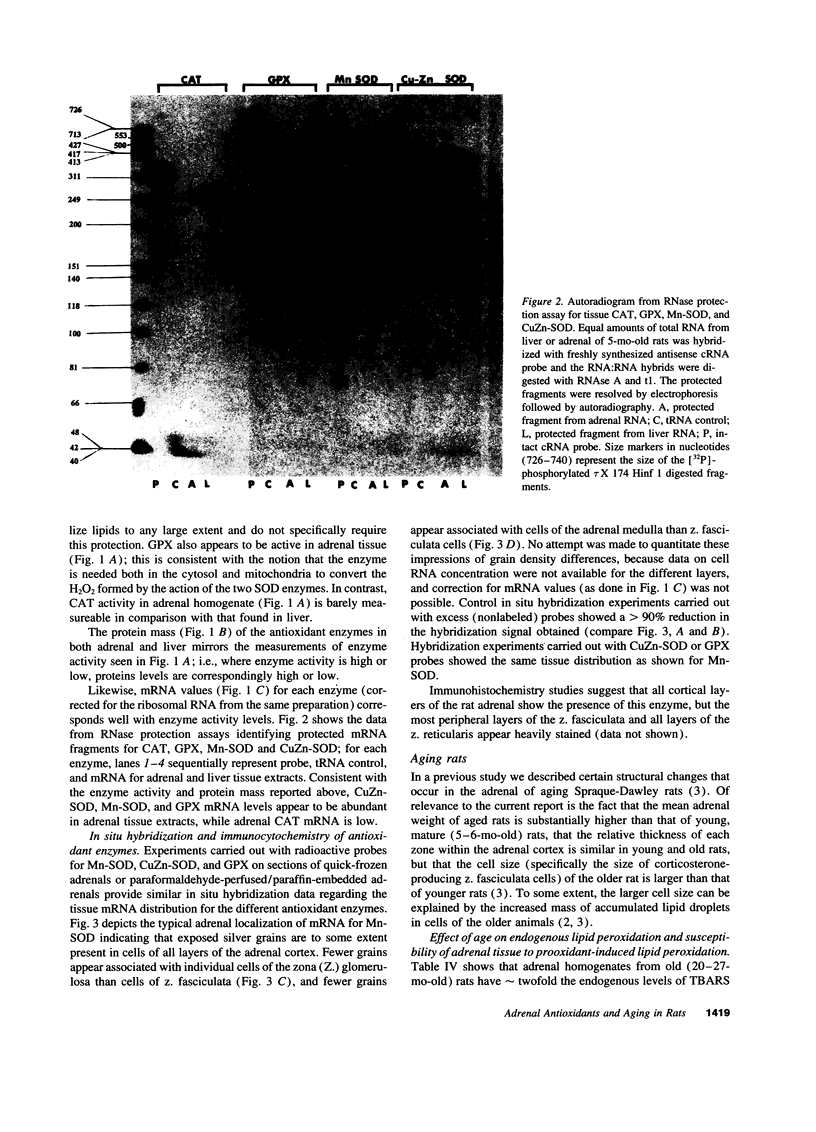
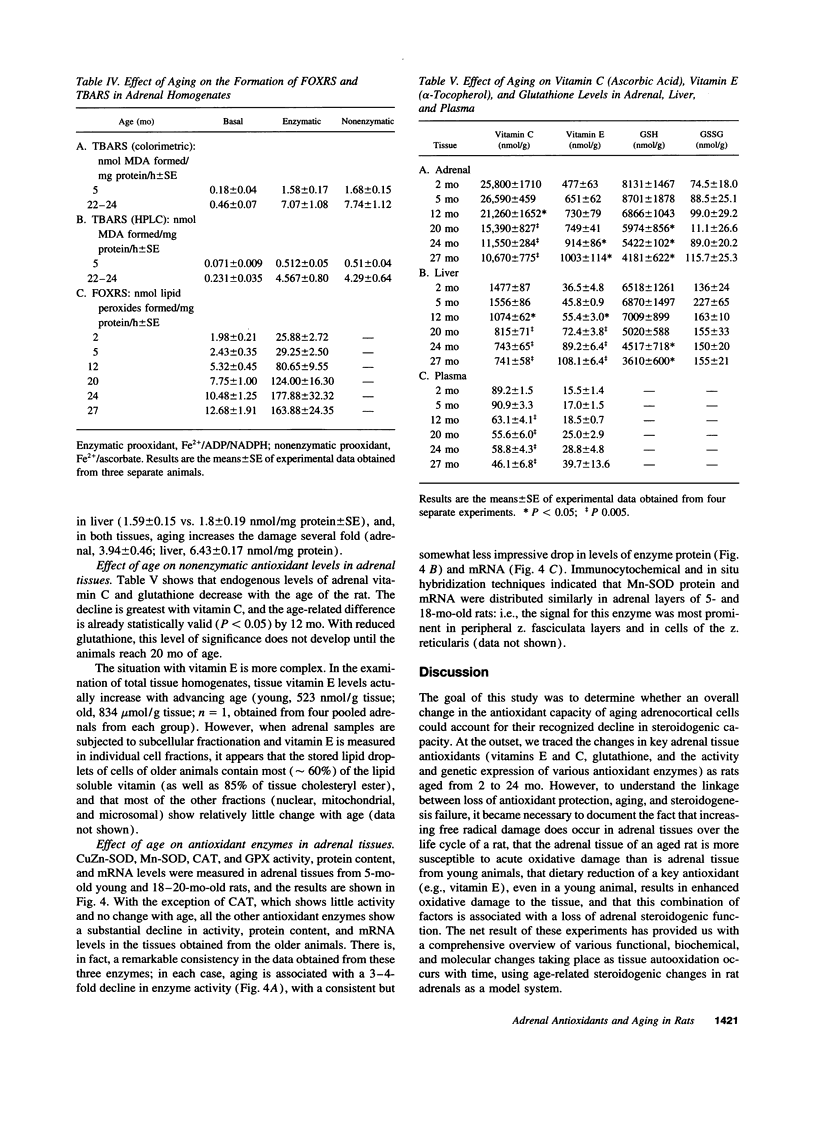
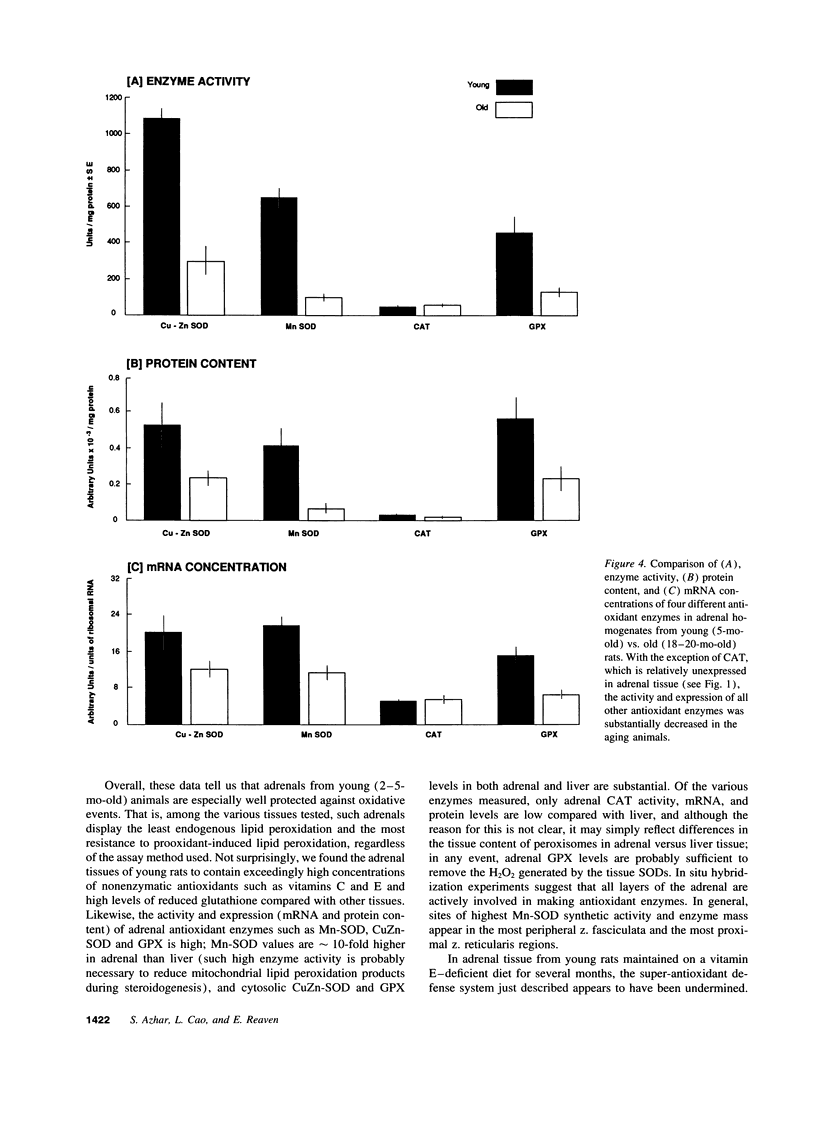
The goal of this study was to determine to what extent aging affects the antioxidant defense system of the rat adrenal and to evaluate the impact of any change in this system on the recognized age-related decline in steroidogenic capacity of adrenocortical cells. The studies were conducted on young (2-5 mo) and aging (12-27 mo) Sprague-Dawley rats and involved procedures measuring steroidogenesis; oxidative damage to tissue; non enzymatic antioxidants such as vitamin C, E, and glutathione; and tissue antioxidant enzyme (Mn and CuZn superoxide dismutases, catalase, and glutathione peroxidase) activity and expression (mRNA, protein mass, and location). Some measurements were made also on rats maintained on vitamin E-deficient diets. The data show that adrenals from young animals are especially well protected against oxidative events; i.e., these adrenals show the least endogenous lipid peroxidation and the highest level of resistance to prooxidant-induced damage (of various tissues measured) and show exceedingly high levels of tissue antioxidants. Aging, on the other hand, results in oxidative changes in adrenal tissue that are generally linked in time to a reduction in efficiency of the normally protective antioxidant defense system and to the decline in corticosterone production. We speculate that these events are causally related, i.e., that the age-related reduction in oxidative mechanisms in adrenal tissues leads to oxidative damage of membrane or cytosolic factors important to cholesterol transport, and, as a consequence of this damage, cholesterol cannot reach appropriate mitochondrial cholesterol side chain cleavage sites, and corticosterone production fails.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Atalla L., Fernandez M. A., Rao N. A. Immunohistochemical localization of catalase in ocular tissue. Curr Eye Res. 1987 Oct;6(10):1181–1187. doi: 10.3109/02713688709025227. [DOI] [PubMed] [Google Scholar]
- Azhar S., Reaven E. Effect of age on cholesterol uptake and utilization by rat adrenals: I. Internalization of lipoprotein-derived cholesteryl esters. Mech Ageing Dev. 1994 Nov 25;77(1):13–25. doi: 10.1016/0047-6374(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Behrman H. R., Aten R. F. Evidence that hydrogen peroxide blocks hormone-sensitive cholesterol transport into mitochondria of rat luteal cells. Endocrinology. 1991 Jun;128(6):2958–2966. doi: 10.1210/endo-128-6-2958. [DOI] [PubMed] [Google Scholar]
- Bethea C. L., Walker R. F. Age-related changes in reproductive hormones and in Leydig cell responsivity in the male Fischer 344 rat. J Gerontol. 1979 Jan;34(1):21–27. doi: 10.1093/geronj/34.1.21. [DOI] [PubMed] [Google Scholar]
- Beyer R. E. The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and coenzyme Q. J Bioenerg Biomembr. 1994 Aug;26(4):349–358. doi: 10.1007/BF00762775. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Traber M. G. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- Carlson J. C., Wu X. M., Sawada M. Oxygen radicals and the control of ovarian corpus luteum function. Free Radic Biol Med. 1993 Jan;14(1):79–84. doi: 10.1016/0891-5849(93)90511-r. [DOI] [PubMed] [Google Scholar]
- Cheng B., Kowal J. Analysis of adrenal cholesteryl esters by reversed phase high performance liquid chromatography. J Lipid Res. 1994 Jun;35(6):1115–1121. [PubMed] [Google Scholar]
- Chikaraishi D. M., Buchanan L., Danna K. J., Harrington C. A. Genomic organization of rat rDNA. Nucleic Acids Res. 1983 Sep 24;11(18):6437–6452. doi: 10.1093/nar/11.18.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coursin D. B., Cihla H. P., Oberley T. D., Oberley L. W. Immunolocalization of antioxidant enzymes and isozymes of glutathione S-transferase in normal rat lung. Am J Physiol. 1992 Dec;263(6 Pt 1):L679–L691. doi: 10.1152/ajplung.1992.263.6.L679. [DOI] [PubMed] [Google Scholar]
- Di Mascio P., Murphy M. E., Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991 Jan;53(1 Suppl):194S–200S. [PubMed] [Google Scholar]
- Draper H. H., Squires E. J., Mahmoodi H., Wu J., Agarwal S., Hadley M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med. 1993 Oct;15(4):353–363. doi: 10.1016/0891-5849(93)90035-s. [DOI] [PubMed] [Google Scholar]
- Driskell W. J., Neese J. W., Bryant C. C., Bashor M. M. Measurement of vitamin A and vitamin E in human serum by high-performance liquid chromatography. J Chromatogr. 1982 Sep 10;231(2):439–444. doi: 10.1016/s0378-4347(00)81869-1. [DOI] [PubMed] [Google Scholar]
- Duckworth P. F., Vlahcevic Z. R., Studer E. J., Gurley E. C., Heuman D. M., Beg Z. H., Hylemon P. B. Effect of hydrophobic bile acids on 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity and mRNA levels in the rat. J Biol Chem. 1991 May 25;266(15):9413–9418. [PubMed] [Google Scholar]
- Fawcett T. W., Sylvester S. L., Sarge K. D., Morimoto R. I., Holbrook N. J. Effects of neurohormonal stress and aging on the activation of mammalian heat shock factor 1. J Biol Chem. 1994 Dec 23;269(51):32272–32278. [PubMed] [Google Scholar]
- Furuta S., Hayashi H., Hijikata M., Miyazawa S., Osumi T., Hashimoto T. Complete nucleotide sequence of cDNA and deduced amino acid sequence of rat liver catalase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):313–317. doi: 10.1073/pnas.83.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990 Apr;15(4):129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956 Jul;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harris E. D. Regulation of antioxidant enzymes. FASEB J. 1992 Jun;6(9):2675–2683. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]
- Higami Y., Shimokawa I., Okimoto T., Ikeda T. An age-related increase in the basal level of DNA damage and DNA vulnerability to oxygen radicals in the individual hepatocytes of male F344 rats. Mutat Res. 1994 Aug;316(2):59–67. doi: 10.1016/0921-8734(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Crapo J. D. Nucleotide sequences of cDNAs coding for rat manganese-containing superoxide dismutase. Nucleic Acids Res. 1987 Dec 10;15(23):10070–10070. doi: 10.1093/nar/15.23.10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. S., Crapo J. D. cDNA and deduced amino acid sequence of rat copper-zinc-containing superoxide dismutase. Nucleic Acids Res. 1987 Aug 25;15(16):6746–6746. doi: 10.1093/nar/15.16.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. E., Bernstein C., Bernstein H. Oxidative and other DNA damages as the basis of aging: a review. Mutat Res. 1992 Sep;275(3-6):305–315. doi: 10.1016/0921-8734(92)90034-m. [DOI] [PubMed] [Google Scholar]
- Hornsby P. J., Crivello J. F. The role of lipid peroxidation and biological antioxidants in the function of the adrenal cortex. Part 1: A background review. Mol Cell Endocrinol. 1983 Apr;30(1):1–20. doi: 10.1016/0303-7207(83)90197-1. [DOI] [PubMed] [Google Scholar]
- Hornsby P. J., Crivello J. F. The role of lipid peroxidation and biological antioxidants in the function of the adrenal cortex. Part 2. Mol Cell Endocrinol. 1983 May;30(2):123–147. doi: 10.1016/0303-7207(83)90043-6. [DOI] [PubMed] [Google Scholar]
- Hornsby P. J. Steroid and xenobiotic effects on the adrenal cortex: mediation by oxidative and other mechanisms. Free Radic Biol Med. 1989;6(1):103–115. doi: 10.1016/0891-5849(89)90163-9. [DOI] [PubMed] [Google Scholar]
- Ji L. L., Stratman F. W., Lardy H. A. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch Biochem Biophys. 1988 May 15;263(1):150–160. doi: 10.1016/0003-9861(88)90623-6. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Species, tissue and subcellular distribution of non Se-dependent glutathione peroxidase activity. J Nutr. 1978 Feb;108(2):211–215. doi: 10.1093/jn/108.2.211. [DOI] [PubMed] [Google Scholar]
- Liao C., Reaven E., Azhar S. Age-related decline in the steroidogenic capacity of isolated rat Leydig cells: a defect in cholesterol mobilization and processing. J Steroid Biochem Mol Biol. 1993 Jul;46(1):39–47. doi: 10.1016/0960-0760(93)90207-d. [DOI] [PubMed] [Google Scholar]
- Lin T., Murono E., Osterman J., Allen D. O., Nankin H. R. The aging Leydig cell: 1. Testosterone and adenosine 3',5'-monophosphate responses to gonadotropin stimulation in rats. Steroids. 1980 Jun;35(6):653–663. doi: 10.1016/0039-128x(80)90090-2. [DOI] [PubMed] [Google Scholar]
- Liscum L., Dahl N. K. Intracellular cholesterol transport. J Lipid Res. 1992 Sep;33(9):1239–1254. [PubMed] [Google Scholar]
- Malamed S., Carsia R. V. Aging of the rat adrenocortical cell: response to ACTH and cyclic AMP in vitro. J Gerontol. 1983 Mar;38(2):130–136. doi: 10.1093/geronj/38.2.130. [DOI] [PubMed] [Google Scholar]
- Markowska A., Rebuffat P., Gottardo G., Mazzochi G., Nussdorfer G. G. Age-dependent changes in the function and morphology of mitochondria of rat adrenal zona fasciculata. Histol Histopathol. 1994 Apr;9(2):263–268. [PubMed] [Google Scholar]
- Musicki B., Aten R. F., Behrman H. R. Inhibition of protein synthesis and hormone-sensitive steroidogenesis in response to hydrogen peroxide in rat luteal cells. Endocrinology. 1994 Feb;134(2):588–595. doi: 10.1210/endo.134.2.7507829. [DOI] [PubMed] [Google Scholar]
- Nohl H. Involvement of free radicals in ageing: a consequence or cause of senescence. Br Med Bull. 1993 Jul;49(3):653–667. doi: 10.1093/oxfordjournals.bmb.a072638. [DOI] [PubMed] [Google Scholar]
- Nourooz-Zadeh J., Tajaddini-Sarmadi J., Wolff S. P. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem. 1994 Aug 1;220(2):403–409. doi: 10.1006/abio.1994.1357. [DOI] [PubMed] [Google Scholar]
- Omaye S. T., Turnbull J. D., Sauberlich H. E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- Pacifici R. E., Davies K. J. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology. 1991;37(1-3):166–180. doi: 10.1159/000213257. [DOI] [PubMed] [Google Scholar]
- Popplewell P. Y., Azhar S. Effects of aging on cholesterol content and cholesterol-metabolizing enzymes in the rat adrenal gland. Endocrinology. 1987 Jul;121(1):64–73. doi: 10.1210/endo-121-1-64. [DOI] [PubMed] [Google Scholar]
- Popplewell P. Y., Butte J., Azhar S. The influence of age on steroidogenic enzyme activities of the rat adrenal gland: enhanced expression of cholesterol side-chain cleavage activity. Endocrinology. 1987 Jun;120(6):2521–2528. doi: 10.1210/endo-120-6-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popplewell P. Y., Tsubokawa M., Ramachandran J., Azhar S. Differential effects of aging on adrenocorticotropin receptors, adenosine 3'5'-monophosphate response, and corticosterone secretion in adrenocortical cells from Sprague-Dawley rats. Endocrinology. 1986 Nov;119(5):2206–2213. doi: 10.1210/endo-119-5-2206. [DOI] [PubMed] [Google Scholar]
- Pyles L. A., Stejskal E. J., Einzig S. Spectrophotometric measurement of plasma 2-thiobarbituric acid-reactive substances in the presence of hemoglobin and bilirubin interference. Proc Soc Exp Biol Med. 1993 Apr;202(4):407–419. doi: 10.3181/00379727-202-43552. [DOI] [PubMed] [Google Scholar]
- Reaven E., Cao L., Azhar S. Effect of age on cholesterol uptake and utilization by rat adrenals: II. Lipoproteins from young and old rats. Mech Ageing Dev. 1994 Nov 25;77(1):27–41. doi: 10.1016/0047-6374(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Reaven E., Kostrna M., Ramachandran J., Azhar S. Structure and function changes in rat adrenal glands during aging. Am J Physiol. 1988 Dec;255(6 Pt 1):E903–E911. doi: 10.1152/ajpendo.1988.255.6.E903. [DOI] [PubMed] [Google Scholar]
- Reaven E., Tsai L., Spicher M., Shilo L., Philip M., Cooper A. D., Azhar S. Enhanced expression of granulosa cell low density lipoprotein receptor activity in response to in vitro culture conditions. J Cell Physiol. 1994 Dec;161(3):449–462. doi: 10.1002/jcp.1041610308. [DOI] [PubMed] [Google Scholar]
- Rebuffat P., Belloni A. S., Rocco S., Andreis P. G., Neri G., Malendowicz L. K., Gottardo G., Mazzocchi G., Nussdorfer G. G. The effects of ageing on the morphology and function of the zonae fasciculata and reticularis of the rat adrenal cortex. Cell Tissue Res. 1992 Nov;270(2):265–272. doi: 10.1007/BF00328012. [DOI] [PubMed] [Google Scholar]
- Reddy A. P., Hsu B. L., Reddy P. S., Li N. Q., Thyagaraju K., Reddy C. C., Tam M. F., Tu C. P. Expression of glutathione peroxidase I gene in selenium-deficient rats. Nucleic Acids Res. 1988 Jun 24;16(12):5557–5568. doi: 10.1093/nar/16.12.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick A. Z., Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- Roberts J. C., Francetic D. J. The importance of sample preparation and storage in glutathione analysis. Anal Biochem. 1993 Jun;211(2):183–187. doi: 10.1006/abio.1993.1254. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Hagen T. M., Ames B. N. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz D. R., Elwell J. H., Sun Y., Oberley L. W., Oberley T. D., Sullivan S. J., Roberts R. J. Oxygen toxicity in control and H2O2-resistant Chinese hamster fibroblast cell lines. Arch Biochem Biophys. 1990 Jun;279(2):249–260. doi: 10.1016/0003-9861(90)90489-l. [DOI] [PubMed] [Google Scholar]
- Spitz D. R., Oberley L. W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989 May 15;179(1):8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Stocco D. M., Wells J., Clark B. J. The effects of hydrogen peroxide on steroidogenesis in mouse Leydig tumor cells. Endocrinology. 1993 Dec;133(6):2827–2832. doi: 10.1210/endo.133.6.8243310. [DOI] [PubMed] [Google Scholar]
- Sun Y., Colburn N. H., Oberley L. W. Decreased expression of manganese superoxide dismutase mRNA and protein after immortalization and transformation of mouse liver cells. Oncol Res. 1993;5(3):127–132. [PubMed] [Google Scholar]
- Supakar P. C., Jung M. H., Song C. S., Chatterjee B., Roy A. K. Nuclear factor kappa B functions as a negative regulator for the rat androgen receptor gene and NF-kappa B activity increases during the age-dependent desensitization of the liver. J Biol Chem. 1995 Jan 13;270(2):837–842. doi: 10.1074/jbc.270.2.837. [DOI] [PubMed] [Google Scholar]
- Tsitouras P. D., Kowatch M. A., Harman S. M. Age-related alterations of isolated rat leydig cell function: gonadotropin receptors, adenosine 3',5'-monophosphate response, and testosterone secretion. Endocrinology. 1979 Dec;105(6):1400–1405. doi: 10.1210/endo-105-6-1400. [DOI] [PubMed] [Google Scholar]
- Weglicki W. B., Luna Z., Nair P. P. Sex and tissue specific differences in concentrations of alpha-tocopherol in mature and senescent rats. Nature. 1969 Jan 11;221(5176):185–186. doi: 10.1038/221185a0. [DOI] [PubMed] [Google Scholar]
- Yu B. P. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994 Jan;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]