Abstract
To identify attentional and neural mechanisms affecting global and local feature extraction, we devised a global-local hierarchical letter paradigm to test the hypothesis that aging reduces functional cerebral lateralization through corpus callosum (CC) degradation. Participants (37 men and women, 26–79 years) performed a task requiring global, local, or global+local attention and underwent structural MRI for CC measurement. Although reaction time (RT) slowed with age, all participants had faster RTs to local than global targets. This local precedence effect together with greater interference from incongruent local information and greater response conflict from local targets each correlated with older age and smaller callosal genu (anterior) areas. These findings support the hypothesis that the CC mediates lateralized local-global processes by inhibition of task-irrelevant information under selective attention conditions. Further, with advancing age smaller genu size leads to less robust inhibition, thereby reducing cerebral lateralization and permitting interference to influence processing. Sex was an additional modifier of interference, in that callosum-interference relationships were evident in women but not in men. Regardless of age, smaller splenium (posterior) areas correlated with less response facilitation from repetition priming of global targets in men, but with greater response facilitation from repetition priming of local targets in women. Our data indicate the following dissociation: Anterior callosal structure was associated with inhibitory processes (i.e., interference from incongruency and response conflict), which are vulnerable to the effects of age and sex, whereas posterior callosal structure was associated with facilitation processes from repetition priming dependent on sex and independent of age.
Keywords: age, sex, interhemispheric, visuospatial, corpus callosum, hierarchical letters
1. Introduction
Visuospatial perception is based on the extraction of higher-order features (the “gestalt”) on a global level and details on a local level. Studies employing hierarchical stimuli to examine whole-part perception and patterns of facilitation and interference among concurrent cognitive processes (Christman, 2001; Robertson and Lamb, 1991) indicate that processing on the local level is slower when the irrelevant global level carries incongruent information about the target. Earlier accounts assumed that such interference results from the processing priority of global features, i.e., global precedence, in which processing at the global level either precedes or finishes earlier than processing at the local level (e.g., Navon, 1977). Current theories assume parallel and concurrent processing of the different dimensions of local-global stimuli (Hoffman, 1980; Madden et al., 1996), where global interference arises from the inhibition of local pathways by those carrying global information (Christman, 2001; Hughes, 1986; Kitterle et al., 1993). Evidence from brain lesion studies (Delis et al., 1986, 1988; Lamb et al., 1989; Robertson et al., 1988, 1991; Yamaguchi et al., 2000) and neuroimaging studies in healthy participants (Evans et al., 2000; Fink et al., 1996, 1999; Han et al., 2002; Weber et al., 2000; Yamaguchi et al., 2000) indicates that local and global processing proceeds in parallel, with the left hemisphere assuming a local and the right hemisphere a global processing advantage.
However, reaction time (RT) studies show diverging evidence for visual field effects supporting lateralized global/local processing (Blanca et al., 1994; Evert and Kmen, 2003; Hübner, 1997; Yovel et al., 2001; for meta-analysis see Van Kleeck, 1989), whereas nearly all published electrophysiological studies using compound letters in event-related designs report hemispheric asymmetries in the expected direction (Heinze and Münte, 1993; Heinze et al., 1998; Malinowski et al., 2002; Yamaguchi et al., 2000). In an event-related potential (ERP) study with both central and lateralized stimulus presentations Han and colleagues (2002) found hemispheric differences only for centrally presented stimuli. Thus, stimulus position is most likely responsible for different findings in RT studies usually using a lateral stimulus presentation in contrast to ERP studies with central presentations. The competition hypothesis (Han et al., 2002; Volberg and Hübner, 2004) assumes that with central stimulation both hemispheres have simultaneous access to the same visual information and the specialized hemisphere assigns more resources to a given local or global target level bringing hemispheric differences for local and global feature processing to light. With lateralized presentations visual information has to cross over to the cerebral hemisphere ipsilateral to the stimulated visual hemifield. Thus, the contralateral hemisphere receives the information prior to the ipsilateral hemisphere that receives information after interhemispheric transfer through the corpus callosum. The resulting time difference in hemispheric processing may eliminate competition between the hemispheres, and also hemispheric differences associated with local and global feature processing (Han et al., 2002, 2003; Heinze and Münte, 1993; Heinze et al., 1998; Malinowski et al., 2002; Yamaguchi et al., 2000).
Some studies supporting the relative hemispheric specialization in local and global processing used divided attention paradigms (Heinze and Münte, 1993; Heinze et al., 1998; Roalf et al., 2005), whereas others used selective attention designs directing attention to the global or the local level (Han et al., 2000b; Heinze et al., 1998; Lux et al., 2004; Roberston et al., 1993; Weissman et al., 2002). Global precedence is typically observed when selective attention is drawn to the respective processing level, but this bias is less likely under divided attention conditions (Roalf et al., 2005). Thus, competition between target levels and resource assignment to local or global target levels may differ between selective and divided attention designs.
1.1. Lateralized processing and age
The competition hypothesis is further supported by neuroimaging studies reporting smaller corpora callosa with older age (Pfefferbaum et al., 2000; Salat et al., 2005; Sullivan et al., 2006) and bihemispheric activation patterns even in less complex tasks that were unilaterally processed in young participants (Cabeza et al., 2002; Reuter-Lorenz et al., 2000). In a meta-analysis of three separate fMRI studies, Konishi and colleagues (2001) found that additional activity in the less-relevant hemisphere occurred early during processing but then disappeared, which is indicative of an active inhibition by the task-relevant/dominant hemisphere. In older subjects, bilateral involvement may arise when callosal inhibitory interactions responsible for maintaining hemispheric dominance of particular brain functions are degraded (Kinsbourne, 1974; Kinsbourne and Byrd, 1985). Examples include local-global interference, distractor incongruency and attentional selection of task-relevant from irrelevant information, all of which have also been associated with frontal cortex functions (Banich et al., 2000; MacDonald et al., 2000; Weissman et al., 2003, 2005). Given that aging is associated with selective degradation of anterior callosal subregions as assessed by means of Magnetic Resonance Imaging (Raz et al.,1995; Salat et al., 2001; Sullivan et al., 2002) and MR diffusion tensor imaging (Pfefferbaum and Sullivan, 2003; 2005; Pfefferbaum et al., 2000, 2005; Salat et al., 2005; Sullivan et al., 2001), processes of the executive attention system, i.e., local-global interference, may be particularly affected in older participants (Anderson et al., 2000; Cabeza, 2001, 2002; Cabeza et al., 1997).
Alternatively to the explanation that recruitment of additional brain areas (in the less-relevant hemisphere) results from less callosal inhibition in older participants (competition hypothesis) is the possibility that additional recruitment serves a compensatory function to overcome age-related cognitive decline (cooperation hypothesis) (Cabeza et al., 1997; Madden et al., 1999; McIntosh et al., 1999; Reuter-Lorenz et al., 2000). Compensatory recruitment of additional brain areas likely requires integrity of the corpus callosum, and smaller corpora callosa would be associated with poorer performance. The cooperation hypothesis is supported by reaction time studies showing that older participants profited more from bilateral stimulation conditions, requiring callosal transfer, than from unilateral conditions (Reuter-Lorenz and Stanczak, 2000; Reuter-Lorenz et al., 1999), and by functional imaging studies showing bilateral activation in high but not low performing older adults (Cabeza et al., 2002; Rosen et al., 2002). In addition, whether callosal interhemispheric interaction is inhibitory or cooperative may likely depend on task demands, task complexity and the specific process (e.g., inhibition vs. facilitation) and lateralization of functions (e.g., verbal-spatial, local-global).
1.2. Interference and facilitation in global-local processing
Interference may emerge from at least three sources: the stimulus content at global and local levels, i.e., when local and global stimuli are incongruent compared with when they are congruent (Kotchoubey et al., 1997; Proverbio et al., 1998); from response conflict when feature processing at the unattended level suggests a different response from the attended stimulus attribute (Hübner and Malinowski, 2002; Malinowski et al., 2002; Volberg and Hübner, 2004; Weissman et al., 2003); or from sequential processing when the target location switches between local and global levels in two consecutive trials (level switching) (Han et al., 2000a; Schatz and Erlandson, 2003). Behaviorally, interference effects are usually defined as costs in response time (RT) and facilitation effects as benefits in RTs. Facilitation may emerge from (a) concurrent processing of matching local-global information, e.g., when the unattended level carries a target in addition to a target at the attended level (response facilitation), and (b) from sequential processing of target level repetition (repetition priming). In this procedure, the hierarchical level of the target (trial n) is considered in conjunction with the target level of the prior stimulus (trial n-1) (Kim et al., 1999; Kinsbourne and Byrd, 1985; Slavin et al., 2002). Such sequential priming effects, measured by the level-repetition procedure, are largely independent from effortful attention manipulation biasing perception to the global or local level (Lamb and Yund, 1996; Lamb et al., 1998, 2000). For example, Lamb and colleagues (2000) showed that pretrial cues that provided information about the level of the upcoming target affected performance with RT benefits for valid and costs for invalid cues relative to noninformative neutral cues. Facilitation effects from sequential priming were assumed to occur relatively early, at a postsensory but preattentive stage (Han et al., 2000a; Kim et al., 1999; Lamb et al., 1998, 2000; Robertson, 1996; Schatz and Erlandson, 2003), whereas interference effects often have been associated with later response-related stages (Han et al., 1999; Kaufmann et al., 2005; van Veen and Carter, 2005).
1.3. The role of the corpus callosum in interference and facilitation processes
A possible neural mechanism for facilitation and interference between global and local processing levels involves interhemispheric interactions (Corballis et al., 2004; Forster and Corballis, 2000). Evidence from a split-brain study (Robertson et al., 1993) suggests that the corpus callosum mediates local-global interference. Degradation of connectivity between brain areas occurs with age (postmortem evidence: Aboitiz et al., 1992; Kemper, 1994) and involves the corpus callosum and its cortical connections, especially in prefrontal areas (in vivo evidence: Madden et al., 2004; Pfefferbaum et al., 2005; Raz et al., 1997, 2000; Salat et al., 1997; Sullivan et al., 2006). Callosal thinning without disconnection, determined with MRI, can contribute to impairment in tasks requiring interhemispheric sensorimotor processing (Schulte et al., 2004). Further, slowing of interhemispheric transfer time in simple tasks in older (60 – 82 years) compared with younger participants has been related to age-related callosal thinning (Hopper et al., 1994; Janowsky et al., 1996; Jeeves and Moes, 1996; Thompson et al., 2000).
The purpose of this study was to test the hypothesis that age affects the corpus callosum and influences component processes susceptible to local-global interference and facilitation. If a larger callosum in younger age enables faster interhemispheric communication, then the two hemispheres may readily collaborate when solving complex tasks (Banich and Belger, 1990; Banich et al., 1990); similarly, larger callosal size would facilitate global/local level cooperation. Alternatively, inhibitory callosal projections may protect lateralized local/global processes, and greater callosal size would be associated with more robust inhibition of task-irrelevant information, thereby reducing interference. Thus, a larger CC would be related to better performance emerging from faster interhemispheric cooperation or from more robust inhibition of task-irrelevant information. Slower interhemispheric communication would be related to older age, and thinning of callosal size would be related to less facilitation and greater interference between global and local levels and greater difficulty in switching between levels (Lux et al., 2004; Miller et al., 2000; Robertson et al., 1993; Yazgan et al., 1995).
We devised a novel paradigm of global/local stimuli to probe the effects of age and regional callosal size on these different processing levels and attentional demands. Accordingly, this study tested 1) the contribution of regional callosal size to component hierarchical stimulus processes, 2) whether age or sex modulates callosal-hierarchical processing relations, and 3) whether anterior (genu), medial (body), and posterior (splenium) callosal pathways are differentially related to components of hierarchical stimulus processing defined by competition vs. cooperation between local and global levels (interference, response conflict and switching vs. response facilitation and repetition priming) and attentional demands (selective, divided).
2. Results
2.1. Corpus callosum size, sex and age
Age correlated significantly with callosal genu area (r = −.43, p < .004, one-tailed), but not body (r = −.17, ns, one-tailed) or splenium (r = .01, ns) area (Figure 2). Although callsoal subareas did not significantly differ between women and men (MANOVA Pillai’s Trace F(3,33) = 1.26, ns; genu F(1,35) = 0.31, ns; body F(1,35) = 0.33, ns; splenium F(1,35) = 1.47, ns), women tended to have larger splenia (ICV-corrected Z-scores) than men relative to the genu and body areas (sex-by-CC area interaction F(1,35) = 3.42, p = .073; repeated measures ANOVA) (Figure 1).
Figure 2. Precedence effects.

Model of parallel processing with global letters preferentially processed in the right cerebral hemisphere and local letters in the left cerebral hemisphere (upper middle panel). Mean reaction times (RT) and standard errors (SE) as a function of attention condition (selective vs. divided). Left and right panels: Examples of stimuli (global vs. local targets) used for the calculation of the precedence effects under (a) selective attention (left panel) and (b) divided attention conditions (right panel). Green circles: attention to local features; red circles: attention to global features. Gg: Global attention block/global target; Ll: Local attention block/local target; Bg: Both (global+local) attention block/global target; Bl: Both (global+local) attention block/local target. Correlations between the precedence effect (difference RTs to global minus local features) and age (selective attention: r = .32, p = .05; divided attention: r = −.05, ns) and the size of the genu area of the corpus callosum (CC) selective attention: r = −.34, p = .03; divided attention: r = −.21, ns).
Figure 1.
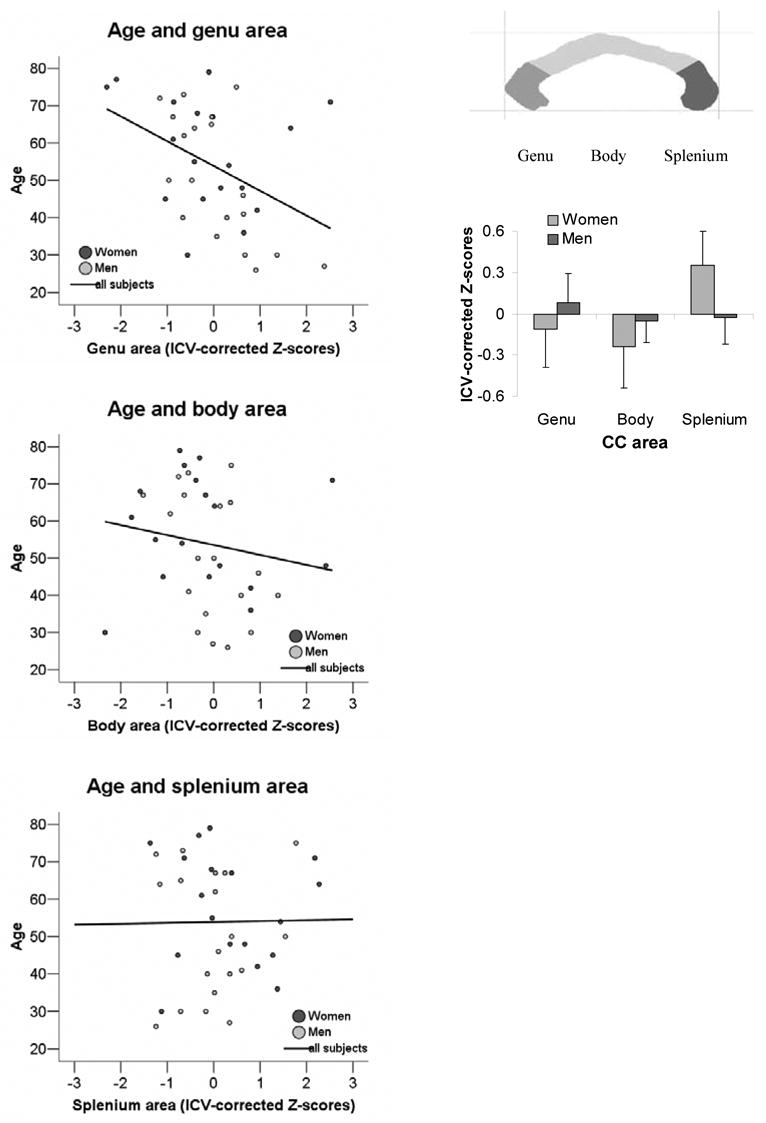
Correlations between age and the mean area size of the subregions genu (r = −.56, p < .0001), body (r = −.32, p = .05), and splenium (r = −.26, n.s.) of the corpus callosum, the thick band of white matter fibers connecting the two cerebral hemispheres (left panel). Comparison of callosal area (genu, body, splenium) between women and men (right panel). CC-area are expressed as Z-scores corrected for intracranial volume (IVC), for women and men.
2.2. Specific effects of local-global processing and relation to callosal area
2.2.1. Error analysis and overall RT
The mean overall error rate was less than 2.5 % (6.9 ± 5 out of 288 trials). Women and men did not significantly differ in error rate (women 6.7 ± 6; men 7.1 ± 4; t(35) = .24, ns). A positive correlation between overall RTs and error rate (r = .33, p < .05) indicated a lack of speed-accuracy tradeoff, which would have been indicated by a negative correlation (Salo et al., 2001; Schulte et al., 2005). Error rate was not correlated with age (r = .24, ns). Because of the small overall error rate, error rates were not analyzed further. Longer overall RT was correlated with older age (r = .63, p = .0001). Overall RTs did not differ between women (809.1 ± 147 msec) and men (837.6 ± 141 msec) (t(35) = 0.6, ns). Similarly, women and men did not differ in their RTs for selective (women: 723 ± 144 msec, men: 753.6 ± 131 msec, F(1,35) = 0.23, ns) and divided attention conditions (women: 963.4 ± 159 msec, men: 1005.5 ± 184 msec, F(1,35) = 0.55, ns).
2.2.2. Precedence effects
The precedence effect describes whether the global or local level is processed faster and was tested with a 2 × 2 × 2 ANOVA, with sex (women/men) as between subjects factor and attention (selective/divided) and target level (local/global) as within subject factors. For the selective attention condition, mean RTs to local targets in the local instruction block were compared with mean RTs to global targets in the global instruction block. For the divided attention condition, mean RTs to local targets were compared with RTs to global targets in blocks requiring attention to both levels (see Figure 5).
Figure 5.

Design of the local-global paradigm: 3 attention blocks (1. Attend the global level, 2. Attend the local level, 3. Attend both levels) with 4 randomly intermixed conditions (global targets, local targets, both global and local targets, neither global nor local targets) were repeatedly presented. Target letters were Es and Ts, non-target letters Fs and Ls. Participants answered the question: “Is there an E or a T?” by pressing a Yes key for targets (E and T) and a No key for non-targets (F and L) at the attended level(s).
Data analysis revealed a local precedence effect (local: 815 ± 156 msec; global: 887 ± 168 msec; F(1,35) = 24.5, p < .0001), and a selective attention effect (selective: 706 ± 137 msec; divided: 996 ± 184 msec; F(1,35) = 386.2, p < .0001). A significant target level-by-attention interaction (F(1,35) = 5.67, p = .023) indicated that local precedence effects were greater under the divided than selective attention condition (precedence/selective attention: global: 733.8 ± 152 msec, local: 688 ± 132 msec; t(36) = 4.11, p < .0001, post-hoc analysis; precedence/divided attention: global: 1056 ± 201 msec, local: 954 ± 198 msec; t(36) = 4.18, p < .0001, post-hoc analysis) (Figure 2, center). Sex effects were not observed (F(1,35) = 0.67, ns).
2.2.2.1. Age, corpus callosum area, and precedence effects
For selective attention conditions, greater local precedence effects correlated significantly with older age and smaller callosal genu areas (Table 1, Figure 2 left). Multiple regression analysis revealed that genu area and age together accounted for 15% of the total variance (F(2,34) = 3.09, p = .059) and that neither age (t = 1.19, ns) nor genu area (t = −1.47, ns) contributed independently to the precedence effect for selective attention. Under divided attention conditions the precedence effect was not correlated with age or any regional callosal area (Table 2, Figure 2 right).
Table 1.
Selective attention to global OR local features; Pearson bivariate correlations (two-tailed) between four effects of local-global processing (precedence, interference between processing levels, response conflict and facilitation), age and callosal area genu, body and splenium (ICV-corrected Z-values for women and men).
| Precedence | Interference | Response conflict | Response facilitation | ||||
|---|---|---|---|---|---|---|---|
| local | global | local | global | local | global | ||
| age | .32* | .20 | .28 | −.11 | .31 | .15 | .10 |
| genu | −.34* | .02 | −.48** | −.01 | −.43** | −.01 | −.03 |
| body | −.12 | −.11 | −.20 | .17 | −.14 | −.13 | −.12 |
| splenium | .02 | −.02 | −.18 | −.16 | −.15 | −.06 | −.01 |
Significance level was set on
p < .05,
p < .01,
* p < .001.
Table 2.
Divided attention to global AND local features; Pearson bivariate correlations (two-tailed) between four effects of local-global processing (precedence, interference between global and local processing levels, repetition priming and switching), age and callosal area genu, body and splenium (ICV-corrected Z-values for women and men).
| Precedence | Interference | Repetition priming | Switch | |||
|---|---|---|---|---|---|---|
| local | global | to local | to global | |||
| age | −.05 | .14 | −.14 | −.09 | .00 | −.01 |
| genu | −.21 | −.33* | .01 | .06 | −.06 | .21 |
| body | −.03 | .14 | −.01 | .12 | .02 | −.07 |
| splenium | −.14 | −.28 | −.24 | .22 | .15 | .20 |
Significance level was set on
p < .05,
p < .01,
* p < .001.
2.2.3. Interference between incongruent global and local processing levels
When target letters (E, T) appeared at local and global levels, they were either congruent (local E – global E, local T – global T) or incongruent (local E – global T, local T – global E). Interference describes longer RTs to incongruent than congruent information. Moreover, both letters (E and T) required a YES response, i.e., the response remained the same independent of the congruent/incongruent level information.
A 2 × 2 × 2 ANOVA with sex as between subjects factor and congruency (congruent/ incongruent) and selective attention to the local or the global level as within subject factors revealed a significant congruency effect with faster RTs to congruent than to incongruent target levels (F(1,35) = 17.04, p < .0001), no effect for selective attention (global/local) (F(1,35) = 0.69, ns), and no congruency-by-attention interaction (F(1,35) = 2.51, ns). Interference measures did not differ between women and men (F(1,35) = 0.05, ns). Similarly, under divided attention conditions RTs were longer to incongruent than to congruent information (RT difference: 70.5 ± 90 msec) (F(1,34) = 20.6, p < .0001) (Figure 3, left) and did not differ between women and men (F(1,34) = 1.29, ns).
Figure 3. Interference, response conflict and facilitation effects.

Model of interhemispheric local and global feature processing. Examples of the stimuli used to calculate (a) interference effects, (b) response conflict, and (c) response facilitation effects from targets at the unattended spatial scale. Mean difference reaction times and standard errors (SE) as a function of congruity between targets at either level (a), or the presence of unattended targets alone (b) or together with attended targets (c). Gg: Global attention block/global target; Gl: Global attention block/local target; Gb: Global attention block/global+local target; Gn: Global attention block/no target; Ll: Local attention block/local target; Lg: Local attention block/global target; Lb: Local attention block/global+local target; Ln: Local attention block/no target. Correlations between genu area of the corpus callosum (CC) and interference from incongruency (left down) (r = −.48, p = .003), genu area and response conflict (middle down) (r = −.43, p = .008) and age and response conflict (right down) (r = .31, p = .06).
2.2.3.1. Age, corpus callosum area and interference from incongruency
For selective attention conditions, correlation analysis yielded that greater local interference while attending to global features was related to smaller callosal genu area (Table 1) and a trend for a relation with older age (r = .28, p = .09). For divided attention conditions, correlation analysis also yielded an association between greater interference and smaller genu area (r = −.33, p = .05) and a trend for splenium area (r = −.28, p = .09) (Table 2). Multiple regression analyses (F(2,34) = 5.31, p = .01) revealed that age and genu area together accounted for 24% of the total variance of interference in the global instruction block and that genu area (t = −2.69, p = .011) contributed independently over and above that contributed from age (t = .52, ns).
Separate analyses for women and men showed that interference from local targets (global attention block) was significantly correlated with genu and splenium area in women (genu: r = −.73, p < .001; splenium: r = −.62, p < .006) but not in men (genu: r = −.17, ns; trend for splenium: r = .45, p = .055). Interference–genu and interference–splenium correlations differed significantly between women and men (genu: z = 2.12, p < .02; splenium: z = 3.37, p < .0004). To mitigate the potential effect of outliers, nonparametric Spearman Rho correlations were calculated and confirmed the relations between interference from local targets (global block), age, and callosal area (all subjects: age Rho = .30, p = .064; genu Rho = −.40, p = .013; women: genu Rho = −.50, p = .035, splenium Rho = −.41, p = .09).
2.2.4. Response conflict
Usually, conflict occurs for stimuli for which information at each level is associated with a different response (see Hübner and Malinowski, 2002). Such response conflict was tested when the target was on the unattended level but not on the attended level. Global and local directed attention abilities were tested separately. When no-targets (F or L) appeared at the attended level, the correct response was to press the NO button even though the simultaneous presence of targets (E or T) at the not-to-be-attended level may suggest a YES-response. Thus, processing of unattended targets could lead to response conflict. Such conflicting trials were compared with non-conflicting trials, i.e., with no targets (F or L) at either level. Difference values index response conflict elicited by unattended targets and were calculated for the global and the local instruction block separately (Figure 3, center).
A 2 × 2 × 2 ANOVA with sex as between subjects factor and selective attention (local/global) and response conflict (target at unattended level/no target at attended level) as within subject factors revealed a significant effect for response conflict, with faster RTs to trials with non-conflicting than with conflicting information (F(1,35) = 32.5, p < .0001), i.e., when the unattended level contained targets. This analysis further revealed an effect for selective attention (global/local) (F(1,35) = 5.65, p = .023) and a trend for a response conflict-by-attention interaction (F(1,35) = 3.59, p = .067) with greater response conflict induced by local (unattended) targets in the global instruction block (64 ± 89 msec) than by global targets in the local instruction block (33.4 ± 51 msec) (Figure 3, center). Trends were also observed for women who showed greater response conflict than men (F(1,35) = 3.11, p = .087) and a greater disparity in RTs between local and global attention blocks (fastest RTs in local attention block) (F(1,35) = 3.65, p = .064).
2.2.4.1. Age, corpus callosum area and response conflict
Greater response conflict from local targets (global instruction block) correlated significantly with smaller callosal genu area (Table 1, Figure 3) and a trend for a relation with older age (r = .31, p = .06). Multiple regression analyses with all subjects (F(2,34) = 4.39, p = .02) revealed that age and genu area together accounted for 21% of the total variance of response conflict in the global instruction block and that genu area (t = −2.16, p = .038) contributed independently over and above that from age (t = .92, ns). Separate analyses for women and men reveled that greater response conflict from local targets (global attention block) was related to older age (trend) and smaller genu and splenium areas in women (age: r = .43, p = .066; genu: r = −.68, p < .002; splenium: r = −.58, p = .013) but not in men (age: r = .14, ns; genu: r = .01, ns; trend for splenium: r = .41, p = .08). Response conflict–genu and response conflict–splenium correlations differed significantly between women and men (genu: z = −2.33, p < .01; splenium: z = −3.06, p < .002). The relationships involving age, callosal area and response conflict were confirmed with Spearman Rho correlations (all subjects: age Rho = .31, p = .055; genu Rho = −.30, p = .07; women: age Rho = .42, p = .07, genu Rho = −.55, p = .018, splenium Rho = −.50, p = .036).
2.2.5. Response facilitation
When targets (E or T) appeared at the attended level, the simultaneous presence of them at the unattended level may facilitate the response compared with when the non-attended level presented non-target letters (F or L). Difference values between trials with two targets relative to those with only one target index the amount of response facilitation elicited by unattended targets. Facilitation effects were calculated for the two selective attention conditions: for the global block by comparing RTs to global targets with RTs to targets at both spatial scales and for the local block by comparing RTs to local targets with RTs to targets at both spatial scales (Figure 3, right).
A 2 × 2 × 2 ANOVA with sex as between subjects factor and response facilitation (targets at attended and unattended levels/target at attended level only) and selective attention (local/global) as within subject factors yielded a significant effect for response facilitation, with faster RTs to trials with two targets than with one target presented at the attended level only (F(1,35) = 11.5, p < .002). Also observed was an effect for selective attention (global/local) (F(1,35) = 11.0, p < .002) with faster RTs when attention was directed to the local than the global level, and a significant response facilitation-by-attention interaction (F(1,35) = 9.02, p < .005) with greater facilitation from (unattended) local (34.5 ± 52 msec, global block; t(36) = 4.03, p < .0001, post-hoc analysis) than global targets (9.8 ± 44 msec, local block; t(36) = 1.37, ns, post-hoc analysis) (Figure 3, right). A significant 3-way interaction among sex, response facilitation, and selective attention (F(1,35) = 5.08, p = .021) showed that women in contrast to men exhibted no response facilitation from global targets in the local attention block (women: −8.7 ± 30 msec; men: 27.4 ± 49 msec).
2.2.5.1. Age, corpus callosum area and response facilitation
Correlations between response facilitation and age or callosal area were not significant for local targets (global block) or for global targets (local block) (Table 1) neither for women or men.
2.2.6. Sequential repetition priming and switching between global and local levels
Sequential repetition priming describes a shortening in RTs when a target repeatedly appears at the same processing level (local – local or global – global) in two consecutive trials n-1 and n under divided attention conditions. By contrast, level switching describes a prolongation in RT when targets appear at different levels in two consecutive trials (n-1, n), requiring an attentional switch from local to global or global to local target processing (Figure 4). Repetition priming is assumed to underlie automatic processing (Kim et al., 1999; Lamb et al., 2000), whereas level switching may require voluntary control alternating the attentional focus between the two processing levels (Fink et al., 1997; Wecker et al., 2005; Wilkinson et al., 2001).
Figure 4. Repetition priming and switching effects.

Examples of the stimuli used to calculate repetition priming (left) and switching effects (right) (a) from local to global and (b) from global to local, under divided attention conditions. Mean reaction times and standard errors (SE) of the two consecutive trials (n-1, n) for global and local repetition, and for global and local switch are displayed. Repetition and switch effects, i.e., mean difference reaction times and SE between n-1 and n trials. Correlation between the callosal splenium area and global (left panel) (men: r = −.53, p < .02; women r = −.00, ns) and local (right panel) (men: r = −.02, ns; women r = −.50, p = .035) repetition priming calculated as mean RT difference between RTs between n-1 and n trials for global and for local target repetitions.
2.2.6.1. Facilitation from level repetition
A 2 × 2 × 2 ANOVA with sex as between subjects factor and repetition (trial n-1, trial n) and target level (local, global) as within subject factors revealed a significant effect for repetition, with faster RTs to trials n than to trials n-1 at the same target level (F(1,35) = 37.13, p < .0001), and an effect for target level (global/local) (F(1,35) = 5.93, p = .02). Also observed was a trend for a repetition-by-target level interaction (F(1,35) = 3.91, p = .056) with greater facilitation from repetition at the local (141.9 ± 131 msec) than the global level (98.1 ± 124 msec) (Figure 4). Sex was not related to RT facilitation from level repetition (F(1,35) = 0.7, ns).
2.2.6.1.1. Age, corpus callosum area and facilitation from level repetition
Correlations between facilitation from level repetition, age or callosal area were not significant for local or global target repetition when all subjects were considered (Table 2). However, subgroup analyses revealed that facilitation effects from repetition of local targets correlated significantly with smaller splenium areas in women (r = −.50, p = .035; Rho = −.42, p = .08) but not men (r = −.02, ns), whereas facilitation effects from repetition of global targets correlated significantly with larger splenium areas in men (r = .53, p = .019; Rho = .42, p = .07) but not in women (r = −.00, ns) (Figure 4). Trends for significantly different correlations between women and men were observed for global (z = 1.62, p = .055) and local (z = 1.34, p = .09) repetition priming – splenium relationships.
2.2.6.2. Inhibition from switching between processing levels
A 2 × 2 × 2 ANOVA with sex as between subjects factor and switching (RT n-1/RT n) and selective attention (local/global) as within subject factors confirmed a switching effect with longer RTs to trials n than to trials n-1 at different target levels (F(1,34) = 66.4, p < .0001), but no effect for target level (to global/ to local) (F(1,34) = .18, ns). The switching-by-target level interaction (F(1,34) = 8.59, p < .006) was significant, indicating higher RT-costs when switching from local to global (244.9 ± 187 msec) than from global to local levels (86.2 ± 195 msec) (Figure 4). A significant sex-by-switch interaction (F(1,34) = 5.43, p = .026) indicated greater switch effects in men (199 ± 152 msec) than women (107 ± 60 msec).
2.2.6.2.1. Age, corpus callosum area and switching
Neither switching from the local to the global nor from the global to the local level correlated with age or callosal area (Table 2).
3. Discussion
The aim of this study was to identify the differential effects of age and age-related disruption of corpus callosum integrity on components of visual attentional processes elicited in a global-local hierarchical letter paradigm with different task demands. Depending on attentional task conditions (selective or divided), various processes are involved, including precedence of processing level, interference, facilitation, and response conflict. Larger local precedence effects, greater interference from incongruent local information and greater response conflict from local targets on the ignored level each correlated with older age and smaller areas of the genu of the corpus callosum measured on MRI. These findings support the hypothesis that the corpus callosum mediates lateralized local-global processes by inhibition of task-irrelevant information under selective attention conditions. With advancing age, however, a smaller anterior callosal size is associated with a less robust inhibition and possibly reduced lateralized function. Moreover, regardless of age, less response facilitation from repetition priming of global targets correlated with smaller splenium areas in men, whereas in women greater response facilitation from repetition priming of local targets correlated with smaller splenium areas. Our data indicate the following dissociation based on the differential effects of age on anterior and posterior callosal structure and lateralized local-global functions: Anterior callosal structure was associated with inhibitory processes (i.e., interference from local incongruent information, and response inhibition from conflicting local information while processing global information) vulnerable to the effects of age, whereas posterior callosal structure was associated with facilitation processes from repetition priming dependent on sex and independent of age.
3.1. Precedence effects
The precedence effect denotes which spatial scale – global or local – is processed faster and depends on task characteristics influencing stimulus salience or presentation time (Bruyer and Scailquin, 2000; Hübner and Malinowski, 2002). Shorter RTs to local than global targets indicated a local precedence effect. Factors that can potentially facilitate a local processing bias are stimulus characteristics (Boles and Karner, 1996; Ivry and Robertson, 1998; Lamb and Robertson, 1988, 1989; Wilkinson et al., 2001) and task demands (Kimchi, 1992). In contrast to other studies that used time constraints for stimulus presentation and reported global precedence effects (Navon, 1977; Slavin et al., 2002; Weissman et al., 2002), stimuli in the present study remained on the screen until the subject pressed the response button. This procedure ensured sufficient processing time for older participants, who commonly have prolonged RTs (Cabeza, 2001; Madden et al., 1996; Salthouse, 1996) as they did herein, but may also have afforded time to process beyond the global level. Further, in our paradigm black local letters were superimposed on a gray global letter presented on white background. This set of contrasts possibly led to changes in the spatial frequency spectrum (Ivry and Robertson, 1998) to more contrast balanced stimuli that produced larger effects on global than local processing and resulted in local precedence (Han et al., 2002; Lamb and Yund, 1993, 1996). Finally, Kimchi (1992) observed that global precedence becomes more likely with peripheral stimulus presentation and is less likely when stimuli are presented centrally, as they were in the present design.
The local precedence effect was significantly more pronounced with advancing age, consistent with studies that found local precedence effects in older participants (Bruyer and Scailquin, 2000; Polster and Rapcsak, 1994; Slavin et al., 2002). Moreover, a smaller genu area was associated with a greater local precedence effect. Age and genu area together (but not independently) predicted the local precedence effect. One possible explanation is that the corpus callosum insulates lateralized local-global processes and anterior callosal decline with older age and leads to a less robust inhibition permitting interference, particularly from the faster (local) process, to influence precedence effects. Alternatively, the genu may serve to integrate holistic stimuli rather than to insulate lateralized local-global processes. Local stimuli may be available to both hemispheres, whereas interhemispheric integration may be needed for the perception of global stimuli when requiring visual exploration. A decline in callosal function with older age would mean that holistic stimuli are not well integrated interhemispherically, thus, global function would decline more than local function. Finally, structural genu area and functional global/local processing are affected by a common factor, i.e., age, and it is possible that genu size is more an index of aging than of a processing bias leading to local over global precedence.
Correlations between age and genu area with the local precedence effect were evident in selective but not divided attention condition. However, the longer RTs in the divided compared with the selective attention conditions suggest greater task difficulty when participants have to direct their attention to the local and the global level than when they can focus on one level. Thus, the greater local precedence effect may be a result of greater task difficulty. One may further assume bilateral processing and callosal involvement with greater difficulty, especially in older participants, to recruit compensatory brain reserve and overcome age-related cognitive decline (Cabeza et al., 2002; Reuter-Lorenz et al., 1999, 2000) (compensatory hypothesis). We did not, however, find such a relationship. One possible explanation is inadequate statistical power. Another possibility is that global and local information are processed in parallel intrahemispherically and that the genu-precedence relationship under selective attention conditions reflects interhemispheric communication from top-down selective attentional control rather than from precedence processing itself. Thus, callosal inhibitory functions would serve top-down attentional control mechanisms when selectively directing attention to the relevant level and suppressing the irrelevant level; such gating functions would not be required when attention is directed to both levels simultaneously (Figure 1).
3.2. Interference from incongruent local-global information and response conflict
Our paradigm permitted testing of two types of interference: 1) interference from incongruent local-global information and 2) interference from response conflict created by stimuli for which information at each level was associated with a different response. As expected, both types of interference resulted in prolonged responses. Interference from local targets while attending the global level correlated with age and independently with genu size. The anterior cingulate cortex is involved in the regulation of attention and in monitoring response conflict (Botvinick et al., 2001, 2004; Carter et al., 1998), and is thought to detect conflicts occurring at later or response-related processing levels and signals the need to engage top-down attentional processes (van Veen and Carter, 2002; van Veen et al., 2001; Weissman et al., 2003). Our findings possibly add to identification of neural substrates of such attentional processes by demonstrating the influence of age-related genu thinning on interference control. The special role that this callosal region may play is in the preservation of lateralized local-global processes that become less robust with age. Callosal inhibition from age-related genu-thinning would allow interference from task-irrelevant information. Sex was an additional modifier of interference, in that ‘callosal structure - interference function’ relationships were evident in women but not in men. This observation adds to lateralization research demonstrating that functional cerebral asymmetry of visuospatial processes is more pronounced in men and tends to be more symmetrical in women (Corballis and Sidey, 1993; Hausmann and Güntürkün, 1999; McGlone, 1980; Rasmjou et al., 1999). Thus, in women, a smaller callosal size with older age may compromise mechanisms recruited to reduce interference using interhemispheric processes, whereas such interference may be resolved unilaterally in men.
Although one component of response conflict may be thought to be related to the integrity of motor areas and their callosal connections, we did not observe any correlations based on our measure of the callosal body. Further subdivision of the callosal body (Seltzer and Pandya, 1986) using marcostructural or microstructural neuroimaging methods of fiber integrity (Hofer and Frahm, 2006; Robertson et al., 1991) may be useful for future research on motor components of local-global processes.
No relations emerged among interference measures in the local instruction block, callosal area, and age. One possible explanation is that processing of the dominant level must be suppressed when processing at the nondominant level is required, and that the corpus callosum mediates such interhemispheric equilibration processes depending on task demands (Lux et al., 2004). Suppression of local precedence was also associated with smaller genu area, suggesting that with advancing age smaller anterior callosal volume increases local interference (global block) and amplifies an unbalanced inhibition pattern.
3.3. Response facilitation
Response facilitation effects, i.e., shorter RTs when targets appear at attended and unattended levels than when targets appear at attended levels only, were greater for local than global unattended targets and were independent of age and callosal size. Although the interpretation of non-significant results is problematic, one possibility is that facilitation effects from targets at both levels may originate intrahemipherically because each hemisphere is capable of processing local and global information but with different levels of efficacy (Robertson and Lamb, 1991; Yamaguchi et al., 2000).
3.4. Sequential repetition priming
Faciliation effects from repetition priming, i.e., shorter RTs to a trial (n) compared with the previous trial (n-1), were evident for both processing levels. Thus, identifying a target letter on a trial n-1 decreased identification time on the following trial n. This facilitation effect was higher for local than global targets, providing support for level-specific sequential priming (Kim et al., 1999). In the present study, repetition effects from global target repetition correlated with greater splenium area in men, whereas local target repetition effects correlated with smaller splenium area in women. The splenium connects posterior brain areas associated with primary visual and visuo-attentional processes. This finding supports the assumption of an early mechanism, perhaps at a postsensory but preattentive stage, in the visual processing stream (Kim et al., 1999; Lamb et al., 2000) that operates differently in men and women. One explanation for these sex effects in level-specific priming is that ‘left-hemispheric’ local processing advantages in women and ‘right-hemispheric’ global processing advantages in men are mediated by posterior callosal connectivity, here expressed as splenium size, which was proportionally larger in women than in men (Davatzikos and Resnick, 1998; DeLacoste-Utamsing and Holloway, 1982; Witelson, 1989).
In contrast to repetition priming, switch effects describe longer RTs to trial n than trial n-1 when the two trials contained targets at different levels. Cost effects from level switching emerged for both levels, although switching to the global level elicited higher costs than switching to the local level. According to the Attentional Print model (Robertson, 1996, 1999), information regarding the target level leaves a “print” that weights the high- and low-spatial-frequency channel to resolve whether targets are on a local or global level. The channel with the last resolved target receives a greater weight or “print” to resolve the following target, thus resulting in level-specific priming. With switch-level trials this weighting leads to a filtering of information through the “wrongly” primed channel that must be inhibited to resolve the target accurately. The local precedence effect in our study may reflect a higher weighting for the high-spatial-frequency channel that reduced the costs for switches towards the local level and enhanced costs for switches away from the local towards the global level. Lesion studies have associated the left inferior parietal cortex with level-switch effects (Robertson et al., 1988; Robertson and Rafal, 2000), but functional MRI studies in healthy participants report bilateral parietal (Wilkinson et al., 2001) and occipito-temporal involvement (Han et al., 2000a; Schatz and Erlandson, 2003). Although one would expect interhemispheric inhibition of wrongly primed spatial-frequency information of lateralized local-global processes via callosal structure, callosal size did not correlate with switch effects.
3.5. Conclusion
Interhemispheric functions underlying components of local-global processing are differentially affected by callosal size, age, and sex. According to the competition hypothesis, use of central stimulus presentations, which simultaneously provides each hemisphere with the same information, should lead to local-global competition such that each hemisphere assigns more resources to its specialized target level. In support of the competition hypothesis, precedence effects were related to callosal genu area under selective but not divided attention conditions. Resource assignment occurs when attention is directed to one target level but not when attentional resources are divided between local and global features. Anterior callosal size is also relevant in the presence of conflicting or incongruent local information while attention is directed to the global level. Averting such interference from local unattended targets requires the integrity of the anterior callosum, and both resistance to local interference and integrity of the anterior callosum decline with age. Response facilitation from repetition priming, however, correlates with splenium area regardless of age and differentially for men and women. Finally, our data indicate the following dissociation: Anterior callosal structure is associated with inhibitory processes (i.e., interference from local incongruent information, and response inhibition from conflicting local information while processing global information), which are vulnerable to the effects of age and sex, whereas posterior callosal structure is associated with facilitation processes from repetition priming dependent on sex but independent of age.
4. Experimental Procedure
4.1. Participants
The study sample comprised 37 healthy adults (18 women and 19 men), age 26 to 79 years, with normal or corrected to normal visual acuity. Women and men did not differ significantly in age (women: 58 ± 15 years, men: 51 ± 17 years, t(35) = 1.34, ns), handedness (Crovitz and Zener, 1962; 14–32 right handed, 50–70 left handed; women: 20 ± 7, men: 22 ± 12, t(35) = 0.54, ns), education (women: 17 ± 3 years, men: 16 ± 3 years, t(35) = 1.16, ns), or verbal intelligence (NART IQ, women: 115 ± 6, men: 117 ± 5, t(28) = 0.8, ns). All participants received a Structural Clinical Interview for DSM-IV diagnosis (American Psychiatric Association, 1994) by trained clinicians to rule out psychiatric and neurological disease. Written informed consent was obtained from all participants and the study was approved by the local ethics committee. The sample of 37 reflects the exclusion of six subjects, four women who were inattentive while performing the task, i.e., three were talking and one had RTs in the global selective attention task exceeding the group’s mean by more than three standard deviations (SD), and two subjects (1 woman, 1 man) who had ventricle sizes that were more than four standard deviations (SD) from the normal average.
4.2. Stimuli and procedures
Stimuli consisted of the letters E, T, L and F, for example, a global F made out of local Es. Local letters were black; global letters had a light grayish background color to enhance their salience. Local and global letters were presented superimposed on white background. The letters E and T were targets, L and F non-targets. Stimuli were presented at the center of a 21-inch computer screen. Global stimuli were 9.5 cm high and 7 cm wide; local stimuli were 1 cm by 0.5 cm. With a subject-monitor distance of approximately 55 cm, global stimuli subtended ± 5° visual angle vertically and ± 3.5° visual angle horizontally. Local stimuli measured 1° by 0.5° visual angle.
Hierarchical letters were presented in two selective attention blocks of trials and one divided attention block. In the selective attention blocks participants attended to either the global or local spatial scale. In the divided attention block participants attended to both spatial scales simultaneously. Stimuli were the same for each block, only the attention instruction differed.
There were four target conditions: a target letter on the global, the local, both, or neither level (Figure 5). When targets appeared on both spatial scales, they could be a) congruent, i.e., the same letter was presented at the global and local spatial scale, or b) incongruent with different target letters at the two spatial scales, i.e., a global E made out of local Ts.
Participants were instructed to identify target letters (E, T) among distractors (F, L) and pressed a YES button with the index finger of their dominant hand when a target letter appeared at the attended level, and a NO button with the middle finger of the same hand when a non-target appeared at the attended level. Response buttons were the N and M keys on a regular computer keyboard that was aligned to the participants’ body midline. Subjects were instructed to sit about 55 cm from the screen under free viewing conditions (i.e. without a chin rest). Stimuli remained on the screen until the participant pressed the response button ensuring individually optimized processing time for both local and global processing levels. Between trials a blank screen occurred for 30 msec. RTs and errors were collected for each trial. A total of 288 stimuli were presented. Each of the three blocks comprised 32 stimuli with 8 stimuli in each of the four target conditions, and each block was presented three times in the following fixed order: G-L-GL-G-L-GL-G-L-GL. Overall error rates and median RT values for each condition were calculated from all three measurements. Participants performed short practice blocks of trials for each attention condition before testing.
4.3. MRI protocol
The midsagittal area of the corpus callosum and its subareas (genu, body, and splenium) were measured in 37 participants. T1-weighted SPGR images (TR = 26 msec, TE = 5 msec, 30° flip angle, field of view = 24 cm, 256 × 196 matrix, acquired resolution = 0.9 × 1.2 × 2 mm, reconstructed and resampled resolution, 1.0 × 1.0 × 1.0 mm) were acquired on a 1.5T GE Signa MRI system. As previously described (Schulte et al., 2004), measurements were made on a midline slice extracted from the native data after interpolation, alignment, and reslicing. For extraction of the aligned midline slice, the anterior commissure (AC) was identified on the native sagittal slice on which it was best visualized. The sagittal slices were then stacked and interpolated to high isotropic resolution (1 mm). The left/right midline was determined from a coronal reconstruction at the level of the AC. The AC and posterior commissure (PC) were identified on a resliced midline sagittal image. Head tilt angle was defined on a high resolution coronal image and head rotation was defined on a high resolution axial image using an interactive rotating cursor. The resultant landmarks and angles were used to align the volume in a uniform space and orientation anchored on the AC. The midsagittal image was then extracted for semi-automated edge identification of the corpus callosum.
Regional callosal areas were defined geometrically as follows (Sullivan et al., 2002). The corpus callosum silhouette was rotated to a plane parallel to the inferior extremes of the rostrum anteriorly and splenium posteriorly. The midpoint along this plane between the anterior extreme of the genu and posterior extreme of the splenium was used as the center of a circle, and radii were projected at +30 and +150° angles from the plane, thus dividing the corpus callosum into genu + rostrum, body, and splenium.
Because men and women have fundamentally different size brains and substructures (Dekaban and Sadowsky, 1978), we used a linear regression model to correct the size of the corpus callosum for normal variation in intracranial volume (ICV) (Pfefferbaum et al., 1994; Sullivan et al., 2001), based on a sample of 121 healthy men and women who spanned the adult age range of 20 to 81 years (Pfefferbaum et al., 2006); the subjects of the present sample are a subset of the full sample. The corpus callosum and its substructures were then expressed as ICV-corrected standardized Z-scores, with an expected mean of 0 and a standard deviation of 1. In a previous MRI study, which also examined the ventricular system, we found that one man and one woman included in the current report had ventricular volumes that were greater than four standard deviations from the mean of the remaining controls and thus were deemed statistical outliers and excluded from further analyses.
4.4. Statistical analysis
Non-paired Student’s t-tests and χ2 tests were used for group comparison (men vs. women) of demographic data. RT analysis of global and local information processing was based on correct responses. Interference and facilitation effects for concurrent (1a,b; 2a) and sequential global-local (1c; 2b) processing were tested: 1) interference from a) incongruent targets at the global and local level (e.g., local E, global T) compared with congruent targets (e.g., local E, global E) (Navon and Norman, 1983), b) targets at the non-attended level that suggest a different response from targets at the attended level, c) switching between target levels in two consecutive trials (global to local, local to global), and 2) facilitation from a) two targets compared with one target at the attended level, and b) target repetition at the same level in two consecutive trials (repetition priming). First, a series of analysis of variance (ANOVAs) used sex as between-subject variable, and the repeated measures component tested for the following effects: precedence, interference between local and global processing level, response conflict and response facilitation, and level repetition and level switching. Secondly, relations of these effects with age and with regional callosal area (genu, body, splenium; Z-scores using regression analyses (Pfefferbaum et al., 1994) corrected for intracranial volume, ICV, for women and men) were tested with two-tailed Pearson correlations, which permitted age to be modeled as a continuous rather than discrete variable. Thirdly, for significant correlations, the predictive value and independent or joint contribution of age and callosal area for the special effects of global-local processing was tested using multiple regression analysis. The alpha level was set to 0.05 for all hypotheses tested (SPSS 10.0).
Acknowledgments
This research was supported by grants from the National Institute on Aging (AG17919) and the National Institute on Alcohol Abuse and Alcoholism (AA10723, AA12388, AA05965). The authors thank Anne O’Reilly, Ph.D., Anjali Deshmukh, M.D., Stephanie Sassoon, Ph.D., Andrea Spadoni, B.A., Julia Sandler, B.A., and Marya Schulte, B.A. for help with recruiting and screening study participants and assistance in data collection.
Text Abbreviations
- CC
corpus callosum
- RT
reaction time
- SE
standard errors
- ERP
event-related potential
- AC
anterior commissure
- PC
posterior commissure
- ICV
intracranial volume
- Gg
global attention block/global target
- Gl
global attention block/local target
- Gb
global attention block/local+global targets
- Gn
global attention block/no target
- Ll
local attention block/local target
- Lg
local attention block/global target
- Lb
local attention block/local+global targets
- Ln
local attention block/no target
- Bg
both (global+local) attention block/global target
- Bl
both (global+local) attention block/local target
- Bb
both (global+local) attention block/local+global targets
- Bn
both (global+local) attention block/no target (Bn)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Scheibel AB, Zaidel E. Morphometry of the Sylvian fissure and the corpus callosum, with emphasis on sex differences. Brain. 1992;115:1521–1541. doi: 10.1093/brain/115.5.1521. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FI. The effects of divided attention on encoding- and retrieval-related brain activity: A PET study of younger and older adults. J Cogn Neurosci. 2000;12:775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Banich MT, Belger A. Interhemispheric interaction: how do the hemispheres divide and conquer a task? Cortex. 1990;26:77–94. doi: 10.1016/s0010-9452(13)80076-7. [DOI] [PubMed] [Google Scholar]
- Banich MT, Goering S, Stolar N, Belger A. Interhemispheric processing in left- and right-handers. Int J Neurosci. 1990;54:197–208. doi: 10.3109/00207459008986636. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, et al. Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Brain Res Cogn Brain Res. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Blanca MJ, Zalabardo C, Garcia-Criado F, Siles R. Hemispheric differences in global and local processing dependent on exposure duration. Neuropsychologia. 1994;32:1343–1351. doi: 10.1016/0028-3932(94)00067-0. [DOI] [PubMed] [Google Scholar]
- Boles DB, Karner TA. Hemispheric differences in the global versus local processing: Still unclear. Brain Cogn. 1996;30:232–243. doi: 10.1006/brcg.1996.0015. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bruyer R, Scailquin JC. The fate of global precedence with age. Experimental Aging Research. 2000;26:285–314. doi: 10.1080/036107300750015705. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: Contributions of functional neuroimaging. Scand J Psychol. 2001;42:277–286. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Christman R. Individual differences in Stroop and local-global processing: a possible role of interhemispheric interaction. Brain Cogn. 2001;45:97–118. doi: 10.1006/brcg.2000.1259. [DOI] [PubMed] [Google Scholar]
- Corballis MC, Corballis PM, Fabri M. Redundancy gain in simple reaction time following partial and complete callosotomy. Neuropsychologia. 2004;42:71–81. doi: 10.1016/s0028-3932(03)00152-0. [DOI] [PubMed] [Google Scholar]
- Corballis MC, Sidey S. Effects of concurrent memory load on visual-field differences in mental rotation. Neuropsychologia. 1993;31:183–197. doi: 10.1016/0028-3932(93)90046-3. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Zener K. A group test for assessing hand- and eye-dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- Davatzikos C, Resnick SM. Sex differences in anatomic measures of interhemispheric connectivity: correlations with cognition in women but not men. Cereb Cortex. 1998;8:635–640. doi: 10.1093/cercor/8.7.635. [DOI] [PubMed] [Google Scholar]
- Dekaban A, Sadowsky D. Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Annals of Neurology. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- DeLacoste-Utamsing C, Holloway RL. Sexual dimorphism in the human corpus callosum. Science. 1982;216:1431–1432. doi: 10.1126/science.7089533. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kiefner M, Fridlund AJ. Visuospatial dysfunction following unilateral brain damage: Dissociations in hierarchical and hemispatial analysis. J Clin Exp Neuropsychol. 1988;10:421–431. doi: 10.1080/01688638808408250. [DOI] [PubMed] [Google Scholar]
- Delis DC, Robertson LC, Efron R. Hemispheric specialization of memory for visual hierarchical stimuli. Neuropsychologia. 1986;24:205–214. doi: 10.1016/0028-3932(86)90053-9. [DOI] [PubMed] [Google Scholar]
- Evans MA, Shedden JM, Hevenor SJ, Hahn MC. The effect of variability of unattended information on global and local processing: Evidence for lateralization at early stages of processing. Neuropsychologia. 2000;38:225–239. doi: 10.1016/s0028-3932(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Evert DL, Kmen M. Hemispheric asymmetries for global and local processing as a function of stimulus exposure duration. Brain Cogn. 2003;51:115–142. doi: 10.1016/s0278-2626(02)00528-6. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997;120:1779–1791. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Dolan RJ. Hemispheric asymmetries in global/local processing are modulated by perceptual salience. Neuropsychologia. 1999;37:31–40. doi: 10.1016/s0028-3932(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Forster B, Corballis MC. Interhemispheric transfer of colour and shape information in the presence and absence of the corpus callosum. Neuropsychologia. 2000;38:32–45. doi: 10.1016/s0028-3932(99)00050-0. [DOI] [PubMed] [Google Scholar]
- Han S, He X, Woods DL. Hierarchical processing and level-repetition effect as indexed as indexed by early brain potentials. Psychophysiology. 2000a;37:817–830. [PubMed] [Google Scholar]
- Han S, Fan S, Chen L, Zhuo Y. Modulation of brain activities by hierarchical processing: A high density ERP study. Brain Topogr. 1999;11:171–183. doi: 10.1023/a:1022244727182. [DOI] [PubMed] [Google Scholar]
- Han S, Liu W, Yund EW, Woods DL. Interactions between spatial attention and global/local feature selection: An ERP study. Neuroreport. 2000b;11:2753–2758. doi: 10.1097/00001756-200008210-00029. [DOI] [PubMed] [Google Scholar]
- Han S, Weaver JA, Murray SO, Kang X, Yund EW, Woods DL. Hemispheric asymmetry in global/local processing: Effects of stimulus position and spatial frequency. Neuroimage. 2002;17:1290–1299. doi: 10.1006/nimg.2002.1255. [DOI] [PubMed] [Google Scholar]
- Han S, Yund EW, Woods D L. An ERP study of the global precedence effect: The role of spatial frequency. Clin Neurophysiol. 2003;114:1850–1865. doi: 10.1016/s1388-2457(03)00196-2. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Güntürkün O. Sex differences in functional cerebral asymmetries in a repeated measures design. Brain Cogn. 1999;41:263–275. doi: 10.1006/brcg.1999.1126. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Münte TF. Electrophysiological correlates of hierarchical stimulus processing: Dissociation between onset and later stages of global and local target processing. Neuropsychologia. 1993;31:841–852. doi: 10.1016/0028-3932(93)90132-j. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Hinrichs H, Scholz M, Burchert W, Mangun GR. Neural mechanisms of global and local processing: A combined PET and ERP study. J Cogn Neurosci. 1998;10:485–498. doi: 10.1162/089892998562898. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hoffman J. Interaction between global and local levels of a form. J Exp Psychol Hum Percept Perform. 1980;6:222–234. doi: 10.1037//0096-1523.6.2.222. [DOI] [PubMed] [Google Scholar]
- Hopper KD, Patel S, Cann TS, Wilcox T, Schaeffer JM. The relationship of age, gender, handedness, and sidedness to the size of the corpus callosum. Acad Radiol. 1994;1:243–248. doi: 10.1016/s1076-6332(05)80723-8. [DOI] [PubMed] [Google Scholar]
- Hübner R. The effect of spatial frequency on global precedence and hemispheric differences. Percept Psychophys. 1997;59:187–201. doi: 10.3758/bf03211888. [DOI] [PubMed] [Google Scholar]
- Hübner R, Malinowski P. The effect of response competition on functional hemispheric asymmetries for global/local processing. Percept Psychophys. 2002;64:1290–1300. doi: 10.3758/bf03194772. [DOI] [PubMed] [Google Scholar]
- Hughes HC. Asymmetric interference between components of suprathreshold compound gratings. Percept Psychophys. 1986;40:241–250. doi: 10.3758/bf03211503. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Robertson LC. The Two Sides of Perception. MIT Press; London: 1998. [Google Scholar]
- Janowsky JS, Kaye JA, Carper RA. Atrophy of the corpus callosum in Alzheimer’s disease versus healthy aging. J Am Geriatr Soc. 1996;44:798–803. doi: 10.1111/j.1532-5415.1996.tb03736.x. [DOI] [PubMed] [Google Scholar]
- Jeeves MA, Moes P. Interhemispheric transfer time differences related to aging and gender. Neuropsychologia. 1996;34:627–636. doi: 10.1016/0028-3932(95)00157-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann L, Koppelstaetter F, Delazer M, Siedentopf C, Rhomberg P, Golaszewski S, Felber S, Ischebeck A. Neural correlates of distance and congruity effects in a numerical Stroop task: an event-related fMRI study. Neuroimage. 2005;25:888–898. doi: 10.1016/j.neuroimage.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kemper T. Neuroanatomical and neuropathological changes during aging and dementia. 2. Oxford University Press; New York: 1994. [Google Scholar]
- Kim N, Ivry RB, Robertson LC. Sequential priming in hierarchically organized figures: Effects of target level and target resolution. J Exp Psychol Hum Percept Perform. 1999;25:715–729. doi: 10.1037//0096-1523.25.3.715. [DOI] [PubMed] [Google Scholar]
- Kimchi R. Primacy of wholistic processing and global/local paradigm: A critical review. Psychol Bull. 1992;112:24–38. doi: 10.1037/0033-2909.112.1.24. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Mechanisms of hemispheric interaction in the brain. In: Kinsbourne M, Smith WL, editors. Hemispheric disconnection and cerebral function. Thomas; Springfield: 1974. pp. 26c–285. [Google Scholar]
- Kinsbourne M, Byrd M. Word load and visual hemifield shape recognition: priming and interference effects. In: Posner MI, Marin OSM, editors. Mechanisms of Attention: Attention and Performance XI. Erlbaum; Hillsdale, New Jersey: 1985. pp. 529–543. [Google Scholar]
- Kitterle F, Christman S, Conesa J. Hemispheric differences in the interference among components of compound gratings. Percept Psychophys. 1993;54:785–793. doi: 10.3758/bf03211803. [DOI] [PubMed] [Google Scholar]
- Konishi S, Donaldson DI, Buckner RL. Transient activation during block transition. Neuroimage. 2001;13:364–374. doi: 10.1006/nimg.2000.0691. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B, Wascher E, Verleger R. Shifting attention between global features and small details: an event-related potential study. Biol Psychol. 1997;46:25–50. doi: 10.1016/s0301-0511(96)05236-2. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Robertson LC. The processing of hierarchical stimuli: Effects of retinal locus, locational uncertainty, and stimulus identity. Percept Psychophys. 1988;44:172–181. doi: 10.3758/bf03208710. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Robertson LC. Do response time advantage and interference affect the order of processing of global and local-level information? Percept Psychophys. 1989;47:489–496. doi: 10.3758/bf03208087. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Robertson LC, Knight RT. Attention and interference in the processing of global and local information: Effects of unilateral temporal-parietal junction lesions. Neuropsychologia. 1989;27:471–483. doi: 10.1016/0028-3932(89)90052-3. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Yund EW. The role of spatial frequency in the processing of hierarchically organized stimuli. Percept Psychophys. 1993;54:773–784. doi: 10.3758/bf03211802. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Yund EW. Spatial frequency and attention: Effects of level-, target-, and location-repetition on the processing of global and local forms. Percept Psychophys. 1996;58:363–373. doi: 10.3758/bf03206812. [DOI] [PubMed] [Google Scholar]
- Lamb MR, London B, Pond HM, Whitt KA. Automatic and controlled processes in the analysis of hierarchical structure. Psychol Sci. 1998;9:14–19. doi: 10.1037//0096-1523.26.1.234. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Pond HM, Zahir G. Contributions of automatic and controlled processes to the analysis of hierarchical structure. J Exp Psychol Hum Percept Perform. 2000;26:234–245. doi: 10.1037//0096-1523.26.1.234. [DOI] [PubMed] [Google Scholar]
- Lux S, Marshall JC, Ritzl A, Weiss PH, Pietrzyk U, Shah NJ, et al. A functional magnetic resonance imaging study of local/global processing with stimulus presentation in the peripheral visual hemifields. Neuroscience. 2004;124:113–120. doi: 10.1016/j.neuroscience.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Pierce TW, Allen PA. Adult age differences in the use of distractor homogeneity during visual search. Psychol Aging. 1996;11:454–474. doi: 10.1037//0882-7974.11.3.454. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Malinowski P, Hübner R, Keil A, Gruber T. The influence of response competition on cerebral asymmetries for processing hierarchical stimuli revealed by ERP recordings. Exp Brain Res. 2002;144:136–139. doi: 10.1007/s00221-002-1057-1. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McGlone J. Sex differences in human brain asymmetry: a critical survey. Behav Brain Sci. 1980;3:215–263. [Google Scholar]
- McIntosh AR, Sekuler AB, Penpeci C, Rajah MN, Grady CL, Sekuler R, Bennett PJ. Recruitment of unique neural systems to support visual memory in normal aging. Curr Biol. 1999;9:1275–1278. doi: 10.1016/s0960-9822(99)80512-0. [DOI] [PubMed] [Google Scholar]
- Miller SM, Liu GB, Ngo TT, Hooper G, Riek S, Carson RG, Pettigrew JD. Interhemispheric switching mediates perceptual rivalry. Curr Biol. 2000;10:383–392. doi: 10.1016/s0960-9822(00)00416-4. [DOI] [PubMed] [Google Scholar]
- Navon D. Forest before trees: The precedence of global features in visual perception. Cognit Psychol. 1977;9:353–383. [Google Scholar]
- Navon D, Norman J. Does global precedence really depend on visual angle? J Exp Psychol Hum Percept Perform. 1983;9:955–965. doi: 10.1037//0096-1523.9.6.955. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Chapter 33: Diffusion MR imaging in neuropsychiatry and aging. In: Gillard J, Waldman A, Barker P, editors. Clinical MR Neuroimaging: Diffusion, Perfusion and Spectoscopy. Cambridge University Press; Cambridge: 2005. pp. 558–578. [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magn Reson Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, et al. Contribution of alcoholism to brain dysmorphology in HIV infection: Effects on the ventricles and corpus callosum. NeuroImage. 2006 doi: 10.1016/j.neuroimage.2006.05.052. in press. [DOI] [PubMed] [Google Scholar]
- Polster MR, Rapcsak SZ. Hierarchical stimuli and hemispheric specialization: Two case studies. Cortex. 1994;30:487–497. doi: 10.1016/s0010-9452(13)80344-9. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Minniti A, Zani A. Electrophysiological evidence of a perceptual precedence of global vs. local visual information. Brain Res Cogn Brain Res. 1998;6:321–334. doi: 10.1016/s0926-6410(97)00039-6. [DOI] [PubMed] [Google Scholar]
- Rasmjou S, Hausmann M, Güntürkün O. Hemispheric dominance and gender in the perception of an illusion. Neuropsychologia. 1999;37:1041–1047. doi: 10.1016/s0028-3932(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Briggs SD, Spencer WD, Thornton AE, Loken WJ, Gunning FM, McQuain JD, Driesen NR, Acker JD. Selective neuroanatomic abnormalities in Down’s syndrome and their cognitive correlates: evidence from MRI morphometry. Neurology. 1995;45:356–366. doi: 10.1212/wnl.45.2.356. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc Res Tech. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L. Differential effects of aging on the functions of the corpus callosum. Dev Neuropsychol. 2000;18:113–137. doi: 10.1207/S15326942DN1801_7. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller AC. Neural recruitment and cognitive aging: two hemispheres are better than one especially as you age. Psychol Sci. 1999;10:494–500. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Roalf D, Lowery N, Turetsky BI. Behavioral and physiological findings of gender differences in global-local visual processing. Brain Cogn. 2005;60:32–42. doi: 10.1016/j.bandc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Robertson LC. Attentional persistence for features of hierarchical patterns. J Exp Psychol Gen. 1996;125:227–249. doi: 10.1037//0096-3445.125.3.227. [DOI] [PubMed] [Google Scholar]
- Robertson LC. Spatial frequencies as a medium for guiding attention: comment on Lamb, Yund, Pond, 1999. J Exp Psychol Gen. 1999;128:95–98. doi: 10.1037//0096-3445.128.1.95. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR. Neuropsychological contributions to theories of part/whole organization. Cognit Psychol. 1991;23:299–330. doi: 10.1016/0010-0285(91)90012-d. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR, Knight RT. Effects of lesions of temporal-parietal junction on perceptual and attentional processing in humans. J Neurosci. 1988;8:3757–3769. doi: 10.1523/JNEUROSCI.08-10-03757.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR, Knight RT. Normal global-local analysis in patients with dorsolateral frontal lobe lesions. Neuropsychologia. 1991;29:959–67. doi: 10.1016/0028-3932(91)90060-l. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR, Zaidel E. Interhemispheric realtions in processing hierarchical patterns: Evidence from normal and commissurotomized subjects. Neuropsychology. 1993;7:325–342. [Google Scholar]
- Robertson LC, Rafal R. Disorders of visual attention. In: Gazzangia MS, editor. The New Cognitive Neurosciences. MIT Press; Cambridge: 2000. pp. 633–649. [Google Scholar]
- Rosen AC, Prull MW, O’Hara R, Race EA, Desmond JE, Glover GH, et al. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Salat D, Ward A, Kaye JA, Janowsky JS. Sex differences in the corpus callosum with aging. Neurobiol Aging. 1997;18:191–197. doi: 10.1016/s0197-4580(97)00014-6. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA Janowsky JS. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Arch Neurol. 2001;58:1403–1408. doi: 10.1001/archneur.58.9.1403. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salo R, Henik A, Robertson LC. Interpreting Stroop interference: An analysis of differences between task versions. Neuropsychology. 2001;15:462–471. doi: 10.1037//0894-4105.15.4.462. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schatz J, Erlandson F. Level-repetition effects in hierarchical stimulus processing: timing and location of cortical activity. Int J Psychophysiol. 2003;47:255–269. doi: 10.1016/s0167-8760(02)00158-7. [DOI] [PubMed] [Google Scholar]
- Schulte T, Pfefferbaum A, Sullivan EV. Parallel interhemispheric processing in aging and alcoholism: Relation to corpus callosum size. Neuropsychologia. 2004;42:257–271. doi: 10.1016/s0028-3932(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: evidence from a Stroop Match-to-Sample task. Biol Psychiatry. 2005;57:67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Posterior parietal projections to the intraparietal sulcus of the rhesus monkey. Exp Brain Res. 1986;62:459–469. doi: 10.1007/BF00236024. [DOI] [PubMed] [Google Scholar]
- Slavin MJ, Mattingley JB, Bradshaw JL, Storey E. Local-global processing in Alzheimer’s disease: An examination of interference, inhibition and priming. Neuropsychologia. 2002;40:1173–1186. doi: 10.1016/s0028-3932(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A. Sex differences in corpus callosum size: Relationship to age and intracranial size. Neurobiology of Aging. 2001;22:603–611. doi: 10.1016/s0197-4580(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Adalsteinsson E, Swan GE, Carmelli D. Differential rates of regional brain change in callosal and ventricular size: A 4-year longitudinal MRI study of elderly men. Cereb Cortex. 2002;12:438–445. doi: 10.1093/cercor/12.4.438. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Mega MS, Narr KL, Sowell ER, Blanton RE, Toga AW. Brain image analysis and atlas construction. In: Fitzpatrick M, Sonka M, editors. SPIE Handbook on Medical Image Analysis. Society of Photo-Optical Instrumentation Engineers (SPIE) Press; 2000. pp. 1063–1131. [Google Scholar]
- Van Kleeck MH. Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: new data and a meta-analysis of previous studies. Neuropsychologia. 1989;27:1165–1178. doi: 10.1016/0028-3932(89)90099-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. Neuroimage. 2005;27:497–504. doi: 10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Volberg G, Hübner R. On the role of response conflicts and stimulus position for hemispheric differences in global/local processing: an ERP study. Neuropsychologia. 2004;42:1805–1813. doi: 10.1016/j.neuropsychologia.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Weber B, Schwarz U, Kneifel S, Treyer V, Buck A. Hierarchical visual processing is dependent on the oculomotor system. Neuroreport. 2000;11:241–247. doi: 10.1097/00001756-200002070-00004. [DOI] [PubMed] [Google Scholar]
- Wecker NS, Kramer JH, Hallam BJ, Delis DC. Mental Flexibility: Age Effects on Switching. Neuropsychology. 2005;19:345–352. doi: 10.1037/0894-4105.19.3.345. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage. 2003;19:1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Mangun GR, Woldorff MG. A role for top-down attentional orienting during interference between global and local aspects of hierarchical stimuli. Neuroimage. 2002;17:1266–1276. doi: 10.1006/nimg.2002.1284. [DOI] [PubMed] [Google Scholar]
- Wilkinson DT, Halligan PW, Marshall JC, Büchel C, Dolan RJ. Switching between the forest and the trees: Brain systems involved in local/global changed-level judgements. Neuroimage. 2001;13:56–67. doi: 10.1006/nimg.2000.0678. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Yamagata S, Kobayashi S. Cerebral asymmetry of the “top-down” allocation of attention to global and local features. J Neurosci. 2000;20:RC72. doi: 10.1523/JNEUROSCI.20-09-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazgan MY, Wexler BE, Kinsbourne M, Peterson B, Leckman JF. Functional significance of individual variations in callosal area. Neuropsychologia. 1995;33:769–779. doi: 10.1016/0028-3932(95)00018-x. [DOI] [PubMed] [Google Scholar]
- Yovel G, Levy J, Yovel I. Hemispheric asymmetries for global and local visual perception: effects of stimulus and task factors. J Exp Psychol Hum Percept Perform. 2001;27:1369–1385. [PubMed] [Google Scholar]


