Abstract
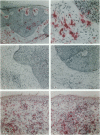
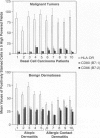
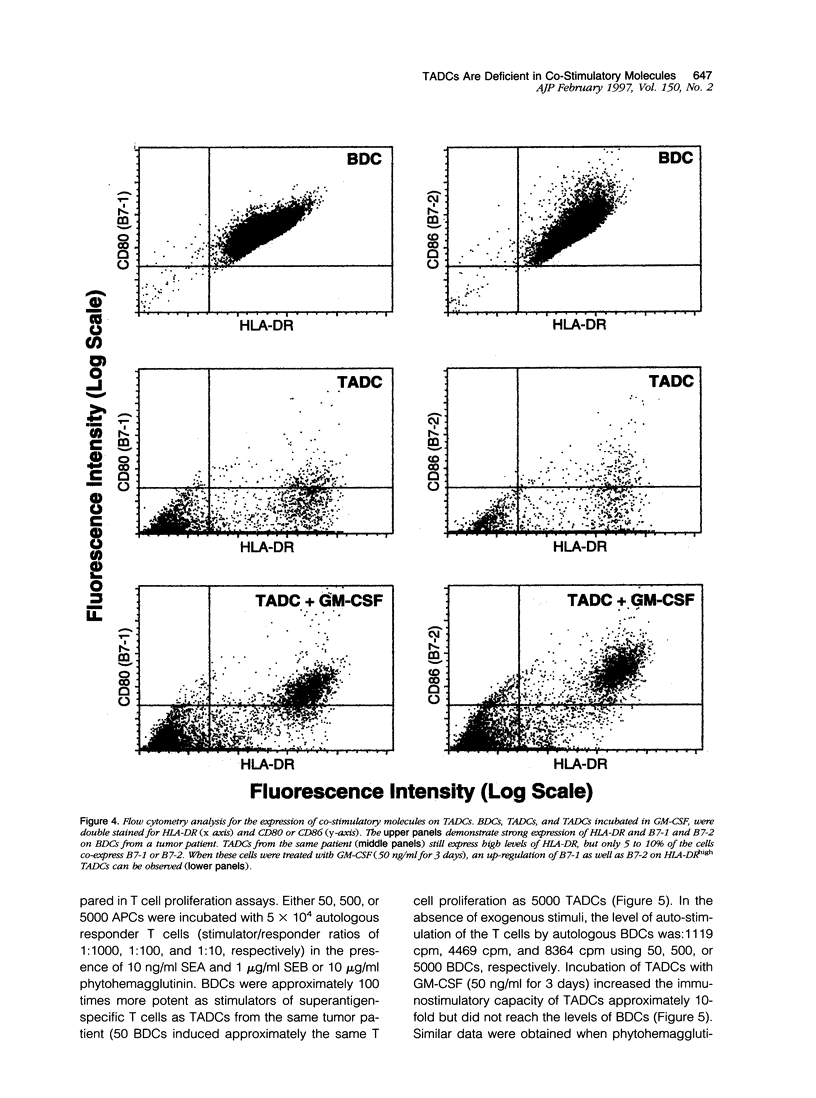
Immune surveillance of skin cancer involves the stimulation of effector T cells by tumor-derived antigens and antigen-presenting cells (APCs). An effective APC must not only display processed antigen in the context of MHC molecules but also express co-stimulatory molecules that are required to fully activate T cells. One of the most common cutaneous neoplasms is basal cell carcinoma. To investigate expression of the co-stimulatory molecules CD80 (B7-1) and CD86 (B7-2) on tumor-associated dendritic cells (TADCs), cryosections from basal cell carcinomas were immunostained. In basal cell carcinomas, only 1 to 2% of intratumor and 5 to 10% of peritumor APCs expressed CD80 or CD86. In contrast, biopsies of immunological/inflammatory dermatoses revealed that 38 to 73% of APCs expressed CD80 and CD86. To further evaluate their phenotype and function, TADCs were isolated from tissue samples of basal cell carcinomas; they were non-adherent to plastic, displayed a typical dendritic morphology, and expressed high levels of major histocompatibility class II molecules on their surface. When TADCs were compared with dendritic cells from blood for presentation of superantigens (staphylococcal enterotoxins A and B) to resting autologous T cells, TADCs were consistently weaker stimulators of T cell proliferation than blood dendritic cells. When analyzed by flow cytometry, TADCs expressed high levels of HLA-DR, but only 5 to 10% co-expressed CD80 or CD86. A 3-day culture in granulocyte/macrophage colony-stimulating factor-containing medium partially reconstituted the TADC expression of CD80 and CD86 as well as their immunostimulatory capacity. Thus, in this common skin cancer, although there are prominent collections of HLA-DR-positive APCs in and around tumor cells, the TADCs are deficient in important co-stimulatory molecules as well as being weak stimulators of T cell proliferation. The paucity of co-stimulatory molecule expression and functional activity of TADCs may explain why the local T lymphocytic infiltrate fails to become fully activated to eradicate adjacent tumor cells. From a clinical perspective, these findings suggest a novel immunotherapeutic strategy targeting T cell co-stimulatory molecules on professional APCs in cutaneous oncology.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beissert S., Hosoi J., Grabbe S., Asahina A., Granstein R. D. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J Immunol. 1995 Feb 1;154(3):1280–1286. [PubMed] [Google Scholar]
- Beissert S., Ullrich S. E., Hosoi J., Granstein R. D. Supernatants from UVB radiation-exposed keratinocytes inhibit Langerhans cell presentation of tumor-associated antigens via IL-10 content. J Leukoc Biol. 1995 Aug;58(2):234–240. doi: 10.1002/jlb.58.2.234. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991 Dec 1;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechner S. A. Intralesional interferon alfa-2b in the treatment of basal cell carcinoma. Immunohistochemical study on cellular immune reaction leading to tumor regression. J Am Acad Dermatol. 1991 May;24(5 Pt 1):731–734. doi: 10.1016/0190-9622(91)70111-e. [DOI] [PubMed] [Google Scholar]
- Chen L., Linsley P. S., Hellström K. E. Costimulation of T cells for tumor immunity. Immunol Today. 1993 Oct;14(10):483–486. doi: 10.1016/0167-5699(93)90262-J. [DOI] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni G., Giovarelli M., Cavallo F., Consalvo M., Allione A., Modesti A., Musiani P., Colombo M. P. Cytokine-induced tumor immunogenicity: from exogenous cytokines to gene therapy. J Immunother Emphasis Tumor Immunol. 1993 Nov;14(4):253–257. doi: 10.1097/00002371-199311000-00001. [DOI] [PubMed] [Google Scholar]
- Gascón J., Corachan M., Valls M. E., Gene A., Bombi J. A. Cyanobacteria-like body (CLB) in travellers with diarrhea. Scand J Infect Dis. 1993;25(2):253–257. doi: 10.3109/00365549309008493. [DOI] [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Gray G., Nadler L. M. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Huang A. Y., Bruce A. T., Pardoll D. M., Levitsky H. I. Does B7-1 expression confer antigen-presenting cell capacity to tumors in vivo? J Exp Med. 1996 Mar 1;183(3):769–776. doi: 10.1084/jem.183.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. Y., Golumbek P., Ahmadzadeh M., Jaffee E., Pardoll D., Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994 May 13;264(5161):961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- Hunt M. J., Halliday G. M., Weedon D., Cooke B. E., Barnetson R. S. Regression in basal cell carcinoma: an immunohistochemical analysis. Br J Dermatol. 1994 Jan;130(1):1–8. doi: 10.1111/j.1365-2133.1994.tb06873.x. [DOI] [PubMed] [Google Scholar]
- Inaba K., Metlay J. P., Crowley M. T., Steinman R. M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990 Aug 1;172(2):631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Bluestone J. A., Nadler L. M., Thompson C. B. The B7 and CD28 receptor families. Immunol Today. 1994 Jul;15(7):321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Kim J., Modlin R. L., Moy R. L., Dubinett S. M., McHugh T., Nickoloff B. J., Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995 Aug 15;155(4):2240–2247. [PubMed] [Google Scholar]
- Kripke M. L., Cox P. A., Alas L. G., Yarosh D. B. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Hendrix R., Linsley P. S., Hathcock K. S., Hodes R. J., Lowry R. P., Pearson T. C. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994 Jun 1;152(11):5208–5219. [PubMed] [Google Scholar]
- Matsuda M., Salazar F., Petersson M., Masucci G., Hansson J., Pisa P., Zhang Q. J., Masucci M. G., Kiessling R. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994 Dec 1;180(6):2371–2376. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R. S., Judge T. A., Nestle F. O., Turka L. A., Nickoloff B. J. Psoriatic skin-derived dendritic cell function is inhibited by exogenous IL-10. Differential modulation of B7-1 (CD80) and B7-2 (CD86) expression. J Immunol. 1995 Mar 15;154(6):2668–2677. [PubMed] [Google Scholar]
- Nestle F. O., Thompson C., Shimizu Y., Turka L. A., Nickoloff B. J. Costimulation of superantigen-activated T lymphocytes by autologous dendritic cells is dependent on B7. Cell Immunol. 1994 Jun;156(1):220–229. doi: 10.1006/cimm.1994.1166. [DOI] [PubMed] [Google Scholar]
- Nestle F. O., Turka L. A., Nickoloff B. J. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J Clin Invest. 1994 Jul;94(1):202–209. doi: 10.1172/JCI117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F. O., Zheng X. G., Thompson C. B., Turka L. A., Nickoloff B. J. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993 Dec 1;151(11):6535–6545. [PubMed] [Google Scholar]
- Pardoll D. M. Cancer vaccines. Immunol Today. 1993 Jun;14(6):310–316. doi: 10.1016/0167-5699(93)90051-L. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Rivas J. M., Ullrich S. E. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992 Dec 15;149(12):3865–3871. [PubMed] [Google Scholar]
- Romani N., Gruner S., Brang D., Kämpgen E., Lenz A., Trockenbacher B., Konwalinka G., Fritsch P. O., Steinman R. M., Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994 Jul 1;180(1):83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Koide S., Crowley M., Witmer-Pack M., Livingstone A. M., Fathman C. G., Inaba K., Steinman R. M. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989 Mar 1;169(3):1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Kunkel S. L., Burdick M. D., Wilke C. A., Orringer M. B., Whyte R. I., Strieter R. M. Production of interleukin-10 by human bronchogenic carcinoma. Am J Pathol. 1994 Jul;145(1):18–25. [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Taylor J. R., Vincek V., Kurimoto I., Shimizu T., Tie C., Golomb C. Immune surveillance and sunlight-induced skin cancer. Immunol Today. 1994 Apr;15(4):174–179. doi: 10.1016/0167-5699(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Mulé J. J. Experimental and clinical studies of cytokine gene-modified tumor cells. Hum Gene Ther. 1994 Feb;5(2):153–164. doi: 10.1089/hum.1994.5.2-153. [DOI] [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Tykocinski M. L., Kaplan D. R., Medof M. E. Antigen-presenting cell engineering. The molecular toolbox. Am J Pathol. 1996 Jan;148(1):1–16. [PMC free article] [PubMed] [Google Scholar]