Abstract
Previous studies using a cardiac-specific metallothionein-overexpressing transgenic mouse model have demonstrated that metallothionein protects the heart from doxorubicin toxicity. The present study was undertaken to determine cellular and subcellular distribution of metallothionein and located the antioxidant action of this protein in the transgenic heart. Using light microscopic immunoperoxidase method, it was identified that the overexpressed metallothionein is localized exclusively in cardiomyocytes. The electron microscopic immunogold method revealed that elevated metallothionein is in nucleus, myofibers, and sarcoplasm. In contrast with these distributions, metallothionein in nontransgenic myocardium was undetectable by immunoperoxidase light microscopy and was seldom found in nucleus and myofibers by immunogold electron microscopy. Treatment with doxorubicin induced cytoplasmic vacuolization and severe damages in myofilaments and nucleus in nontransgenic myocardium. The most prominent injury, however, occurred in mitochondria, including striking size and shape changes, focal swelling and loss of cristae. These damages were rarely found in the doxorubicin-treated transgenic myocardium. In particular, the internal morphology of mitochondria was maintained essentially normal, although metallothionein was not localized in this compartment in transgenic hearts. This study thus demonstrates that although the subcellularly localized action of metallothionein is important, it also plays a significant role in protection against oxidative injury by doxorubicin in remote organelles.
In previous studies, we used a cardiac-specific metallothionein (MT)-overexpressing transgenic mouse model and demonstrated that MT functions in protection against oxidative heart injury. 1,2 MT is a highly conserved, low-molecular-weight, thiol-rich protein. The mammalian MT has 61 amino acids, including 20 cysteine residues, but no aromatic amino acids, histidine, or leucine. 3 The basal level of MT in biological systems is very low, although it may vary with age and type of tissue. 4 However, this protein is highly inducible when the system is challenged by heavy metals, starvation, heat, inflammation, or other stress conditions. 5,6 Because MT can both bind to and be induced by heavy metal ions, it is generally agreed that MT is somehow involved in metal metabolism and toxicity. 7
That MT functions as an antioxidant has been proposed for more than a decade. Studies in vitro have shown that MT directly interacts with reactive oxygen species and acts as a scavenger of these toxic radicals. 8,9 The same reaction between MT and reactive oxygen species has not been demonstrated in intact animals. However, recent studies have provided direct evidence to show that MT functions in vivo as an antioxidant. 1,2,10,11 For instance, in the MT-overexpressing transgenic mouse heart, oxidative injury induced by doxorubicin (DOX), a most effective anticancer agent that causes severe cardiotoxicity through reactive oxygen species, is significantly inhibited. 1 It is, therefore, important to know the mechanism by which MT protects the heart from DOX toxicity.
Subcellular localization of MT would play an important role in the detoxification of DOX. Cytoplasmic MT would prevent DOX-induced lipid peroxidation and damages to adjacent organelles, and nuclear MT would inhibit DOX-induced DNA damage. It is, therefore, important to determine the localization of the overexpressed MT in the myocardial cells to provide basic information for elucidating mechanisms by which MT provides protection against DOX toxicity. Similarly, it is important to know which cell types in the heart are targeted to express the transgenic MT.
MT has been detected by using immunocytochemical technique in various tissues of humans and animals, but its distribution displays some species differences. 12-19 The subcellular localization of MT has been studied most in the liver. It is localized exclusively in the cytoplasm of hepatocyte in adult animals. 20,21 It is also found in the nucleus when this protein is induced by chemical or physical stresses 22-26 or partial hepatectomy. 21,27
Cellular and subcellular localization of MT in the heart is seldom studied. One of the reasons for the lack of this kind of study is that the heart contains very low basal levels of MT, almost undetectable by immunohistochemical methods. The constitutively cardiac-specific MT-overexpressing transgenic mouse model should provide a unique experimental tool to fill this gap. On the other hand, understanding MT distribution in the transgenic mouse heart would provide significant insights into mechanisms of MT protection against oxidative heart injury. The present study was thus undertaken to examine cellular and subcellular distribution of MT in the transgenic mouse heart and its relation to protection against DOX toxicity among organelles in the cardiomyocytes.
Materials and Methods
Animals
Cardiac-specific MT-overexpressing transgenic and nontransgenic mice aged 8 weeks and weighing about 25 g were used for this study. Production of the transgenic mice was previously described. 1 All animals were housed in the animal quarters at the University of Louisville Research Resources Center. They were maintained at 22°C with a 12-hour light/12-hour dark cycle and free access to rodent chow and deionized water. The experimental procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care.
Chemicals and Reagents
Monoclonal antibody to MT, biotinylated rabbit anti-mouse IgG1, HRP-streptavidin, and DAB kit were purchased from Zymed Laboratories, Inc. (San Francisco, CA). Ten-nanometer gold conjugates of goat anti-mouse IgG and sheep anti-digoxigenin were obtained from BBInternational (Cardiff, UK). ApopTag apoptosis detection kit was the product of Intergen (Purchase, NY). All routine chemicals and reagents were obtained from Sigma Chemical (St. Louis, MO) unless otherwise stated.
Tissue MT Assay
Tissue MT concentrations were estimated by a cadmium-hemoglobin affinity assay. 28 Briefly, tissues were homogenized in 4 volumes of 10 mmol/L Tris-HCl buffer, pH 7.4, at 4°C. After centrifugation of the homogenate at 10,000 × g for 15 minutes, 200 μl of supernatant were transferred to microtubes for MT analysis as described previously. 1
Light Microscopic Immunocytochemistry
The hearts of anesthetized mice were perfused in situ as described previously, 1 then removed and cut into slices about 3 mm thick that were fixed with 10% formalin in phosphate buffered saline (PBS), 0.01 mol/L, pH 7.4, for 20 hours at room temperature. Kidney, liver, and pancreas tissues were also subjected to the same preparation procedure. The tissue slices were dehydrated in graded ethanol and embedded in Paraplast at 55°C. Tissue sections of 5 μm were cut and mounted on silanized slides.
Tissue sections were deparaffinized and rehydrated in graded ethanol. Endogenous peroxidase activity was blocked with 3% H2O2 in PBS for 10 minutes. Sections were incubated in 5% rabbit serum in PBS for 20 minutes for reducing nonspecific binding. After tapping the excess rabbit serum solution, sections were incubated overnight at 4°C in the presence of monoclonal antibody to MT (Clone E9, mouse IgG1) diluted 1:300 in antibody diluent. Sections were then incubated for 20 minutes in biotinylated rabbit anti-mouse IgG1, followed by incubation with HRP-streptavidin for 20 minutes. The antibody binding sites were visualized by incubation with DAB-H2O2 solution using a DAB kit. Finally, sections were counterstained in 0.5% methyl green.
Electron Microscopic Immunogold Procedure
Heart tissues taken from left ventricles of normal and transgenic mice were cut into pieces about 1 mm 3 and fixed in 2% freshly depolymerized paraformaldehyde with 0.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.4, at 4°C for 2 hours. After rinsing in sodium cacodylate buffer, the samples were partially dehydrated with ethanol and embedded in LR White. Ultrathin sections were cut on a LKB ultratome and collected on gold grids. The ultrathin sections were incubated with normal rabbit sera for 20 minutes to block nonspecific reactions and then incubated in the presence of monoclonal antibody to MT diluted 1:50 overnight at 4°C. After rinsing in immunogold buffer (0.01 mol/L PBS with 1% normal serum, 1% bovine serum albumin, 0.1% Tween 20, and 0.1% Na3N, pH 8.2), the ultrathin sections were incubated in 10 nm gold-conjugated goat anti-mouse IgG diluted in immunogold buffer for 2 hours. The ultrathin sections were then rinsed in distilled water and counterstained with uranyl acetate and lead citrate. The labeled ultrathin sections were observed with a Philips transmission electron microscope. For observation of general fine structures, some heart tissues were processed for conventional electron microscopy. Briefly, left ventricles were cut into pieces 1 mm 3 and fixed with 3% glutaraldehyde for 2 hours and postfixed with 1% osmium tetraoxide for 1 hour. Tissues were then dehydrated with graded ethanol and embedded in LR White, and the ultrathin sections were stained with uranyl acetate and lead citrate.
Immunocytochemical Controls
The labeling specificity of both light and electron microscopic immunocytochemistry was assessed by substituting MT antibody with antibody diluent.
DOX-Induced Cardiotoxicity and Myocardial Apoptosis
Ultrastructural changes of cardiomyocytes induced by DOX were observed by conventional electron microscopy. The DNA fragmentation was monitored by immunogold TUNEL assay. Transgenic and nontransgenic mice received an i.v. injection of 15 mg/kg doxorubicin hydrochloride. Four days after the treatment, the heart tissue from left ventricles was sampled. The ultrathin sections for conventional electron microscopy were stained with uranyl acetate and lead citrate as described above. The fragmented DNA on the ultrathin sections were labeled with the ApopTag apoptosis detection kit with some modulations. Briefly, the ultrathin sections were incubated with 10× diluted working strength terminal deoxynucleotidyl transferase (TdT) enzyme at 37°C for 10 minutes. After rinsing in immunogold buffer, the ultrathin sections were incubated with 10 nm gold conjugate of sheep anti-digoxigenin diluted 1:30 in immunogold buffer. The labeled ultrathin sections were observed with a Philips transmission electron microscope. Negative controls were performed by omitting the TdT enzyme. Besides the immunogold TdT-mediated dUTP nick end labeling (TUNEL) assay, a light microscopic TUNEL procedure was also performed. Briefly, the myocardial tissue slides were pretreated with H2O2 and incubated with the reaction mixture containing TdT and digoxigenin-conjugated dUTP for 1 hour at 37°C. Labeled DNA was visualized with peroxidase-conjugated anti-digoxigenin antibody with DAB as the chromagen. Rat mammary gland tissue provided in the kit was used as positive control. For negative control, TdT was routinely omitted from the reaction mixture.
Results
MT concentrations in the heart of transgenic mice were about 40-fold higher than those in nontransgenic controls, as shown in Table 1 ▶ . Light microscopic immunocytochemistry was used to identify cell populations that contain MT in the heart of transgenic mice, in comparison with that of nontransgenic mice. The results shown in Figure 1 ▶ demonstrate that strongly positively stained cardiac cells were found in both atrium and ventricle in transgenic mice, in contrast with the negative staining of nontransgenic myocardium. Almost all of the cells were positive, although the intensity of the positive staining varied among cells (Figure 1) ▶ . The staining sites were mainly on the cytoplasm, whereas nuclear staining was found in some cells. Strongly positively stained cells were also observed at the opening of pulmonary veins and vena cava, which consist of cardiomyocytes, but not in pulmonary arteries, aorta, or cardiac valves (Figure 2) ▶ , strongly indicating that only cardiomyocytes were targeted to express the transgenic MT.
Table 1.
MT Concentrations in the Heart and Other Tissues in Transgenic Mice in Comparison with That in Nontransgenic Mice
| Mouse type | MT (μg/g tissue) | ||
|---|---|---|---|
| Heart | Liver | Kidney | |
| Transgenic | 205.7 ± 11.9 | 122.6 ± 10.8 | 73.7 ± 4.6 |
| Nontransgenic | 5.9 ± 1.6 | 116.8 ± 9.7 | 71.4 ± 3.9 |
Values are means ± SD (n = 12).
Figure 1.
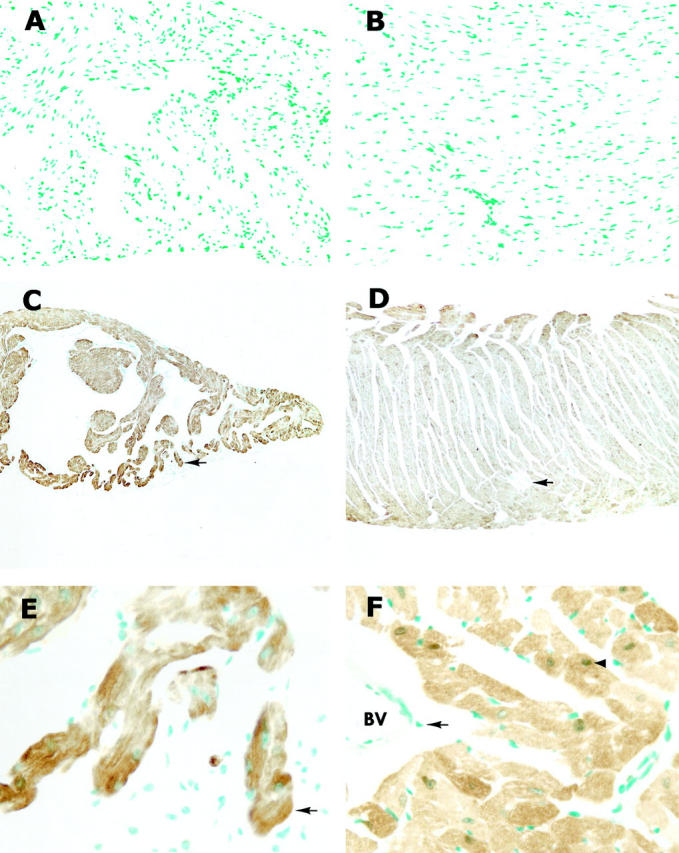
Photomicrographs of immunocytochemical localization of MT in atria and ventricles of nontransgenic and transgenic mice. Both atrium (A) and ventricle (B) in nontransgenic mice showed negative staining, whereas strongly positive staining was found in transgenic atrium (C) and ventricle (D). At high magnification of C and D, positive staining was seen exclusively on the cardiomyocytes in either atrium (E) or ventricle (F) and also found in the nucleus (arrowhead in F). Arrows indicate the same structure in C and E and in D and F. BV, blood vessel. Original magnifications, ×170 (A and B), ×65 (C and D), ×520 (E and F).
Figure 2.
Photomicrographs of immunocytochemical localization of MT in the cardiac blood vessels and valves of transgenic mice. Positive staining was observed in the pulmonary vein (A) and vena cana (B), but not in the pulmonary artery (C), aorta (D), or cardiac valves (C and D). Arrows in C and D indicate the direction of blood flow. PV, pulmonary vein; VC, vena cava; PA, pulmonary artery; Ao, aorta; PVa, pulmonary valve; AV, Aortic valve. Original magnification, ×130.
To confirm the specificity of MT expression in the heart of transgenic mice, MT concentrations in the kidney and liver of transgenic mice were compared with those in nontransgenic mice, as shown in Table 1 ▶ . The immunocytochemical staining on kidney, liver, and pancreas tissues demonstrated a similar pattern between transgenic and nontransgenic mice. In the kidney, moderate staining was found on the epithelial cells of distal convoluted tubules and the thick limb of Henle’s loop (Figure 3) ▶ . The staining on the proximal convoluted tubules was very weak. Liver tissue showed a weak staining specifically on the hepatocytes. Weak staining was also observed on the acinar cells in pancreas, with both cytoplasmic and nuclear immunoreactivity.
Figure 3.

Photomicrographs of immunocytochemical localization of MT in the kidney, liver, and pancreas of nontransgenic and transgenic mice. In nontransgenic mice, the main staining sites of kidney were on the epithelia of distal convoluted tubules in the cortex (A) and thick limb of Henle’s loop in the medulla (B). Positive staining in the liver (E) and pancreas (G) was on the hepatocytes and the acinar cells, respectively. The transgenic mice showed similar staining pattern in the kidney (C and D), liver (F), and pancreas (H) to the nontransgenic mice. Nuclear staining was observed on the acinar cells of pancreas (arrows in G and H). G, glomerulus; D, distal convoluted tubule; P, proximal convoluted tubule; H, Henle’s loop; C, collecting duct. Original magnifications, ×260 (A−D) and ×520 (E−H).
Observation of ultrastructures between transgenic and nontransgenic myocardia by using conventional electron microscopy revealed no differences. Electron microscopic immunocytochemistry was then applied to identify subcellular localization of MT in the transgenic heart. High density of immunogold staining was found in myofibers, sarcoplasm, and nucleus (Figure 4) ▶ . Only a few of the positive staining cells were found in myofibers and nucleus in cardiomyocytes of nontransgenic mice (Figure 4) ▶ . In either transgenic or nontransgenic cardiomyocytes, it was clearly shown that mitochondria were totally negative (Figure 4) ▶ .
Figure 4.

Electron micrographs of cardiomyocyte ultrastructure and immunogold labeling of MT in nontransgenic and transgenic mice. No differences were found in the ultrastrucure of cardiomyocytes of nontransgenic (A) and transgenic (B) mice by conventional electron microscopy. MT immunogold labeling on the cardiomyocytes of nontransgenic mouse only demonstrated a few gold particles on the myofibers (C) and nucleus (D), as indicated by arrows. In transgenic mice, strong gold labeling was observed on the myofibers, sarcoplasm, and nucleus, but not on the mitochondria (E and F). Mf, myofiber; Mt, mitochondria; Nu, nucleolus. Original magnifications, ×21,000 (A and B), ×36,000 (C and D), and ×48,000 (E and F).
DOX induced dramatic morphological changes in the nontransgenic mouse heart (Figure 5) ▶ . Subcellular organelles showed different degrees of structural changes. Moderate nuclear chromatin margination with many pieces of coarse chromatin clumping was observed. Immunogold TUNEL assay also identified fragmented DNA within the condensed chromatin structure, indicating apoptotic myocytes. Myofilaments show disarray with loss of Z-bands. Sarcoplasmic reticula were dilated and cytoplasmic vacuolization was apparent. Myelin figures were also observed. The most prominent structural changes, however, occurred in mitochondria, such as striking variation in size and shape with focal swelling and loss of cristae. In contrast to these findings, the chromatin was distributed homogeneously within the nucleus and no apoptotic myocytes were detected in the DOX-treated MT-overexpressing transgenic mouse heart. Mitochondrial membrane and cristae were intact. No myelin figures were found. Therefore, there was no obvious alteration in the morphology of the DOX-treated MT-overexpressing transgenic mouse heart. The inhibitory effect of MT on DOX-induced apoptosis was also determined by the light microscopic TUNEL assay. As shown in Figure 6 ▶ , the number of TUNEL-positive cells in the MT-overexpressing myocardium was significantly decreased.
Figure 5.

Electron micrographs of ultrastructural and EM-TUNEL observations on the cardiomyocytes of nontransgenic and transgenic mice treated with DOX. A−D and E−F represent the results of conventional electron microscopy and EM-TUNEL, respectively. Saline-treated nontransgenic myocardium showed normal ultrastructure (A). DOX-treated nontransgenic myocardium showed cytoplasmic vacuolization and mitochondrial damage (B) and myofilament disarray with loss of Z-band (C). However, no obvious ultrastructural changes were found in the DOX-treated transgenic mice (D). EM-TUNEL revealed fragmented DNA in the condensed chromatin structure (arrows) in the cardiomyocyte nucleus of nontransgenic (E), but not in the transgenic mice (F). Mf, myofiber; Mt, mitochondria; Nu, nucleolus. Original magnifications, ×15,000 (A−D) and ×20,000 (E and F).
Figure 6.
Light microscopic TUNEL assay of DNA fragmentation in the hearts of nontransgenic and transgenic mice treated with DOX at a single i.v. injection of 15 mg/kg. Both saline-treated controls of nontransgenic (A) and transgenic (B) mice showed negative staining. Many positive cells were found in the heart of nontransgenic mice treated with DOX (C), whereas the positive cells were occasionally observed in the transgenic mice treated with DOX (D). Original magnifications, ×180.
Discussion
Many studies have been done to examine MT subcellular distribution in hepatocytes and tumor cells, but little work has been undertaken to study its localization in cardiomyocytes. The present study thus took advantage of the cardiac-specific MT-overexpressing transgenic mouse model to address this fundamental issue of MT metabolism and function in the heart. Light microscopic immunocytochemical examination revealed that all of the cells that were positively stained with the anti-MT antibody were localized in the tissues consisting of cardiomyocytes including atrium, ventricle, and the opening of pulmonary veins and vena cava in the transgenic mouse heart. The tissues that are not composed of cardiomyocytes, such as pulmonary arteries, aorta, and all of the valves of the heart, were not reactive to the anti-MT antibody. This result thus demonstrates that the elevated MT driven by the transgene, which contains human MT-IIa gene that linked behind the mouse α-cardiac myosin heavy chain promoter, 1 is localized exclusively in the cardiomyocytes. Although all of the cardiomyocytes were positively stained, the intensity among the cells was different, indicating that different levels of MT expression exist from one cardiomyocyte to another.
MT concentrations in the heart, as reflected by immunoperoxidase light microscopic examination, were significantly elevated in transgenic mice. In contrast, its basal level in the heart of nontransgenic mice was almost undetectable by the same procedure. On the other hand, MT concentrations in kidney, liver, and pancreas were significantly higher than in the heart in nontransgenic mice. This result agrees with the observation that much lower MT concentrations were found in the heart than in the liver and kidney in mice as shown in Table 1 ▶ and as previously reported. 1
The immunogold labeling and electron microscopic examination revealed that the elevated MT was localized in myofibers, sarcoplasm, and nucleus, but not in mitochondria. These subcellular distributions of MT are quite different from those observed in hepatocytes. In hepatocytes, MT is exclusively localized in cytoplasm under unstressed physiological conditions. 20,21 The nuclear localization of MT in hepatocytes occurs under chemical and physical stress conditions 22-26 and partial hepatectomy. 21,27 Under these conditions, MT concentrations are also significantly elevated in the hepatocytes. 21,29 The nuclear localization of MT is likely functionally regulated, rather than concentration-related, as evidenced by the observation obtained from this study that the heart of nontransgenic mice contains very low concentrations of MT, but its nuclear localization is still obvious.
The present study demonstrates that MT inhibited ultrastructural alterations induced by DOX in many organelles including mitochondria. In the nucleus, DOX caused significant damage to the ultrastructures of this organelle. Nuclear shrinkage, chromatin margination and condensation are among the most distinguished alterations. These changes indicate typical apoptotic processes. This was then confirmed by the immunogold TUNEL assay. Immunogold staining of the fragmented DNA within the condensed and clumped chromatin structures provided further convincing evidence to show DOX-induced apoptosis in cardiomyocytes. These changes in the nucleus were almost completely inhibited in the MT-overexpressing transgenic cardiomyocytes, as evidenced by both immunogold and light microscopic TUNEL assays. This inhibition is associated with the nucleic localization of MT.
High concentrations of transgenic MT were also found in myofibers and sarcoplasm, in which DOX caused remarkable structural changes. These changes can be segregated into three degrees of severity. In some cells, dilation of sarcoplasmic reticula was the only sarcoplasmic change, suggesting a mild damage. Moderate damages were expanded to cytoplasmic vacuolization and the most severe damages included myofilament disarray with loss of Z-bands. All of these changes were almost completely inhibited in the MT-overexpressing transgenic myocardium, although some cells showed a slight dilation of sarcoplasmic reticula.
The transgenic MT is not localized in mitochondria, which is a critical organelle for production of reactive oxygen species from DOX. However, the striking ultrastructural changes in this organelle are mostly suppressed in the transgenic myocardium. It suggests that the MT in the transgenic mouse heart not only provides its localized protection, but also functions in remote organelle’s defense against DOX-induced oxidative injury.
In summary, the data obtained from the present study demonstrate that elevated MT in transgenic mouse heart localizes exclusively in cardiomyocytes with its distributions in myofibers, sarcoplasm, and nucleus, but not in mitochondria. It seems that the MT is located in the same organelle where its antioxidant action occurs, although it also functions in protection against DOX toxicity in remote organelles such as mitochondria.
Acknowledgments
We thank Donald Mosley and Cathie Caple for technical assistance.
Footnotes
Address reprint requests to Dr. Y. James Kang, University of Louisville School of Medicine, Department of Medicine, 511 South Floyd St., MDR 530, Louisville, KY 40202. E-mail: yjkang01@athena.louisville.edu.
Supported in part by National Institutes of Health grant HL59225, American Heart Association Established Investigator Award (9640091N), and Jewish Hospital Foundation, Louisville, Kentucky.
References
- 1.Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN: Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest 1997, 100:1501-1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang YJ, Li G, Saari JT: Metallothionein inhibits ischemia-reperfusion injury in mouse heart. Am J Physiol 1999, 276:H993-H997 [DOI] [PubMed] [Google Scholar]
- 3.Hamer DH: Metallothionein. Ann Rev Biochem 1986, 55:913-951 [DOI] [PubMed] [Google Scholar]
- 4.Bremner I: Nutritional and physiologic significance of metallothionein. Methods Enzymol 1991, 205:25-35 [DOI] [PubMed] [Google Scholar]
- 5.Kagi JHR: Overview of metallothionein. Methods Enzymol 1991, 205:613-626 [DOI] [PubMed] [Google Scholar]
- 6.Sato M, Bremnar I: Oxygen free radical and metallothionein. Free Radical Biol Med 1993, 14:325-337 [DOI] [PubMed] [Google Scholar]
- 7.Templeton DM, Cherian MG: Toxicological significance of metallothionein. Methods Enzymol 1991, 205:11-24 [DOI] [PubMed] [Google Scholar]
- 8.Thornalley PJ, Vasak M: Possible role for metallothionein in protection against radioation-induced oxidative stress: kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochem Biophys Acta 1985, 827:36-44 [DOI] [PubMed] [Google Scholar]
- 9.Thomas JP, Bachowski GL, Girotti AW: Inhibition of cell membrane lipid peroxidation by cadium- and zinc-metallothionein. Biochem Biophys Acta 1986, 884:448-461 [DOI] [PubMed] [Google Scholar]
- 10.Wu H-Y, Kang YJ: Inhibition of buthionine sulfoximine-enhanced doxorubicin toxicity in metallothionein overexpressing transgenic mouse heart. J Pharmacol Exp Ther 1998, 287:515-520 [PubMed] [Google Scholar]
- 11.Wang GW, Kang YJ: Inhibition of doxorubicin toxicity in cultured neonatal mouse cardiomyocytes with elevated metallothionein levels. J Pharmacol Exp Ther 1999, 288:938-944 [PubMed] [Google Scholar]
- 12.Blaaugeers HG, Sillevis Smitt PA, De Jong JM, Troost D: Localization of metallotionein in the mammalian central nervous system. Biol Signals 1994, 3:181-187 [DOI] [PubMed] [Google Scholar]
- 13.Danielson KG, Ohi S, Huang PC: Immunochemical localization of metallothionein in rat liver and kidney. J Histochem Cytochem 1982, :1033-1039 [DOI] [PubMed] [Google Scholar]
- 14.Mididoddi S, McGuirt JP, Sens MA, Todd JH, Sens DA: Isoform-specific expression of metallothionein mRNA in the developing and adult human kidney. Toxicol Lett 1996, 85:17-27 [DOI] [PubMed] [Google Scholar]
- 15.Nishimura H, Nishimura N, Ghaffar A, Tohyama C: Localization of metallothionein in developing rat tissues. J Histochem Cytochem 1989, 37:715-722 [DOI] [PubMed] [Google Scholar]
- 16.Nishimura N, Nishimura H, Ghaffar A, Tohyama C: Localization of metallothionein in female reproductive organs of rat and guinea pig. J Histochem Cytochem 1990, 37:1601-1607 [DOI] [PubMed] [Google Scholar]
- 17.Nishimura N, Nishimura H, Ghaffar A, Tohyama C: Localization of metallothionein in the brain of rat and mouse. J Histochem Cytochem 1992, 40:309-315 [DOI] [PubMed] [Google Scholar]
- 18.Ono S, Cherian MG: Regional distribution of metallothionein, zinc, and copper in the brain of different strains of rats. Biol Trace Elem Res 1999, 69:151-159 [DOI] [PubMed] [Google Scholar]
- 19.Shimada A, Yanagida M, Umemura T: An immunohistochemical study on the tissue-specific localization of metallothionein in dog. J Comp Pathol 1997, 116:1-11 [DOI] [PubMed] [Google Scholar]
- 20.Deng DX, Ono S, Koropatnick J, Cherian MG: Metallothionein and apoptosis in the toxic milk mutant mouse. Lab Invest 1998, 78:175-183 [PubMed] [Google Scholar]
- 21.Tohyama C, Suzuki JS, Hemelraad J, Nishimura N, Nishimura H: Induction of metallothionein and its localization in the nucleus of rat hepatocytes after partial hepatectomy. Hepatology 1993, 18:1193-1201 [PubMed] [Google Scholar]
- 22.Banerjee D, Onotaka S, Cherian MG: Immunohistochemical localization of metallothionein in cell nucleus and cytoplasm of rat liver and kidney. Toxicology 1982, 24:95-105 [DOI] [PubMed] [Google Scholar]
- 23.Dincer Z, Haywood S, Jasani B: Immunocytochemical localization of metallothionein (MT1 and MT2) in copper-enhanced sheep brain. J Comp Pathol 1999, 120:29-37 [DOI] [PubMed] [Google Scholar]
- 24.Evering WE, Haywood S, Elmes ME, Jasani B, Trafford J: Histochemical and immunochemical evaluation of copper and metallothionein in the liver and kidney of copper-overloaded rats. J Pathol 1990, 160:305-312 [DOI] [PubMed] [Google Scholar]
- 25.Leyshon-Sorland K, Stang E: The ultrastructural localization of metallothionein in cadmium exposed rat liver. Histochem J 1993, 25:857-864 [PubMed] [Google Scholar]
- 26.Mullins JE, Fuentealba IC: Immunohistochemical detection of metallothionein in liver, duodenum and kidney after dietary copper overload in rats. Histol Histopathol 1998, 13:627-633 [DOI] [PubMed] [Google Scholar]
- 27.Tsujikawa K, Suzuki N, Sagawa K, Itoh M, Sugiyama T, Kohama Y, Otaki N, Kimura M, Mimura T: Induction and subcellular localization of metallothionein in regeneration rat liver. Eur J Cell Biol 1994, 63:240-246 [PubMed] [Google Scholar]
- 28.Eaton DL, Cherian MG: Determination of metallothionein in tissues by cadmium-hemoglobin affinity assay. Methods Enzymol 1991, 205:83-88 [DOI] [PubMed] [Google Scholar]
- 29.Iszard MB, Liu J, Liu Y, Dalton T, Andrews GK, Palmiter RD, Klaassen CD: Characterization of metallothionein-I-transgenic mice. Toxicol Appl Pharmacol 1995, 133:305-312 [DOI] [PubMed] [Google Scholar]




