Abstract
An integrase dimer can process and integrate a single HIV-1 DNA end in vitro, whereas a tetramer is required to integrate two ends. LEDGF/p75 can potently stimulate integrase activity, but its affects on half- versus full-site integration have not been investigated. Stimulation of half-site but inhibition of full-site integration is revealed here. LEDGF/p75 seems to interfere with integrase oligomerization, but does not inhibit the catalytic activity of pre-assembled complexes. We therefore speculate LEDGF/p75 function is restricted to a point in the viral lifecycle that occurs after the formation of the preintegration synaptic complex, for example, as a chromatin-associated tethering factor.
Keywords: Integrase, full-site integration, HIV-1, LEDGF/p75
Introduction
Integrase (IN) catalyzes two sequential reactions, 3′ processing and DNA strand transfer, first removing a 3′-GT dinucleotide from the U5 and U3 ends of reverse transcribed HIV-1 cDNA. The recessed ends are then used by IN to affect a double-stranded staggered cut in chromosomal DNA, which at the same time joins the viral ends to protruding 5′-phosphates. In vitro, IN can process the 3′ end of a donor (D) oligonucleotide substrate that models the U3 or U5 viral end, and then integrate that end into a second oligonucleotide that serves as surrogate chromosomal DNA (Bushman and Craigie, 1991). Including a heterologous circular target DNA in the reaction helps determine the extent that strand transfer yields the coupled integration of two D ends, referred to here as full-site (FS) integration. Initial experiments revealed that HIV-1 IN prefers to integrate one viral end into one strand of target DNA to generate half-site (HS) reaction products as compared to integrating two ends with a 5 bp spacing, as occurs in vivo (Bushman and Craigie, 1991; Goodarzi et al., 1995). Subsequent work revealed that maintaining relatively low protein concentrations during IN purification (Sinha and Grandgenett, 2005) or increasing D DNA length and ensuring that the reaction proceeds via the 3′ processing step by assaying blunt-ended as compared to pre-cleaved substrates (Li and Craigie, 2005) significantly increased FS integration but did not eliminate HS product formation. Chemically-crosslinked IN dimers and tetramers were furthermore shown to specifically mediate HS and FS integration, respectively, indicating that the multimeric state of IN protein in large part dictates the outcome of the different pathways (Faure et al., 2005). The lentiviral IN-interacting protein lens epithelium-derived growth factor (LEDGF)/p75 binds to tetrameric recombinant IN in human cells, stimulates the in vitro activities of purified IN protein (Cherepanov et al., 2003), and plays a crucial role during HIV-1 integration in vivo (Llano et al., 2006a; Vandekerckhove et al., 2006). To gain insight into the role of LEDGF/p75 during integration, we characterized its influences on HS versus FS HIV-1 product formation in vitro.
Results and Discussion
A 972 bp restriction fragment harboring 191 bp of the downstream end of HIV-1 cDNA served as D DNA. Two aspects of this substrate were previously shown to favor FS integration (Li and Craigie, 2005): (i) a blunt end, requiring IN to process the GT dinucleotide prior to DNA strand transfer, and (ii) its relative length. Supercoiled pGEM-3 (2,876 bp) was included in the reaction; integration of one D end into one strand of pGEM-3 yields a tagged circle HS product (Fig. 1A). A separate D molecule can instead serve as target, resulting in the integration of one D end into another. Depending on the relative location of D/D insertion, the resultant dimer will be a Y-shaped branch (D2*, internal integration site) or migrate as a linear 1.9 kb DNA through agarose (D2, integration close to the DNA end). HS or D2 product formation proceeds via a dimer of IN engaging a processed D end and either pGEM-3 or another D molecule, respectively, in what is referred to here as a non-synaptic complex (NSC) (Faure et al., 2005; Guiot et al., 2006). Repeated HS integration into the same pGEM-3 molecule yields multiple-tagged (MT) circles (Fig. 1A). Similarly, HS reactions utilizing D2 products as target result in formation of donor multimers, Dn. FS integration of two D ends into both strands of pGEM-3 yields a linear 4.8 kb product, reminiscent of viral integration in vivo (Fig. 1A). This pathway proceeds via a synaptic complex (SC), whereby a dimer of IN dimers assembled at two D ends engages one pGEM-3 molecule for integration (Li et al., 2006).
Fig. 1.

In vitro integration assay. (A) Schematic diagram of substrate and reaction products. U5 sequences (short bold lines) are at one end of D DNA (thin parallel lines). D-D integration can yield linear D2 or branched D2* products. Integration of one D end into pGEM-3 (solid bold line) yields HS tagged circles, whereas MT circles arise from additional integrations into HS products. FS integration proceeds via a pair of D ends attacking both strand of target DNA in a concerted fashion. (B) Influence of LEDGF/p75 on IN product formation. Lane 1, labeled 1 kb markers; lane 2, mock reaction lacking proteins; lane 3, integration assay in the absence of BSA and LEDGF/p75; lane 4, assay conducted in the presence of BSA and IN; lane 5, IN and LEDGF/p75. LEDGF/p75 suppressed FS product formation in multiple (n=7) independent integration assays.
Preliminary experiments optimized reaction conditions for maximal levels of IN activity and LEDGF/p75 stimulation. In the absence of added accessory proteins, ~63% of input D was converted to integration products (Fig. 1B, lane 3). The formation of significant levels of apparent end-to-end linear D2 integrants is consistent with results of previous studies that likewise utilized relatively long D substrates (Cherepanov et al., 1999; Li and Craigie, 2005). The level of IN activity and pattern of integration products were unaffected by including the control bovine serum albumin (BSA) protein in the reaction (Fig. 1B, compare lane 4 to lane 3). When LEDGF/p75 was substituted for BSA (lane 5), there was an ~8% increase in the overall level of IN activity (9.6 ± 3.6% for n=5 replicates) as well as a significant shift in the pattern of strand transfer reaction products.
LEDGF/p75 preferentially inhibited the formation of a product that migrated to a position consistent with FS integration (4.8 kb; Fig. 1B, lane 5). To probe the linearity of this species (Fig. 1A) and gain insight into other reaction products, excised gel lanes were electrophoresed in a second orthogonal dimension. The second dimension distinguishes linear DNAs such as input D and FS products, which migrate along an arc, from non-linear, branched DNAs (Dn, HS, and MT integration products) because these migrate above the arc (Friedman and Brewer, 1995). In the absence of LEDGF/p75 (Fig. 2A), three species, D substrate (lower right corner) and D2 and FS products, traveled along the arc defined by the migration positions of co-electrophoresed linear marker fragments. The HS, MT, and branched Dn products in comparison migrated above the arc, as predicted. Comparable levels of HS (51%) and FS (49%) products formed, and the total fraction of NSC-mediated products (HS, MT, and D2–6) was ~78%. This is not unexpected, as NSCs form via tri-molecular events whereas SCs require the interaction of five molecular players for their formation. Analysis of LEDGF/p75-containing reactions confirmed that the host factor stimulated the formation of NSC-mediated products and inhibited the formation of a linear 4.8 kb species (Fig. 2B). Previous studies would indicate that the vast majority of linear 4.8 kb products harbor a 5 bp duplication of target sequence resulting from the coupled integration of two viral DNA ends (Li and Craigie, 2005), though we note an analogous cloning and sequence analysis was not conducted here.
Fig. 2.

2D gel and mutant analyses. (A) IN-alone reaction from lane 3 in Fig. 1B. (B) LEDGF/p75-containing reaction (Fig. 1B, lane 5). Relative orientations of electrophoretic dimensions are shown. The arc indicates the migration path of unlabelled 1 kb linear markers; the FS product migrated between the 4 and 5 kb fragments. *, non-linear D2 and D3 products. Similar results were obtained in three side-by-side comparisons of 2D electrophoretic patterns. (B) LEDGF/p75 mutant analysis. The portion of the gel harboring FS and HS products is shown.
To investigate protein determinants affecting FS product formation, LEDGF/p75 mutants defective for IN (LEDGFD366N) or DNA binding (LEDGF226–530) were analyzed. FS integration was unimpaired by the IN-binding mutant, whereas the 226–530 deletion mutant, which retains IN-binding and stimulates IN activity in vitro (Turlure et al., 2006), suppressed FS integration similar to wild-type LEDGF/p75 (Fig. 3C, lanes 3–6). Suppression of FS integration therefore requires IN-binding and appears to occur independent of LEDGF/p75 DNA binding activity.
Fig. 3.
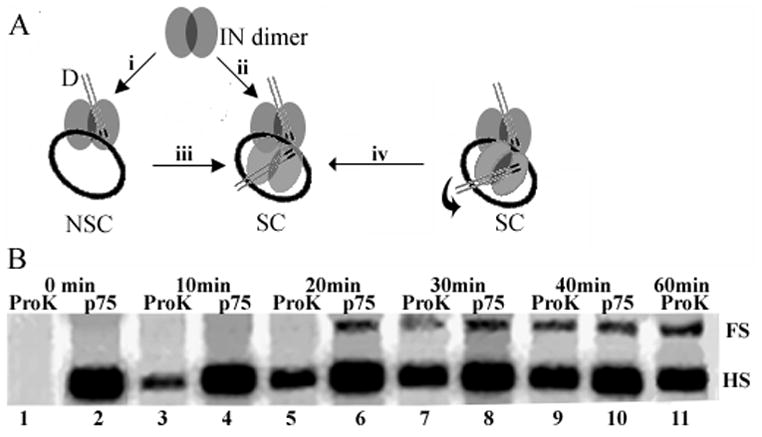
SC assembly and reaction time course. (A) Different schemes to account for SC formation and FS integration. The curved arrow preceding step iv illustrates a theoretical reorientation within the nucleoprotein complex (Wang et al., 2001). See text for additional details. (B) Integration time course. Reactions in lanes 1–11 were treated with proteinase K or LEDGF/p75 at the indicated times. The portion of the gel harboring HS and FS products is presented for simplicity; D2 and MT products were formed at levels proportional to HS levels. Results are representative of those obtained in two independent time course experiments.
Our results indicate that LEDGF/p75 either interferes with SC formation, or affects its disassembly during the course of the reaction. The IN dimer can process and integrate individual D ends (Faure et al., 2005; Guiot et al., 2006) and higher order multimers, including the tetramer, can dissociate into monomers and dimers upon DNA binding (Deprez et al., 2001). Different scenarios may therefore be envisioned for SC formation. IN dimers can bind D DNA, interact with one another to form the active tetramer, and then engage target DNA (Fig. 3A, assembly pathway ii) (Li et al., 2006). Alternatively, the dimeric complex might engage target DNA (Fig. 3A, assembly pathway i) prior to tetramer formation (Fig. 3Aiii). Both D DNA ends within the SC moreover need not concomitantly integrate into target (Li et al., 2006), providing evidence for a theoretical “reorientation” step based on the crystal structure of tetrameric HIV-1 IN1–212 (Wang et al., 2001). The distance between the two active sites most likely to catalyze DNA strand transfer within the tetramer model were further apart (~40 Å) than expected for FS integration into normal B-form DNA (~15 Å). It was therefore proposed that sequential end integration and reorientation of protein subunits within the tetramer might regulate DNA strand transfer such that the second end becomes integrated 5 bp away from the first (Wang et al., 2001). Thus, we envisioned that LEDGF/p75 might interfere with FS product formation by competing for IN dimer-dimer interactions (Fig. 3A, branch ii and/or iii) or interfere with a potential reorientation step within assembled SCs that harbor a single integrated end (branch iv).
To probe if LEDGF/p75 predominantly interferes with SC assembly/function or effects its disassembly, an order-of-addition experiment was performed wherein the host factor was added at different times throughout a 1 h experimental platform. In parallel, proteinase K was added instead of LEDGF/p75. Thus, as a function of time, proteinase K revealed the extent and types of products formed by IN up until that point independent of LEDGF/p75. Comparing these results to those generated in the LEDGF/p75-containing reactions yielded the affects of the host factor on the nucleoprotein complexes that had been formed by IN until that time. In the absence of LEDGF/p75, the ratio of HS:FS products in this experiment was ~3:1 (Fig. 3B, lane 11). As expected, adding proteinase K at the reaction onset negated product formation (Fig. 3B, lane 1). Approximately 30% of IN-alone HS integration had occurred by 10 min (compare lane 3 to lane 11), and this product accumulated over time such that ~58% of it was apparent by 20 min (lane 5), ~77% by 30 min (lane 7), and ~90% by 40 min (lane 9). In stark contrast, appreciable levels of FS integration were not observed until 30 min after the start of the reaction, at which time the product had accumulated to ~35% of its final level (lanes 3, 5, 7, and 11). The time lag required to detect appreciable levels of FS (lane 7) versus HS (lane 3) products indicates that NSC assembly occurs significantly faster and therefore may potentially serve as an intermediate to SC formation under these conditions.
As established above, adding LEDGF/p75 at the start of the reaction (Fig. 3B, lane 2) interfered with FS integration while enhancing the formation of HS products (~27% stimulation of HS integration as compared to IN alone in lane 11). Adding LEDGF/p75 10 min after the start of the reaction (lane 4) likewise prevented FS product formation. Significantly, introducing LEDGF/p75 at 20 min did not prevent FS integration (lane 6). Since significant levels of FS products had failed to form by this time in reactions lacking LEDGF/p75 (lane 5), we infer that the SCs that had formed by 20 min into the reaction but had yet to catalyze integration (lane 5) were not inhibited from completing this task by the presence of LEDGF/p75 during the final 40 min of the time course. The result argues against LEDGF/p75-dependent disassembly of SCs or interference with a potential reorientation step required for FS integration. A substantial boost in the level of HS product formation upon adding LEDGF/p75 at 40 min (compare lane 10 to lane 9) substantiates the conclusion that NSC formation occurs significantly faster than SC formation.
Adopting an assay system (Li and Craigie, 2005) that utilizes relatively long, blunt-ended D DNA to effect FS integration, we determine that LEDGF/p75 stimulates HS product formation yet preferentially inhibits FS HIV-1 integration in vitro. This result was somewhat unanticipated, as LEDGF/p75 binds tetrameric IN in human cells (Cherepanov et al., 2003) and is important for virus integration (Llano et al., 2006a; Vandekerckhove et al., 2006). A variety of reports moreover indicate that the tetramer is likely to catalyze FS HIV-1 integration (Faure et al., 2005; Guiot et al., 2006; Li et al., 2006). The results of order-of-addition experiments allow us to conclude that LEDGF/p75 does not disassemble SCs that are assembled by IN-alone acting on D DNA. Furthermore, any potential reorientation of DNA-bound IN subunits that might be required to bring a second end into a spatial register to complete FS integration (Wang et al., 2001) is not inhibited by LEDGF/p75. Although NSCs form significantly faster than SCs under our reaction conditions, we highlight that our results do not address whether the NSC serves as an intermediate to SC formation.
Numerous studies indicate that LEDGF/p75 functions as a molecular tether in the cell. A conserved PWWP domain and dual copy of the AT-hook DNA-binding motif in the N-terminal half of LEDGF/p75 impart chromatin-binding function (Llano et al., 2006b; Turlure et al., 2006) whereas the IN-binding domain in the C-terminal half of the protein can tether IN (Llano et al., 2006b; Maertens et al., 2003; Vanegas et al., 2005), the cellular factor JPO2 (Maertens et al., 2006), or perhaps the HIV-1 preintegration complex (PIC) to chromatin for integration (Llano et al., 2006a). On the other hand, LEDGF/p75 has been identified as a component of HIV-1 PICs in cytoplasmic extracts of acutely-infected cells (Llano et al., 2004) and can efficiently restore PIC function following an in vitro salt-depletion step (Vandegraaff et al., 2006). Therefore, an important issue to address is whether PIC or chromatin-associated LEDGF/p75 might supply the critical host function in vivo.
Based on the results of this study, we speculate LEDGF/p75 is likely to perform its task as a chromatin-associated factor. In this scenario, SC formation in the PIC is mediated by IN independent of LEDGF/p75 and, upon engaging LEDGF/p75, the IN within the SC would be encouraged to integrate at or nearby the site of LEDGF/p75 binding (Ciuffi et al., 2005). Consistent with this model, both the chromatin and IN-binding functions of LEDGF/p75 were critical for virus integration (Llano et al., 2006a). Our results however do not exclude the possibility that LEDGF/p75 might play its important role as a PIC component. In this case, we would propose that the host factor is recruited to the PIC after IN dimer-dimer formation or, if it is present before this event, that HIV-1 has evolved a mechanism to avoid the LEDGF/p75-IN interaction until the IN tetramer is effectively in place. Additional experiments that employ recently-described LEDGF/p75-depleted cell culture systems (Llano et al., 2006a) should allow more detailed investigations into identifying whether LEDGF/p75 in association with chromatin or the PIC plays the predominant role to effect HIV-1 integration in vivo.
Methods
The 972 bp ScaI-DraIII restriction fragment from pU3U5 (Cherepanov et al., 1999) served as D DNA. Recombinant proteins were purified as described (Turlure et al., 2006). Integration reactions (25 μL) containing 20 mM HEPES (pH 7.5), 5 mM dithiothreitol, 10 mM MgCl2, 20 μM ZnCl2, 100 mM NaCl were assembled by sequential addition of 18 nM D DNA, 11 nM pGEM-3, 0.9–1.8 μM IN, and 0.45–0.7 μM LEDGF/p75 or BSA where indicated, followed by addition of Me2SO to 5% (v/v). After 2 to 4 min at room temperature, polyethylene glycol with an average molecular weight of 8,000 Da was added to 10% (v/v). Reactions incubated at 37°C for 60 min were stopped by addition of EDTA, Na dodecyl sulfate, and proteinase K to the final concentrations of 10 mM, 0.2%, and 1 mg/mL, respectively, followed by 30 min at 37°C. DNAs recovered following precipitation with ethanol were fractionated through 0.6% agarose containing TBE buffer (89 mM Tris base, 89 mM borate, and 2 mM EDTA) at 5 V/cm for 8 h. Dried gels scanned on a STORM 820 Molecular Dynamics phosphorImager were quantified using ImageQuant version 1.2. For the Fig. 3B time course, a scaled-up reaction containing all components except LEDGF/p75 was assembled. Aliquots (10 μL) withdrawn at the indicated times were either treated with proteinase K or LEDGF/p75 (0.7 μM). All reactions were maintained at 37°C for an overall time of 60 min.
Two-dimensional (2D) agarose gel electrophoresis was performed as described (Friedman and Brewer, 1995) with slight modifications. Samples were electrophoresed in the first dimension through 0.4% agarose-TBE at 1 V/cm for 19 h at room temperature. After staining with 0.3 μg/mL ethidium bromide for 1 h, excised lanes were electrophoresed at 5 V/cm for 6 h at 4°C into a 1.1 % agarose-TBE gel. The gel was dried, exposed to a phosphorImager screen, and DNA spots were quantified as described above.
Acknowledgments
This work was funded by NIH grants AI39394, AI70042 (A.E.), and AI60354 (Harvard Medical School Center for AIDS Research).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bushman FD, Craigie R. Activities of human immunodeficiency virus (HIV) integration protein in vitro: Specific cleavage and integration of HIV DNA. Proc Natl Acad Sci USA. 1991;88:1339–1343. doi: 10.1073/pnas.88.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y, De Clercq E, Debyser Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J Biol Chem. 2003;278:372–381. doi: 10.1074/jbc.M209278200. [DOI] [PubMed] [Google Scholar]
- Cherepanov P, Surratt D, Toelen J, Pluymers W, Griffith J, De Clercq E, Debyser Z. Activity of recombinant HIV-1 integrase on mini-HIV DNA. Nucleic Acids Res. 1999;27:2202–2210. doi: 10.1093/nar/27.10.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffi A, Llano M, Poeschla E, Hoffmann C, Leipzig J, Shinn P, Ecker JR, Bushman F. A role for LEDGF/p75 in targeting HIV DNA integration. Nat Med. 2005;11:1287–1289. doi: 10.1038/nm1329. [DOI] [PubMed] [Google Scholar]
- Deprez E, Tauc P, Leh H, Mouscadet JF, Auclair C, Hawkins ME, Brochon JC. DNA binding induces dissociation of the multimeric form of HIV-1 integrase: A time-resolved fluorescence anisotropy study. Proc Natl Acad Sci USA. 2001;98:10090–10095. doi: 10.1073/pnas.181024498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Calmels C, Desjobert C, Castroviejo M, Caumont-Sarcos A, Tarrago-Litvak L, Litvak S, Parissi V. HIV-1 integrase crosslinked oligomers are active in vitro. Nucleic Acids Res. 2005;33:977–986. doi: 10.1093/nar/gki241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman KL, Brewer BJ. Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Methods Enzymol. 1995;262:613–627. doi: 10.1016/0076-6879(95)62048-6. [DOI] [PubMed] [Google Scholar]
- Goodarzi G, Im GJ, Brackmann K, Grandgenett D. Concerted integration of retrovirus-like DNA by human immunodeficiency virus type 1 integrase. J Virol. 1995;69:6090–6097. doi: 10.1128/jvi.69.10.6090-6097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiot E, Carayon K, Delelis O, Simon F, Tauc P, Zubin E, Gottikh M, Mouscadet JF, Brochon JC, Deprez E. Relationship between the oligomeric status of HIV-1 integrase on DNA and enzymatic activity. J Biol Chem. 2006;281:22707–22719. doi: 10.1074/jbc.M602198200. [DOI] [PubMed] [Google Scholar]
- Li M, Craigie R. Processing of viral DNA ends channels the HIV-1 integration reaction to concerted integration. J Biol Chem. 2005;280:29334–29339. doi: 10.1074/jbc.M505367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Mizuuchi M, Burke TR, Craigie R. Retroviral DNA integration: reaction pathway and critical intermediates. EMBO J. 2006;25:1295–1304. doi: 10.1038/sj.emboj.7601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano M, Saenz DT, Meehan A, Wongthida P, Peretz M, Walker WH, Teo W, Poeschla EM. An Essential Role for LEDGF/p75 in HIV Integration. Science. 2006a;314:461–464. doi: 10.1126/science.1132319. [DOI] [PubMed] [Google Scholar]
- Llano M, Vanegas M, Fregoso O, Saenz D, Chung S, Peretz M, Poeschla EM. LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes. J Virol. 2004;78:9524–9537. doi: 10.1128/JVI.78.17.9524-9537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano M, Vanegas M, Hutchins N, Thompson D, Delgado S, Poeschla EM. Identification and characterization of the chromatin-binding domains of the HIV-1 integrase interactor LEDGF/p75. J Mol Biol. 2006b;360:760–773. doi: 10.1016/j.jmb.2006.04.073. [DOI] [PubMed] [Google Scholar]
- Maertens G, Cherepanov P, Pluymers W, Busschots K, De Clercq E, Debyser Z, Engelborghs Y. LEDGF/p75 is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J Biol Chem. 2003;278:33528–33539. doi: 10.1074/jbc.M303594200. [DOI] [PubMed] [Google Scholar]
- Maertens GN, Cherepanov P, Engelman A. Transcriptional co-activator p75 binds and tethers the c-Myc-interacting protein JPO2 to chromatin. J Cell Sci. 2006;119:2563–2571. doi: 10.1242/jcs.02995. [DOI] [PubMed] [Google Scholar]
- Sinha S, Grandgenett DP. Recombinant human immunodeficiency virus type 1 integrase exhibits a capacity for full-site integration in vitro that is comparable to that of purified preintegration complexes from virus-infected cells. J Virol. 2005;79:8208–8216. doi: 10.1128/JVI.79.13.8208-8216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlure F, Maertens G, Rahman S, Cherepanov P, Engelman A. A tripartite DNA-binding element, comprised of the nuclear localization signal and two AT-hook motifs, mediates the association of LEDGF/p75 with chromatin in vivo. Nucleic Acids Res. 2006;34:1663–1675. doi: 10.1093/nar/gkl052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegraaff N, Devroe E, Turlure F, Silver PA, Engelman A. Biochemical and genetic analyses of integrase-interacting proteins lens epithelium-derived growth factor (LEDGF)/p75 and hepatoma-derived growth factor related protein 2 (HRP2) in preintegration complex function and HIV-1 replication. Virology. 2006;346:415–426. doi: 10.1016/j.virol.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove L, Christ F, Van Maele B, De Rijck J, Gijsbers R, Van den Haute C, Witvrouw M, Debyser Z. Transient and stable knockdown of the integrase cofactor LEDGF/p75 reveals its role in the replication cycle of human immunodeficiency virus. J Virol. 2006;80:1886–1896. doi: 10.1128/JVI.80.4.1886-1896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas M, Llano M, Delgado S, Thompson D, Peretz M, Poeschla E. Identification of the LEDGF/p75 HIV-1 integrase-interaction domain and NLS reveals NLS-independent chromatin tethering. J Cell Sci. 2005;118:1733–1743. doi: 10.1242/jcs.02299. [DOI] [PubMed] [Google Scholar]
- Wang JY, Ling H, Yang W, Craigie R. Structure of a two-domain fragment of HIV-1 integrase: implications for domain organization in the intact protein. EMBO J. 2001;20:7333–7343. doi: 10.1093/emboj/20.24.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]


