Abstract
Treatment of children with cranial-spinal radiation (CSR) for brain tumors is associated with adverse intellectual outcome and white matter damage. However, the correlation between IQ and measures of white matter integrity has received little attention. We examined apparent diffusion coefficient (ADC), fractional anisotropy (FA), and intelligence in pediatric patients treated with CSR for medulloblastoma relative to control subjects. ADC and FA measures were obtained for eight patients and eight control children and evaluated in multiple regions of interest in the cerebral hemispheres. Mean ADC and mean FA for each region were calculated, group differences were evaluated, and the relationship between these measures and intelligence were examined. In our study group, decreased IQ was associated with increased ADC and decreased FA (P < 0.01). Mean IQ for the CSR group was lower than that for the control group, but the difference was not significant when controlling for overall mean FA or ADC (P > 0.10). Overall mean FA was lower and ADC was higher in the CSR group relative to controls (P < 0.01). Specifically, FA was lower in the genu of the corpus callosum, the anterior and posterior limbs of the internal capsule, inferior frontal white matter, and high frontal white matter, and ADC was higher in all regions in patients relative to controls (P < 0.01). Compromised white matter integrity was observed for multiple regions within the cerebral hemispheres following CSR. A novel finding was that microscopic damage in normal-appearing white matter, as indexed by higher ADC and lower FA, was related to poor intellectual outcome relative to age-matched controls.
Keywords: cranial radiation, diffusion tensor imaging, intelligence, white matter
Medulloblastomas are malignant tumors that require treatment with surgery, radiation to the cranial-spinal axis (CSR),3 and an additional radiation boost to the posterior fossa (PF), with or without adjuvant chemotherapy. With recent advances in medical treatment, five-year survival rates for children treated for standard-risk medulloblastoma approach 80% (Packer et al., 1999; Strother et al., 2002). Unfortunately, therapeutic CSR has adverse effects on many systems, including the endocrine, skeletal, and central nervous systems. A progressive decline in intelligence (Copeland et al., 1999; Palmer et al., 2001, 2003; Ris et al., 2001; Spiegler et al., 2004) and academic functioning (Mabbott et al., 2005) following CSR in childhood is well documented.
CSR can be associated with injury to the developing brain. The most salient effects are diffuse and multifocal white matter abnormalities, observed as increased signal intensity on T2-weighted images (Edwards-Brown and Jakacki, 1999; Mulhern et al., 2001). The extent of abnormality varies from scattered, small white matter lesions to larger confluent lesions (Edwards-Brown and Jakacki, 1999). Cerebral atrophy can also be observed (Edwards-Brown and Jakacki, 1999; Fouladi et al., 2000). Further, decline in brain volume not evident on clinical scans has been identified, including volume loss in normal-appearing white matter (Mulhern et al., 2001; Reddick et al., 2000), the corpus callosum (Palmer et al., 2002), and the hippocampus (Nagel et al., 2004). The loss of normal-appearing white matter volume predicts lower cognitive outcome (Mulhern et al., 2001; Reddick et al., 2003). Possible mechanisms of radiation injury are alterations of the microvasculature within the brain, damage to the oligodendrocytes that produce myelin, and possibly glial (both microglial and astrocytic) death (Habrand and De Crevoisier, 2001; Schultheiss et al., 1995), resulting in an increase in brain water with the loss of brain tissue (Edwards-Brown and Jakacki, 1999).
Although volumetric MRI techniques have provided important information regarding decline in normal-appearing white matter volume following radiation, they provide little information regarding the potential microstructural changes within this brain tissue. Understanding these changes is important both for early identification of radiation injury and for understanding the complex relationships between mechanisms of radiation injury brain and intellectual outcome. Diffusion tensor magnetic resonance imaging (DTI) is particularly useful for examining changes in white matter microstructure and relating those changes to cognitive outcome (Paus et al., 2001) because of the rotationally invariant ability of DTI to quantify three-dimensional measures of water diffusion. First, DTI is sensitive to developmental changes in white matter: A decrease in mean diffusivity and an increase in anisotropy have been associated with increasing age in newborns and children (Huppi et al., 1998; Li and Noseworthy, 2002; Mukherjee et al., 2001; Neil et al., 1998; Suzuki et al., 2003). These changes have been attributed to the impact of premyelination, reduction in brain water, myelination, increases in fiber diameter, greater cohesiveness, compactness of the fiber tracts, and reduced extra-axonal spaces (i.e., greater packing) as white matter matures over time (Beaulieu, 2002; Huppi et al., 1998; Klingberg et al., 1999; Neil et al., 1998; Suzuki et al., 2003). Second, DTI measures have been used to document tissue breakdown in disorders known to influence white matter microstructure, including multiple sclerosis, brain injury, perinatal cerebral white matter injury, and adrenoleukodystrophy (Huppi et al., 2001; Ito et al., 2001; Jones et al., 2000; Wieshmann et al., 1999). Finally, lower anisotropy values have been found in the posterior fossa (cerebellar hemispheres, pons, and medulla oblongata) and cerebral white matter (corpus callosum, frontal periventricular, parietal periventricular, and corona radiata) for children treated with CSR for medulloblastoma relative to control children (Khong et al., 2003; Leung et al., 2004), and lower values have been related to younger age at diagnosis and larger radiation dose (Khong et al., 2005). Because of the sensitivity to white matter changes with development and/or injury, DTI is an excellent means for evaluating potential damage following treatment with radiation and relations with intellectual outcome.
We evaluated diffusivity by determining apparent diffusion coefficient (ADC) and directionality by determining fractional anisotropy (FA) in normal-appearing white matter in children treated with CSR for medulloblastoma and in normal children. No evaluation of the relationships between DTI measures and intellectual outcome exists in the literature. Examining these relationships is essential for understanding and potentially mediating the mechanism of cognitive decline following cranial-spinal radiation. Our first goal was to examine whether DTI measures are related to adverse intellectual outcome in children treated with CSR. We expected that increased diffusion and decreased anisotropy should be associated with lower intellectual outcome in patients treated with CSR. Second, we compared ADC and FA values of the two samples. We expected increased diffusivity and decreased directionality in patients treated with radiation relative to controls. Other unique contributions of the present study include comprehensive coverage of regions within cerebral white matter, an area where significant loss of volume has been identified (Mulhern et al., 2001; Reddick et al., 2000), and the inclusion of deep gray matter to compare measures in white matter and gray matter structures.
Methods
Patients
Eight patients (seven males) treated for medulloblastoma with CSR were included in the study. Two had high-risk metastatic disease, and two were high risk because of an incomplete resection: These patients were treated with standard-dose (i.e., 3600–3660 cGy) radiation to the cranial-spinal axis. Four patients with average-risk disease (gross total resection without evidence of metatheses) were treated with reduced-dose (i.e., 2340 cGy) radiation to the cranial-spinal axis. All patients received an additional boost to the PF, so that the total PF radiation dose was 5540 cGy. Six patients had a ventriculoperitoneal shunt inserted to treat associated hydrocephalus. All patients received adjuvant chemotherapy, including either etoposide/cisplatin/cyclophosphamide/vincristine or CCNU/vincristine/cisplatin.
The mean age at diagnosis for the patients was 7.48 years (SD = 3.87 years), and the mean time from diagnosis to the study MRI scan was 2.50 years (SD = 0.72 year). A group of eight healthy control children (six males) were recruited from friends and family of the investigators: Children with a history of prior acquired brain injury, developmental delay, or learning disability were not included. The same MRI protocol used for the medulloblastoma patients was followed for these children. Mean age at the time of scan was similar for the patients and the control children (9.98 years, SD = 2.90, vs. 9.84 years, SD = 3.42, respectively; F (variance ratio) = 0.008, P > 0.10). This study conformed to the Tri-Council Ethics Principles and was reviewed and approved by the Research Ethics board of The Hospital for Sick Children.
MR Imaging and Postprocessing
The MRI measurements were performed with a GE LX 1.5T MRI scanner (GE Healthcare, Milwaukee, Wis.) and a single-channel quadrature head coil. Following a routine clinical brain tumor imaging protocol, DTI measurements were acquired for each subject by using a single-shot, spin-echo DTI sequence with an EPI (echo-planar imaging) readout (imaging parameters as follows: TE/TR [echo time/repetition time] = 100/6000 ms, 128 × 128 matrix, number of excitations = 1, field of view = 24 cm, receive bandwidth = 125 kHz, beta (b) = 1000 s/mm2, 25 directions, one b = 0 image). The number of slices and thickness varied across subjects as clinically required: 21 to 42 contiguous axial slices were acquired, and slice thickness ranged from 3 to 5.5 mm across subjects. Total additional scanning time for patients was approximately 5 min.
Postprocessing, including eddy current correction, was conducted with a commercial software program (FUNCTOOL 3.1.10; GE Medical Systems, GE Health-care). ADC and FA were calculated by acquiring the traces and eigenvalues from matrices of diffusion gradients on a pixel-by-pixel basis. ADC and FA were evaluated by using a region of interest (ROI) approach. ROI analysis was conducted using the b = 0 images: ROIs were identified from this image rather than the ADC or FA maps, which avoids the problem of using the dependant variable (i.e., ADC or FA) to define the anatomic regions (Pfefferbaum et al., 2000). As these images were acquired in the same space as the diffusion gradients, image registration was not necessary. ROIs were placed on three successive axial slices to provide comprehensive coverage and were chosen by referring to existing literature documenting radiation-related changes in the regions (Khong et al., 2003; Leung et al., 2004) as well as to provide comprehensive coverage of larger fiber tracts and hemispheric white matter. Specific ROIs for white matter included the genu of the corpus callosum, the anterior and posterior limbs of the internal capsule, inferior frontal white matter, high frontal white matter, and parietal white matter. ROIs were also placed in the thalamus and the putamen to enable comparison of deep gray matter and white matter structures (Fig. 1). All ROIs were reviewed by a neuroradiologist (S.L.) to ensure accuracy of placement: The clinical scans were also reviewed to identify any areas of white matter abnormality. Any areas of white matter hyperintensity on T2 were not included in the ROIs so that the diffusion tensor measures were calculated for normal-appearing white matter only. To conduct qualitative comparisons between the patient and control groups, fiber tractography was performed using the DTIStudio program developed at Johns Hopkins Medical Institute in Baltimore, Md. (http://cmrm.med.jhmi.edu). In this algorithm, the fiber tracts are reconstructed along the eigenvector corresponding to the preferred (largest) diffusion in each voxel. The arrangement of eigenvectors in space forms the so-called diffusion vector field. From this discrete three-dimensional vector field, the fiber tracts within the imaged volume were obtained (Bammer et al., 2003; Mori at al., 1999). Two ROIs were placed within the corpus callosum (in the middle of the genu and splenium): Tractography was started with any voxels with FA greater than 0.60 and discontinued at voxels with FA less than 0.40.
Fig. 1.
Axial T2-weighted images upon which the regions of interest are placed. A. ROIs within the genu of the corpus callosum, the anterior and posterior limbs of the internal capsule, inferior frontal white matter, parietal white matter, the thalamus, and the putamen. B. The ROI within the high frontal white matter.
Intellectual Functioning
Intellectual functioning was assessed for all subjects by using a short-form estimate of the Full Scale IQ from the Wechsler Intelligence Scales for Children (WISC; either the 3rd or 4th edition), comprising the block design, coding, vocabulary, and information subtests (Wechsler, 1991, 2003). Although estimates of full-scale IQ from WISC-III and WISC-IV obtained within the same test group are highly related (r = 0.89) (Wechsler, 2003), we acknowledge that standard scores for these tests are based on different normative samples. Individual test scores were converted to standard scores (based on age-related means and standard deviations from test standardization norms) with a mean of 100 and a standard deviation of 15. Mean time and median time from diagnosis to the follow-up assessment for the patients were 2.38 and 2.07 years, respectively (range, 1.25–5.22 years). Because data for the patient groups were acquired as part of ongoing clinical follow-up, IQ and MRI data were obtained on different occasions. The mean and median times from the study MRI scan to follow-up intellectual testing were 0.63 and 0.54 years, respectively (range, 0.25–1.42 years). For the control sample, IQ data were collected on the same day as the MRI scan. Finally, all of the patients also had an initial assessment of IQ prior to the follow-up assessment: Mean and median times from diagnosis to this initial assessment were 0.78 and 0.50 years, respectively (range, 0.10–3.30 years).
Statistical Analysis
First, differences in intellectual functioning were examined with t tests to provide the context within which to examine the brain behavior relations. IQ at the initial assessment for the patient group was compared to IQ for controls to determine whether any group differences existed at a relatively early point in treatment for the patients. Further, initial and follow-up IQs were compared within the patient group to determine whether intellectual function declined over time, as this is a known effect of cranial radiation (Copeland et al., 1999; Palmer et al., 2001, 2003; Ris et al., 2001; Spiegler et al., 2004).
For imaging analyses, mean ADC and FA across voxels and slices within each ROI were calculated and used in subsequent analyses. First, correlation analyses were used to examine the correlation among IQ, FA, and ADC. Second, mean IQ was examined between the patient group (follow-up assessment) and control group by using one-way analysis of variance. To determine whether potential differences in white matter integrity as measured by ADC and FA were associated with differences in intellectual functioning, these measures were included as covariates in subsequent analyses of covariance comparing IQ of the two groups. Third, a 2 (group) ×8 (ROI) analysis of variance with repeated measures for the ROIs was conducted to compare overall ADC and FA for patients treated with CSR relative to the control sample across the eight ROIs. Further, multiple one-way analyses of variance were conducted for each ROI to evaluate differences between the two groups. To correct for multiple comparisons, all results were considered significant at the P < 0.01 level only.
Results
Neurocognitive Functioning
Mean initial IQ for the patient group was 17.5 points lower than the control group mean IQ (95.25 vs. 112.75), although this was not significant (t = 2.29, P > 0.05). The power limitations of our small sample must be considered in interpreting this finding. The mean decline in IQ from initial to follow-up assessment near the time of scanning for the patient group was 8 points (95.25 vs. 87.25; t = −3.25; P < 0.01).
Neurocognitive Functioning and DTI Measures
To examine the correlation between intellectual functioning and white matter integrity, FA and ADC values were averaged across all ROIs. For FA, only the ROIs including white matter were used to calculate the composite, as these regions were significantly different in the control and the CSR groups (details below). All ROIs were used to calculate the overall mean ADC. Across the entire sample, decreased IQ at the time of scanning was associated with increased ADC (r = −0.60, P < 0.01). The relation between IQ and FA was weak (r = 0.32, P > 0.10). When the scatter plot for FA and IQ was examined, an outlier existed: A single patient demonstrated low IQ and high FA, whereas for all other subjects, both patients and controls, low IQ corresponded with low FA. The correlation analysis was conducted with the outlier removed, and a significant relation was identified between decreased IQ and decreased FA (r = 0.65, P < 0.01).
Mean IQ for the CSR group at the time of scanning was almost 1 SD below the normative mean, and it was significantly lower than that of the control sample (87.50 vs. 112.75; F = 9.53; P < 0.01). This effect was not significant when controlling for overall mean FA (with the outlier removed) or ADC (Fs > 1.42; Ps > 0.10). Hence, FA and ADC appear to be associated with the effect of radiation treatment on intellectual outcome.
Fractional Anisotropy
The FA for the total sample was significantly higher in the corpus callosum and the posterior limb internal capsule relative to all other structures (Table 1): FA for the corpus callosum and FA for the posterior limb internal capsule did not differ. Mean FA for the anterior limb internal capsule was higher than for the inferior frontal white matter, high frontal white matter, thalamus, and putamen, but not different from parietal white matter. Mean FA did not differ among inferior frontal white matter, high frontal white matter, and parietal white matter, but was higher in these areas relative to the thalamus and putamen. Mean FA was not different in the thalamus and putamen. Finally, mean FA across all ROIs was lower in the CSR group relative to controls (0.28 vs. 0.44; P < 0.01; Fig. 2).
Table 1.
Mean FA and ADC within each region of interest, averaged across the patient and control groups
|
FA |
ADC |
|||
|---|---|---|---|---|
| Region of Interest | Mean | SD | Mean | SD |
| Genu of the corpus callosum | 0.56a | 0.14 | 766a | 345 |
| Posterior limb internal capsule | 0.51a | 0.14 | 658b | 337 |
| Anterior limb internal capsule | 0.42b | 0.15 | 645abc | 215 |
| Parietal white matter | 0.37bc | 0.10 | 697abd | 234 |
| Inferior frontal white matter | 0.33c | 0.09 | 667 | 197 |
| High frontal white matter | 0.31c | 0.07 | 632abce | 194 |
| Thalamus | 0.27d | 0.09 | 619 | 159 |
| Putamen | 0.18d | 0.08 | 580 | 151 |
Abbreviations: ADC, apparent diffusion coefficient; CSR, cranial-spinal radiation; FA, fractional anisotropy.
Means with different superscripts within a column differ at P < 0.01.
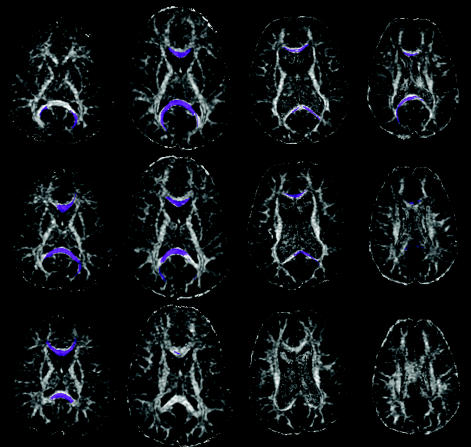
Fig. 2.
FA map, including ROIs, for a control subject (top frame) and patient (bottom frame).
Univariate comparisons (Table 2) showed that mean FA was significantly lower in the corpus callosum, posterior limb internal capsule, anterior limb internal capsule, inferior frontal white matter, and high frontal white matter for patients treated with CSR relative to controls. Tractography was conducted within the corpus callosum to provide qualitative/visual evidence of the differences in FA. Specifically, tractography was conducted for a patient with enlarged ventricles, a patient without enlarged ventricles, and two age-matched controls (Fig. 3). The resulting fiber tracts for the controls were thicker and had greater complexity in connections relative to those of the patients. The significant difference in mean FA for the corpus callosum of the two groups likely reflects differences in axonal/white matter integrity, yielding the impoverishment of tract connections in the patients. No differences in FA were evident between the groups for the parietal white matter, thalamus, and putamen.
Table 2.
Mean FA and ADC within each region of interest for the patient and control groups
|
FA |
ADC |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Control |
CSR |
Control |
CSR |
|||||
| Region of Interest | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Genu of the corpus callosum | 0.66* | 0.07 | 0.46* | 0.12 | 534* | 64 | 998* | 358 |
| Posterior limb internal capsule | 0.60* | 0.02 | 0.42* | 0.15 | 461* | 24 | 857* | 391 |
| Anterior limb internal capsule | 0.54* | 0.06 | 0.30* | 0.10 | 476* | 17 | 814* | 183 |
| Parietal white matter | 0.41 | 0.07 | 0.32 | 0.11 | 532* | 31 | 863* | 231 |
| Inferior frontal white matter | 0.40* | 0.06 | 0.26* | 0.06 | 513* | 17 | 821* | 169 |
| High frontal white matter | 0.36* | 0.05 | 0.26* | 0.06 | 504* | 37 | 778* | 198 |
| Thalamus | 0.30 | 0.05 | 0.23 | 0.10 | 490* | 20 | 749* | 124 |
| Putamen | 0.20 | 0.04 | 0.17 | 0.11 | 479* | 27 | 680* | 158 |
Abbreviations: ADC, apparent diffusion coefficient; CSR, cranial-spinal radiation; FA, fractional anisotropy.
Means are significantly different between the CSR patient and control groups at P <0.01.
Fig. 3.
FA maps with tractography for the genu and splenium of the corpus callosum for two patients (two columns on the right; with and without evidence of hydrocephalus) and two age-matched control subjects (two columns on the left).
Apparent Diffusion Coefficient
Few differences existed in mean ADC values across ROIs (Table 1). Mean ADC was higher for the corpus callosum than for the posterior limb internal capsule and was higher for parietal white matter than for high frontal white matter or anterior limb internal capsule. Mean ADC across all ROIs was higher for the CSR group relative to controls (826 vs. 498; P < 0.01). Univariate comparisons (Table 2) showed mean ADC was significantly higher within each ROI, including the thalamus and putamen, for patients treated with CSR relative to controls (P < 0.01).
Discussion
Although changes in DTI measures of white matter integrity following cranial radiation have been documented (Khong et al., 2003, 2005; Leung et al., 2004), this was the first study to examine the explicit relations between DTI measures and intelligence. A novel finding was the significant association between IQ and measures of underlying tissue property that index brain integrity, including FA and ADC. We found that tissue compromise, as inferred from decreased fiber orientation and increased water diffusion, may mediate adverse intellectual outcome associated with radiation treatment. IQ provides an estimate of overall cognitive functioning, and as such it is reasonable that such a measure is related to relatively diffuse tissue loss. Hence, the biological mechanism relating radiation and adverse intellectual outcome may be microscopic damage to normal-appearing white matter, and possibly other structures, resulting in tissue loss and related decrease in efficiency of cognitive processing.
According to our findings, both FA and ADC appear to be sensitive measures of brain tissue damage following CSR for pediatric brain tumors. FA values across ROIs were consistent with expected anatomical microstructure: For example, the highest FA was observed in the corpus callosum, a structure with substantial directionality in fiber orientation, and the lowest FA was observed in the subcortical nuclei, where little directionality in water diffusion would be expected. Consistent with previous work (Khong et al., 2003, 2005; Leung et al., 2004), our findings showed that FA was reduced in multiple hemispheric sites in patients treated with radiation for medulloblastoma relative to age-matched controls. Specifically, we observed decreased directionality in water diffusion in five of the six regions of white matter, with the largest differences being observed for the large fiber tracts (i.e., genu of the corpus callosum and internal capsule). Demyelination is often a common pathological expression of white matter injury, irrespective of the etiology, as demonstrated in clinical studies of advanced spinal cord injury and animal studies (Schultheiss et al., 1995) and thus must be considered a plausible mechanism accounting for observed FA differences. However, myelin’s role as the primary influence on FA is doubtful: Myelination is not necessary for significant anisotropy to be observed (Beaulieu, 2002). FA likely reflects the primary influence of axonal membranes, including axonal density and fiber packing, which is then attenuated by myelin (Beaulieu, 2002). Hence, mechanisms other than myelin loss should also be considered in accounting for differences in FA between normal and clinical populations treated with radiation. Another potential mechanism in accounting for differences in FA may be the loss of glial progenitor cells that develop into and replenish myelin, as loss of these cells has been implicated in radiation injury (Schultheiss et al., 1995). Thus, differences in FA may reflect a failure of myelin development in addition to actual demyelination. Developing white matter may be vulnerable to the adverse effects of radiation because it results in cell-cycle disruption in growing tissue (Fouladi et al., 2000; Mulhern et al., 2001; Steen et al., 2001). Finally, radiation may also have an impact on astrocytes (Schultheiss et al., 1995). Astrocytes are often characterized as the glue that holds neural tissue together, making various contacts with neurons, synapses, ependyma, and meninges, as well as being one of the structural components of the blood-brain barrier (Schultheiss et al., 1995). Damage to these cells could result in volume loss as well as lead to decreased packing of fibers or fiber density, yielding decreased FA without any direct effects on existing myelin. Current evidence cannot differentiate between these mechanisms. Regardless, our findings regarding FA are consistent with compromised white matter microstructure and/or fiber integrity that is not evident on standard clinical MR imaging sequences or volumetric analyses. This compromise is evident in the very thin and unelaborated fiber tracts of a patient’s corpus callosum following tractography.
Higher ADC values were observed across ROIs, including the thalamus and putamen, in children treated with CSR as compared to controls. This is generally consistent with the findings of Khong et al. (2003), who noted that mean diffusivity was increased in most ROIs for their CSR group relative to the controls, although their differences did not reach statistical significance. Consistent with the FA data in our study, the greatest differences in the Kong et al. (2003) study were observed for the large fiber tracts, including the corpus callosum and internal capsule. An increase in ADC, and presumably increased free water content, is consistent with loss of tissue across both white matter and gray matter nuclei, though this loss is greatest in white matter. The putamen is a component of the lentiform nuclei, and the thalamus has some white matter within it (internal medullary lamina), so the ADC increase may reflect white matter injury as noted previously. Further, this loss may reflect death of glial support cells, as differences are evident in white matter and subcortical nuclei.
Finally, some limitations due to the use of a small and clinically acquired patient sample are relevant for interpreting our findings. First, because of the relatively small sample of patients, we were unable to address important clinical questions, including the influence of age at diagnosis, time since diagnosis, and radiation dose on brain microstructure. Further, we were unable to determine whether severity of intellectual impairment was related to DTI measures. These variables will need to be evaluated in studies with greater power in order to examine the relative risks associated with each. Second, the distribution between males (n = 7) and females (n = 1) in the patient sample is not desirable for accounting for the effects of gender when considering brain behavior correlation following radiation. There is evidence that female cancer patients display greater neurocognitive impairment than males (Brown et al., 1998). As our sample included primarily males, our findings may reflect an underestimate of what might be seen for females when considering DTI measures of white matter and IQ. Third, mean IQ for the control sample was higher than the baseline IQ for a patient sample and was almost 1 SD above the normative mean. Hence, the differences between the control and the patient groups are likely greater than would be expected if a more representative control sample were available, such as a sample comprising siblings of the brain tumor patients. It is notable that despite this potentially exacerbated difference in IQ, controlling for FA and ADC nullified the significant group effect in IQ. It would be expected that an increased difference in IQ between the groups would make it less likely that a covariate would account for any differences. Hence, our findings are robust. Fourth, our study was limited to data for a single scanning time. To evaluate the time course of changes in ADC and FA, we plan to collect longitudinal DTI data in future studies. Finally, to gain greater understanding of the correlation between radiation-related changes in the brain and behavior, more specific measures of cognitive function may be necessary. Intelligence tests have been useful in monitoring the global neurotoxic effects of disease and treatment. However, the mechanism by which damage to white matter may result in adverse intellectual outcome is not well understood. Delineating this mechanism is essential because global intelligence measures are not sufficient for explaining functional outcomes such as academic attainment and vocational success, nor are they suited for determining the basic brain-behavior correlations that are directly affected by treatment (Neisser et al., 1996). There is evidence that attentional difficulties are related to reduced white matter volume (Mulhern et al., 2004) and may mediate the correlation between white matter volume and poor intellectual outcome (Reddick et al., 2000). Quantitative DTI measures are well suited for examining the correlation between brain integrity and specific cognitive functions such as attention, because these measures provide quantitative information regarding tissue microstructure within the normal-appearing white matter that is often examined in volumetric studies, providing a more detailed level of analysis.
Conclusion
In this study, compromised white matter microstructure and/or fiber integrity as indexed by FA and ADC was observed for multiple regions within the cerebral hemispheres of patients treated with cranial-spinal radiation. Further, decreased FA and increased ADC were related to lower intellectual outcome in patients relative to age-matched controls. The correlation between compromised fiber integrity, tissue loss, and lower IQ is consistent with findings in previous studies that have documented that white matter volume loss is related to adverse intelligence and academic outcome (Mulhern et al., 2001; Reddick et al., 2000, 2003). A significant advantage of DTI is that it provides measures sensitive to underlying tissue properties, and hence potential damage is evident even within normal-appearing white matter. Understanding potential changes in brain integrity and associated adverse intellectual outcome is important for identifying children at greatest risk of future cognitive difficulties. Once identified, these children could benefit from early academic intervention before the full impact of late effects manifests. Ultimately, identification of white matter changes early in treatment and the related neurocognitive compromise holds promise in individually tailoring treatment protocols and possibly evaluating the efficacy of potential neuroprotective agents.
Footnotes
This work was supported by a Sick Kids Foundation–Canadian Institutes of Health Research New Investigator Grant and through funding from b.r.a.i.n.child, the Brain Tumor Research Assistance and Information Network at the Hospital for Sick Children. Portions of this data were presented at the 13th Meeting of the International Society for Magnetic Resonance in Medicine.
Note added in proof: While this paper was in production, Khong et al. (2006) identified a correlation between FA and IQ in childhood cancer survivors.
Abbreviations used are as follows: ADC, apparent diffusion coefficient; CSR, cranial-spinal radiation; DTI, diffusion tensor magnetic resonance imaging; FA, fractional anisotropy; PF, posterior fossa; ROI, region of interest; WISC, Wechsler Intelligence Scales for Children.
References
- Bammer R, Acar B, Moseley ME. In vivo MR tractography using diffusion imaging. Eur J Radiol. 2003;45:223–234. doi: 10.1016/s0720-048x(02)00311-x. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Brown RT, Madan-Swain A, Walco GA, Cherrick I, Ievers CE, Conte PM, Vega R, Bell B, Lauer SJ. Cognitive and academic late effects among children previously treated for acute lymphocytic leukemia receiving chemotherapy as CNS prophylaxis. J Pediatr Psychol. 1998;23:333–340. doi: 10.1093/jpepsy/23.5.333. [DOI] [PubMed] [Google Scholar]
- Copeland DR, deMoor C, Moore BD, 3rd and, Ater JL. Neurocognitive development of children after a cerebellar tumor in infancy: A longitudinal study. J Clin Oncol. 1999;7:3476–3486. doi: 10.1200/JCO.1999.17.11.3476. [DOI] [PubMed] [Google Scholar]
- Edwards-Brown MK, Jakacki RI. Imaging the central nervous system effects of radiation and chemotherapy of pediatric tumors. Neuroimaging Clin N Am. 1999;9:177–193. [PubMed] [Google Scholar]
- Fouladi M, Langston J, Mulhern R, Jones D, Xiong X, Yang J, Thompson S, Walter A, Heideman R, Kun L, Gajjar A. Silent lacunar lesions detected by magnetic resonance imaging of children with brain tumors: A late sequela of therapy. J Clin Oncol. 2000;18:824–831. doi: 10.1200/JCO.2000.18.4.824. [DOI] [PubMed] [Google Scholar]
- Habrand JL, De Crevoisier R. Radiation therapy in the management of childhood brain tumors. Childs Nerv Syst. 2001;17:121–133. doi: 10.1007/s003810000365. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, Kikinis R, Jolesz F.A, Volpe JJ. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- Ito R, Melhem ER, Mori S, Eichler FS, Raymond GV, Moser HW. Diffusion tensor brain MR imaging in X-linked cerebral adrenoleukodystrophy. Neurology. 2001;27:544–547. doi: 10.1212/wnl.56.4.544. [DOI] [PubMed] [Google Scholar]
- Jones DK, Dardis R, Ervine M, Horsfield MA, Jeffree M, Simmons A, Jarosz J, Strong AJ. Cluster analysis of diffusion tensor magnetic resonance images in human head injury. Neurosurgery. 2000;47:306–313. doi: 10.1097/00006123-200008000-00008. [DOI] [PubMed] [Google Scholar]
- Khong PL, Kwong DLW, Chan GCF, Sham JST, Chan F.-L, Ooi G.-C. Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: A pilot study. AJNR Am J Neuroradiol. 2003;24:734–740. [PMC free article] [PubMed] [Google Scholar]
- Khong P.-L, Leung LHT, Chan GCF, Kwong DLW, Wong WHS, Cao G, Ooi G.-C. White matter anisotropy in childhood medulloblastoma survivor: Association with neurotoxicity risk factors. Radiology. 2005;236:647–652. doi: 10.1148/radiol.2362041066. [DOI] [PubMed] [Google Scholar]
- Khong P.-L, Leung LH, Fung AS, Fong DY, Qiu D, Kwong DL, Ooi G.-C, McAlanon G, Cao G, Chan GC. White matter anisotropy in post-treatment childhood cancer survivors: Preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Leung LHT, Ooi G.-C, Kwong DLW, Chan GCF, Cao G, Khong P.-L. White-matter diffusion anisotropy after chemo-irradiation: A statistical parametric mapping study and histogram analysis. Neuroimage. 2004;21:261–268. doi: 10.1016/j.neuroimage.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Li T.-Q, Noseworthy MD. Mapping the development of white matter tracts with diffusion tensor imaging. Dev Sci. 2002;5:293–300. [Google Scholar]
- Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, Van Zijl PCM. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BCP, Almli CR, McKinstry RC. Normal brain maturation during childhood: Developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Palmer SL, Reddick WE, Glass JO, Kun LE, Taylor J, Langston J, Gajjar A. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19:472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, White HA, Glass JO, Kun LE, Leigh L. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10:180–189. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Palmer SL, Reddick WE, Glass JO, Helton KJ, Wu S, Xiong X, Kun LE, Gajjar A, Mulhern RK. Abnormal hippocampal development in children with medulloblastoma treated with risk-adapted irradiation. AJNR Am J Neuroradiol. 2004;25:1575–1582. [PMC free article] [PubMed] [Google Scholar]
- Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, Akbudak E, Aronovitz JA, Miller JP, Lee BC, Conturo TE. Normal brain in human newborns: Apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- Neisser U, Boodoo G, Bouchard TJ, Boykin AW, Brody N, Ceci SJ, Halpern DF, Loehlin JC, Perloff R, Sterberg R.J, Urbina S. Intelligence: Knowns and unknowns. Am Psychol. 1996;51:77–101. [Google Scholar]
- Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, Muraszko K, Rorke LB, Wara WM, Cohen BH, Boyett JM. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children‘s Cancer Group study. J Clin Oncol. 1999;17:2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun. L. Merchant, T.E. and, Mulhern RK. Patterns of intellectual development among survivors of pediatric medulloblastoma: A longitudinal analysis. J Clin Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Reddick WE, Glass JO, Gajjar A, Goloubeva O, Mulhern RK. Decline in corpus callosum volume among pediatric patients with medulloblastoma: Longitudinal MR imaging study. AJNR Am J Neuroradiol. 2002;23:1088–1094. [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Gajjar A, Reddick WE, Glass JO, Kun LE, Wu S, Xiong X, Mulhern RK. Predicting intellectual outcome among children treated with 35–40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17:548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Reddick WE, Russell JM, Glass JO, Xiong X, Mulhern RK, Langston JW, Merchant TE, Kun LE, Gajjar A. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magn Reson Imaging. 2000;18:787–793. doi: 10.1016/s0730-725x(00)00182-x. [DOI] [PubMed] [Google Scholar]
- Reddick WE, White HA, Glass JO, Wheeler GC, Thompson SJ, Gajjar A, Leigh L, Mulhern RK. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children’s Cancer Group study. J Clin Oncol. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system (erratum in Int. J. Radiat. Oncol. Biol. Phys. [1995] 32, 1269) Int J Radiat Oncol Biol Phys. 1995;31:1093–1112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- Steen RG, Koury BSM, Granja CI, Xiong X, Wu S, Glass JO, Mulhern RK, Kun LE. Effect of ionizing radiation on the human brain: White matter and gray matter T1 in pediatric brain tumor patients treated with conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:79–91. doi: 10.1016/s0360-3016(00)01351-1. [DOI] [PubMed] [Google Scholar]
- Strother, D.R., Pollack, I.F., Fisher, P.G., Hunter, J.V., Woo, S.Y., Pomeroy, S.L., and Rorke, L.B. (2002) Tumors of the central nervous system. In: Pizzo, P.A., and Poplack D.G. (Eds.), Principles and Practice of Pediatric Oncology, 4th ed. New York: Lippincott Williams & Wilkins, pp. 751–824.
- Suzuki Y, Matsuzawa H, Kwee IL, Nakada T. Absolute eigenvalue diffusion tensor analysis for human brain maturation. NMR Biomed. 2003;16:257–260. doi: 10.1002/nbm.848. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1991) Manual for the Wechsler Intelligence Scale for Children, 3rd ed. San Antonio, Tex.: Psychological Corporation, Harcourt Brace Jovanovich.
- Wechsler, D. (2003) Wechsler Intelligence Scale for Children, 4th ed. San Antonio, Tex.: Psychological Corporation.
- Wieshmann UC, Clark CA, Symms MR, Franconi F, Barker GJ, Shorvon SD. Reduced anisotropy of water diffusion in structural cerebral abnormalities demonstrated with diffusion tensor imaging. Magn Reson Imaging. 1999;17:1269–1274. doi: 10.1016/s0730-725x(99)00082-x. [DOI] [PubMed] [Google Scholar]