Abstract
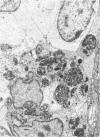
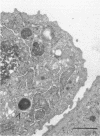
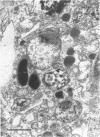
The DNA oligomer 5'-d(TGCGGCCTCTCAGTCCCGCACTTTCATCTTCC)-3' specifically recognizes Haemophilus influenzae 16S rRNA. We report here the use of this oligonucleotide, with a fluorescein label tagged on its 5' end, as a probe for the in situ detection of nonencapsulated nontypeable H. influenzae in sections of adenoid tissue from 10 children who were clinically infection free but were having their adenoids removed because of nasal obstruction. In some cases, the reticular crypt epithelium was focally infiltrated by H. influenzae. The reservoir for these bacterial colonizations, in all likelihood long standing, seemed to be macrophage-like cells found in the subepithelial layers in all 10 cases. These mononuclear cells contained up to 200 intracellular H. influenzae cells. In the transmission electron macroscope, macrophage-like cells with intracellular bacteria with coccoid morphology, at least some of which were dividing, were seen. Adenoid cell suspensions, enriched for macrophages by use of paramagnetic beads coated with monoclonal antibodies against the CD14 marker, yielded up to 1,100 CFU of nontypeable H. influenzae per 10(5) cells after killing of extracellular bacteria with gentamicin followed by mechanical lysis of the cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenkamp S. J., Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992 Apr;60(4):1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Howland E. B., Danielsen S. A., Nierzwicki-Bauer S. A. Development of a rapid method for detecting bacterial cells in situ using 16S rRNA-targeted probes. Biotechniques. 1992 Dec;13(6):928–934. [PubMed] [Google Scholar]
- Brodsky L., Moore L., Stanievich J. The role of Haemophilus influenzae in the pathogenesis of tonsillar hypertrophy in children. Laryngoscope. 1988 Oct;98(10):1055–1060. doi: 10.1288/00005537-198810000-00006. [DOI] [PubMed] [Google Scholar]
- Forsgren J., Samuelson A., Lindberg A., Rynnel-Dagö B. Quantitative bacterial culture from adenoid lymphatic tissue with special reference to Haemophilus [corrected]. Acta Otolaryngol. 1993 Sep;113(5):668–672. doi: 10.3109/00016489309135882. [DOI] [PubMed] [Google Scholar]
- Gates G. A., Avery C. A., Prihoda T. J. Effect of adenoidectomy upon children with chronic otitis media with effusion. Laryngoscope. 1988 Jan;98(1):58–63. doi: 10.1288/00005537-198801000-00013. [DOI] [PubMed] [Google Scholar]
- Giovannoni S. J., DeLong E. F., Olsen G. J., Pace N. R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 1988 Feb;170(2):720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E., Andåker L. Inability of gentamicin and fosfomycin to eliminate intracellular Enterobacteriaceae. J Antimicrob Chemother. 1985 Jun;15(6):723–728. doi: 10.1093/jac/15.6.723. [DOI] [PubMed] [Google Scholar]
- Kuklinska D., Kilian M. Relative proportions of Haemophilus species in the throat of healthy children and adults. Eur J Clin Microbiol. 1984 Jun;3(3):249–252. doi: 10.1007/BF02014895. [DOI] [PubMed] [Google Scholar]
- Maw A. R. Chronic otitis media with effusion (glue ear) and adenotonsillectomy: prospective randomised controlled study. Br Med J (Clin Res Ed) 1983 Nov 26;287(6405):1586–1588. doi: 10.1136/bmj.287.6405.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- St Geme J. W., 3rd, Falkow S., Barenkamp S. J. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Geme J. W., 3rd, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun. 1990 Dec;58(12):4036–4044. doi: 10.1128/iai.58.12.4036-4044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier S., Stenberg K., Svanborg-Edén C. Turnover of nontypable Haemophilus influenzae in the nasopharynges of healthy children. J Clin Microbiol. 1989 Oct;27(10):2175–2179. doi: 10.1128/jcm.27.10.2175-2179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. E., Maskell D. J., Moxon E. R. Relationship between intracellular survival in macrophages and virulence of Haemophilus influenzae type b. J Infect Dis. 1991 Jun;163(6):1366–1369. doi: 10.1093/infdis/163.6.1366. [DOI] [PubMed] [Google Scholar]