Abstract
Objective:
To obtain further analysis regarding specific outcomes and alvimopan doses in bowel resection (BR) patients.
Summary Background Data:
Although postoperative ileus (POI) is common after BR, there is currently no recognized treatment or prevention available. Alvimopan, a novel, peripherally active mu-opioid receptor antagonist, accelerated GI recovery after BR or hysterectomy in 3 phase III trials.
Methods:
A pooled retrospective subset analysis of BR patients in alvimopan phase III trials was performed. Randomized BR patients received alvimopan 6 mg (n = 397), 12 mg (n = 413), or placebo (n = 402) ≥2 hours before surgery and twice daily until hospital discharge for ≤7 days. The primary endpoint of each trial was time to recovery of GI function. Hospital discharge order (DCO) written, readmission, and morbidities were also assessed. Cox proportional hazard models were used to analyze treatment effects on time-to-event endpoints.
Results:
Alvimopan (6 or 12 mg) significantly accelerated GI recovery (GI-3; hazard ratio = 1.28 and 1.38, respectively; P ≤ 0.001 for both). Alvimopan significantly accelerated time to DCO written by 16 hours for 6 mg and 18 hours for 12 mg (P < 0.001 for both) from a mean of 147 hours for placebo. Alvimopan-treated patients had reduced postoperative morbidity compared with placebo, and incidence of prolonged hospital stay or readmission was significantly reduced (P < 0.001). Tolerability profiles were similar among groups.
Conclusions:
Alvimopan significantly accelerated GI recovery in BR patients. A 12-mg dose provided more consistent benefits across both sexes and all ages. Postoperative morbidity rates, prolonged hospital stay, and rates of hospital readmission were significantly reduced. Alvimopan reduces the consequences of POI after BR.
A pooled retrospective subset analysis of patients who received bowel resection in alvimopan phase III efficacy trials was performed. Alvimopan significantly accelerated gastrointestinal recovery and reduced the consequences of postoperative ileus, including postoperative morbidity, prolonged hospital stay, and hospital readmission in bowel resection patients.
In the United States, nearly 350,000 patients undergo colorectal or small bowel resection (BR) annually.1 These patients spend an average of 11 days in the hospital and account for >$15 billion in annual national healthcare costs.1 All of these patients experience postoperative ileus (POI), a temporary impairment of gastrointestinal (GI) function.2–4 Moreover, associated GI complications are common.5 For patients who undergo BR, length of hospital stay (LOS) is dictated mostly by timing of GI recovery. To facilitate GI recovery, some specialized centers have used accelerated care pathways, although readmission rates have been increased in some cases.6–10 Reducing surgical complications and further accelerating GI recovery after BR could increase patient comfort, decrease the average LOS, and reduce costs, readmission rates, and other demands on healthcare resources.11
The etiology of POI is complex, and major intrinsic contributing factors include surgical stress (ie, from physical manipulation of the bowel), secretion of inflammatory mediators and endogenous opioids in the GI tract, and changes in hormone levels and electrolyte and fluid balance.12–15 Opioids are the most widely prescribed analgesics used to treat postoperative pain.16 However, opioids bind to mu-opioid receptors within the gut, exacerbating POI.12,14 No specific treatment currently exists for the treatment or prevention of POI.
Alvimopan is a novel, oral, peripherally acting, mu-opioid receptor (PAM-OR) antagonist that has been studied in patients undergoing abdominal and pelvic surgery.17–19 In 3 phase III multicenter trials, alvimopan accelerated GI recovery after BR or total abdominal hysterectomy (TAH).17–19 Although the results favored alvimopan across studies, statistical significance and magnitude of treatment effect were not consistent with regard to the primary endpoint, a composite assessment that included toleration of solid food and first passage of flatus or stool (GI-3). Further, the individual studies were not powered sufficiently to investigate infrequent but clinically important postoperative morbidities or investigate potential differences between the 6- and 12-mg doses. Hence, a pooled analysis was performed to examine the safety and efficacy of alvimopan within the subgroup of patients who underwent BR.
METHODS
Patients
Inclusion criteria for phase III alvimopan efficacy trials have been reported previously.17–19 After Institutional Review Board approval at each site, patients ≥18 years of age, undergoing laparotomy for partial BR with primary anastomosis or TAH and scheduled for postoperative pain management with patient controlled opioid-based analgesia, were eligible. Patients who had taken opioid analgesics within 4 weeks before surgery (with the exception of a colonoscopy-associated parenteral opioid administration) or who were expected to receive epidural opioids, local anesthetics, or nonsteroidal anti-inflammatory drugs (eg, ketorolac) were excluded. Additional exclusion criteria were severe cardiovascular, pulmonary, renal, hepatic, hematologic, or other systemic disease; clinically significant laboratory abnormalities on screening; complete bowel obstruction; inflammatory bowel disease (eg, Crohn's disease or ulcerative colitis); prior treatment with vinca alkaloids (eg, vinblastine); or a history of illness or behavior (eg, substance abuse) that could pose additional risks in the administration of the study procedures. This analysis is based on the subset of patients who received BR surgery (1212 randomized). Because the randomization of each study was stratified by BR versus TAH, the number of patients who underwent BR within a treatment group is expected to be balanced.
Study Design
This was a pooled analysis of 3 randomized, double-blind, placebo-controlled, phase III, parallel-group, multicenter trials investigating the efficacy and safety of alvimopan after BR.17–19
Treatments
Alvimopan (6 or 12 mg) or placebo was administered ≥2 hours before surgery and twice daily starting on postoperative day 1 until hospital discharge for a maximum of 7 postoperative days. An accelerated postoperative care pathway was used to facilitate GI recovery. Briefly, if kept in place after surgery, the nasogastric tube (NGT) was removed by noon on the day after surgery (day 1), ambulation was encouraged and liquids offered on day 1, and solid food was offered on day 2. Efficacy assessments were performed in the hospital up to 10 days postoperatively, and safety assessments were performed up to 30 days after the last dose of study medication.
Efficacy Assessments
Efficacy analyses were based on the modified intent-to-treat (MITT) population (all randomized and treated patients who received the protocol-specified surgery and had ≥1 efficacy evaluation). Efficacy endpoints for the pooled analysis were the same as those used in the individual trials.17–19 The primary endpoint was recovery of GI function using a 3-component composite endpoint (GI-3) that represents upper and lower GI recovery: GI-3 = maximum (time to first toleration of solid food, minimum [time to first flatus, time to first bowel movement]). Secondary endpoints included GI-2 recovery (maximum [time to first toleration of solid food, time to first bowel movement]), readiness for hospital discharge (HD), and hospital discharge order (DCO) written.
Postoperative Morbidity
Postoperative morbidity (eg, postoperative NGT insertion, POI as a serious adverse event [SAE], and early postoperative small bowel obstruction [EPSBO]) was determined using the incidence of reported events in the safety population (all patients who received study medication and had a safety evaluation). Prolonged POI was determined by the investigator, and POI was defined as an SAE if it was associated with prolonged hospital stay or readmission. Hospital discharge, prolongation of planned hospital stay, and readmission data were also assessed using the safety population.
Safety Assessments
Safety assessments included adverse event (AE) reports, clinical laboratory tests (serum chemistry, biochemical liver tests, hematology, urinalysis), vital sign measurements, and electrocardiograms. Adverse events were defined as treatment-emergent AEs (TEAEs) if they occurred after the first dose of study medication and up to 7 days after the last dose. Serious AEs were defined as those that were immediately life-threatening, required intervention to prevent permanent impairment, or resulted in prolonged hospitalization or readmission, persistent or significant disability, or disruption of the ability to carry out normal life functions.
Analgesia and Opioid Consumption
Daily opioid consumption (in morphine sulfate equivalents) and visual analogue scale pain scores were also recorded. Opioid consumption was summarized for each treatment group by mean daily opioid consumption, and pain was summarized by mean daily and mean maximum pain scores.
Statistical Methods
Data were pooled from 3 phase III placebo-controlled studies.17–19 The validity of pooling data across studies was assessed for the BR subset of the MITT population using a Cox proportional hazards model with terms for treatment group, study, and study by treatment group interaction based on the time to GI-3 recovery endpoint. In the analysis that compared between-group treatment effects for 1 clinical trial versus another, differences were considered statistically significant if P was ≤0.10. Differences in endpoints between studies were considered statistically significant if P was <0.05.
For the primary analysis, treatment effect was assessed for each time-to-event endpoint using a Cox proportional hazards model. For comparisons between alvimopan and placebo groups, hazard ratios (HRs) and their 95% confidence intervals (CIs) and nominal P values were calculated using the Wald χ2 test. Significance conclusions for primary and secondary endpoints were drawn based on Hochberg's step-up method for multiple comparisons controlling the overall type I error to be ≤5% for each endpoint.17–19 The P values for treatment effects on postoperative morbidity were calculated using 2-sided Fisher exact tests, and 95% CIs were calculated using normal approximation. All statistical analyses were performed using Statistical Analysis System Version 8.02 or higher (Cary, NC).
RESULTS
Validity of Pooling Data
For the BR subgroup, differences in endpoint results and comparisons between each treatment and placebo in each trial were not statistically significant (P values ranged from 0.262 to 0.858 for all comparisons).
Patients
Of the 1627 patients who were randomized in the 3 trials, 1212 patients underwent BR and 1165 were in the pooled BR MITT population (Fig. 1). Bowel resection patient demographics were similar between groups (Table 1). Median duration of surgery was 2 hours (data not shown), and patients received an average of 9.5 doses (range, 1–15 doses) of alvimopan. The primary indications for BR surgery were colon or rectal cancer (58%) and diverticular disease (15%).
FIGURE 1. Supplemental figure. Consort diagram. This schematic illustrates the study design for patient randomization and treatment and patient disposition. BR, bowel resection; MITT, modified intent to treat; AE, adverse event; WC, withdrew consent.
TABLE 1. Pooled Demographics for Patients Undergoing Bowel Resection (MITT Population)
Efficacy Results
Alvimopan significantly accelerated GI-3 recovery in the 6-mg (HR = 1.28; P = 0.001) and the 12-mg groups (HR=1.38; P < 0.001) (Fig. 2). Moreover, alvimopan significantly accelerated GI-2 recovery, readiness for HD, and hospital DCO written (P < 0.001 for all). The magnitude of effect of alvimopan treatment ranged from 12 to 15 hours and over 15 to 18 hours earlier recovery for GI-3 and GI-2, respectively (Table 2). Patients who received alvimopan had a time to DCO written that was ≥16 hours earlier than patients who received placebo.
FIGURE 2. Hazard ratios and 95% CIs for time to recovery of GI function endpoints (MITT population). CI, confidence interval; GI, gastrointestinal; MITT, modified intent to treat; GI-3, recovery of bowel movement, passage of flatus, and toleration of solid food; GI-2, recovery of bowel movement and toleration of solid food; HD, hospital discharge based on GI recovery, as determined by the investigator; DCO, discharge order written.
TABLE 2. Kaplan-Meier Mean Time to Recovery of GI Function Endpoints (Bowel Resection MITT Population)
Effects of Sex and Age
In the placebo group, mean time to GI-3 recovery was 111.4 hours for women (n = 200) compared with 126.5 hours for men (n = 183). Among male patients, both alvimopan doses significantly accelerated GI-3 recovery (HR = 1.54, P < 0.001 for 6 mg; HR = 1.51, P < 0.001 for 12 mg). In contrast, female patients had better response to alvimopan 12 mg than to alvimopan 6 mg (HR = 1.06, P = 0.626 for 6 mg; HR = 1.26, P = 0.031 for 12 mg).
Although placebo-treated patients <65 years of age (n = 213) had shorter mean time to GI-3 recovery compared with patients ≥65 years of age (n = 170) (116.9 vs. 121.1 hour, respectively), age was not a significant covariate for GI-3 within any group. Alvimopan significantly accelerated time to GI-3 in patients <65 years of age at both dose levels (HR = 1.32, P = 0.006 for 6 mg; HR = 1.35, P = 0.003 for 12 mg). In contrast, in patients ≥65 years of age, only the 12-mg dose provided a significant benefit (HR = 1.22, P = 0.089 for 6 mg; HR = 1.41, P = 0.003 for 12 mg).
Postoperative Morbidity
The incidence of prolonged POI was lower for both alvimopan groups compared with placebo (5.0% for placebo; 1.6% for 6 mg; 1.4% for 12 mg). Moreover, alvimopan-treated patients had significant reductions in the incidence of POI as an SAE (6.7% for placebo; 1.8% for 6 mg; 1.9% for 12 mg [P < 0.001 for both]) and EPSBO or POI as an SAE (9.2% for placebo; 3.0% for 6 mg [P < 0.001]; 3.9% for 12 mg [P = 0.003]) (Fig. 3). The incidence of postoperative NGT insertion was lower for both alvimopan doses compared with placebo (12.2% for placebo; 6.8% for both 6 and 12 mg [P < 0.05 for both]). The total incidence of anastomotic leak was low, with no difference between groups (1.2% for placebo; 1.5% for 6 mg [P = 0.772]; 1.0% for 12 mg [P = 0.750]).
FIGURE 3. Postoperative morbidity for bowel resection patients (safety population). NGT, nasogastric tube; POI, postoperative ileus; SAE, serious adverse event; EPSBO, early postoperative bowel obstruction.
Compared with placebo, both alvimopan doses reduced the proportion of patients who required hospital readmission (11.7% for placebo; 7.3% for 6 mg [P = 0.040]; 7.7% for 12 mg [P = 0.059]), prolonged hospital stay (13.7% for placebo; 8.6% for 6 mg [P = 0.024]; 7.0% for 12 mg [P = 0.002]), and prolonged stay or readmission combined (24.4% for placebo; 13.6% for 6 mg [P < 0.001]; 14.0% for 12 mg [P < 0.001]). Of those patients who were readmitted to the hospital, the most common reported reasons were GI-related AEs (20.0% for placebo; 16.7% for 6 mg; 25.0% for 12 mg). Fewer patients in the alvimopan groups compared with the placebo group were readmitted with a diagnosis of POI (8.0% for placebo; 3.3% for 6 mg; 3.1% for 12 mg). Moreover, both alvimopan doses significantly reduced the proportion of patients with DCO written ≥7 days after surgery (38.1% for placebo; 24.4% for 6 mg; 19.9% for 12 mg [P < 0.001 for both]). Finally, the majority of AEs that resulted in a DCO written ≥7 days after surgery were GI-related in the placebo group and non–GI-related in the alvimopan groups. Of those patients who were hospitalized ≥7 days, GI-related AEs were reported in 24.6% of placebo patients, 12.9% of alvimopan 6 mg patients, and 13.2% of alvimopan 12 mg patients).
Safety Assessments
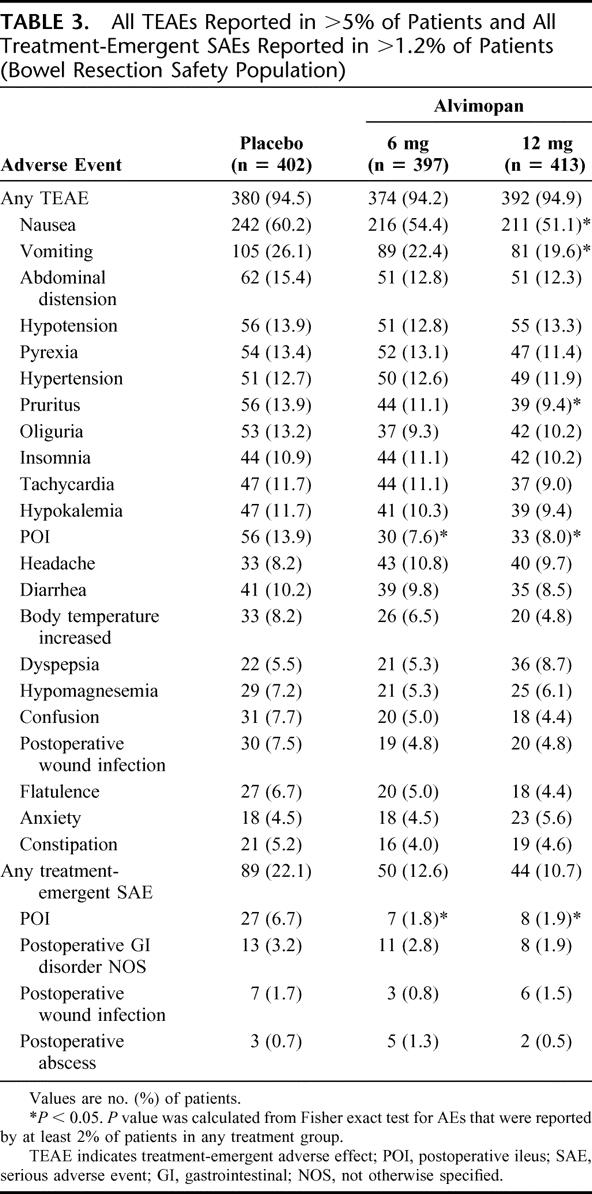
The overall incidence of TEAEs was slightly lower in alvimopan groups compared with the placebo group (Table 3). The most common TEAE was nausea, and its incidence was significantly lower in the alvimopan 12-mg group (60.2% for placebo; 54.4% for 6 mg [P = 0.101]; 51.1% for 12 mg [P = 0.009]). In addition, the incidence of POI as a TEAE was significantly lower for both alvimopan groups compared with placebo (13.9% for placebo; 7.6% for alvimopan 6 mg [P = 0.004]; 8.0% for alvimopan 12 mg [P = 0.007]).
TABLE 3. All TEAEs Reported in >5% of Patients and All Treatment-Emergent SAEs Reported in >1.2% of Patients (Bowel Resection Safety Population)
Analgesia and Opioid Consumption
Mean daily pain intensity scores (29.1 ± 18.4 for placebo; 29.9 ± 17.9 for 6 mg; 29.2 ± 18.0 for 12 mg) and mean maximum pain intensity scores (59.7 ± 25.3 for placebo; 61.2 ± 24.6 for 6 mg; 59.0 ± 25.0 for 12 mg) were comparable between groups. There were no statistically significant differences between the placebo and alvimopan groups for mean daily opioid consumption (26.3 ± 24.0 morphine equivalents for placebo; 28.1 ± 25.5 morphine equivalents for 6 mg [P = 0.298]; 27.3 ± 21.4 morphine equivalents for 12 mg [P = 0.553]).
DISCUSSION
The current pooled analysis demonstrated that alvimopan 6 and 12 mg significantly reduced postoperative GI morbidity and significantly accelerated GI-3 recovery in patients undergoing BR in a cross section of surgical centers. Benefits were significant for both doses, but treatment benefits appeared greater and more consistent for alvimopan 12 mg, which demonstrated benefits in subset analyses in women and elderly patients. Both alvimopan 6 and 12 mg also significantly accelerated GI-2 recovery, an important secondary endpoint based on parameters that can be accurately documented and are important for hospital discharge decisions.20 Moreover, both alvimopan doses produced a significant decrease in the incidence of POI as an SAE and a significant reduction in the proportion of patients who had prolonged hospital stay or readmission. Furthermore, alvimopan 12 mg significantly reduced, by almost 50%, the proportion of patients with prolonged hospital stay, manifested by a DCO written ≥7 days after surgery. The majority of AEs that resulted in DCO written ≥7 days after BR surgery were GI-related in the placebo group and non–GI-related in alvimopan groups, underscoring the potential benefit derived from alvimopan treatment with regard to GI-related adverse events influencing prolonged hospital stay. Alvimopan was generally well tolerated, and pain scores and opioid consumption were similar across all treatment groups. Adverse events were mainly GI-related and expected for patients undergoing BR and receiving postoperative opioid-based analgesia. Most patients in this study received opioid-based anesthetics and analgesia, which contributed to the observed incidence of postoperative nausea and other GI-related adverse events in this study. However, nausea and other adverse events were not more common in either alvimopan group compared with the placebo group. Therefore, alvimopan did not increase the rate of postoperative GI-related adverse events above that of placebo. Overall, the TEAE profile of alvimopan was comparable with that of placebo, and patients treated with alvimopan had a lower incidence of GI-related TEAEs.
The 3 clinical trials included in this analysis strictly implemented a postoperative management protocol for the acceleration of GI recovery. Multimodal management protocols that integrate the use of minimally invasive surgical techniques (eg, laparoscopy instead of laparotomy), opioid-sparing analgesia, early oral nutrition, and physical rehabilitation have been reported to significantly accelerate postoperative GI recovery and reduce LOS.21 However, such practices may result in an increased rate of hospital readmission,22 and opioids provide better pain control compared with other analgesics such as nonsteroidal anti-inflammatory drugs.23 Moreover, in Cochrane Database analyses, although epidural administration of local anesthetics was found to accelerate GI recovery and reduce nausea after abdominal surgery compared with epidural or systemic opioids, it did not reduce LOS compared with patient-controlled opioid analgesia.24,25 However, encouraging early oral nutrition and ambulation in patients undergoing BR or complex abdominal or pelvic colorectal surgery not only accelerated GI recovery but resulted in shorter LOS compared with traditional care and no increase in rehospitalization rates in 2 single-center studies.6,26 Indeed, early oral nutrition and ambulation were encouraged in the alvimopan phase III trials, and the resulting median time to DCO written (6 days) after BR compares favorably with the 7-day mean LOS after colonic surgery that was reported in a recent survey of U.S. clinical practice.27 Therefore, the benefits of alvimopan were in addition to those provided by improved postoperative care pathways.
This pooled analysis of all BR patients in the alvimopan phase III efficacy trial database provides an assessment of GI recovery patterns in patients undergoing BR managed by an accelerated postoperative care pathway. In contrast with the 3 individual trials, which enrolled heterogeneous surgery populations with varying patterns of GI recovery,17–19 the current analysis pooled BR patients from all 3 trials, providing a homogeneous surgery population. In the individual trials, statistical significance and magnitude of effect were inconsistent with respect to GI recovery endpoints in the overall MITT population (BR and TAH). The analysis of pooled BR data revealed that alvimopan provided significant benefit at both doses, as assessed by the time to GI-3 and GI-2 recovery, readiness for HD, and hospital DCO-written endpoints. These results confirm and extend those already reported for the individual trials. Moreover, pooling data allowed further investigation of the potential differences between alvimopan doses. Indeed, alvimopan 12 mg consistently demonstrated greater benefit compared with alvimopan 6 mg for GI recovery, readiness for HD, and hospital DCO written. Alvimopan 12 mg also demonstrated greater benefit in female patients and older patients (age >65 years).
Pooling data allowed further insight into less frequent but clinically important postoperative morbidities and hospital readmission rates. Importantly, the rate of anastomotic leak was low and comparable for all groups, supporting the current view that accelerating GI recovery does not compromise healing of the anastomosis.15 Alvimopan reduced the incidence of clinically important events that are known to increase patient morbidity. Although the severity of GI-associated discomfort is difficult to assess objectively, postoperative NGT insertion is an indication that the patient is experiencing symptoms severe enough to warrant intervention. Both doses of alvimopan significantly reduced the proportion of patients who had postoperative NGT insertion compared with placebo. Moreover, fewer alvimopan-treated patients had POI as an SAE and POI or EPSBO as an SAE. Another indication of postoperative morbidity is prolongation of planned hospital stay or readmission, both of which were reduced with alvimopan treatment. Furthermore, both alvimopan groups had significant reductions of mean time to DCO written compared with the placebo group. These significant reductions were calculated based on the difference between the time of surgical procedure and discharge order written. Reduction in length of hospital stay based on day of hospital admission to day of hospital discharge was approximately 1 day (alvimopan 6 mg, 1 day; alvimopan 12 mg, 0.9 days). In addition to reduced cost associated with occupancy of hospital beds, earlier departure may mean a reduction in monitoring requirements and nursing support. Moreover, earlier departure from the hospital could be meaningful to patients. These reductions and the reduction in hospital readmissions suggest that alvimopan also reduces the average overall hospitalization time associated with BR surgery.
In theory, pooled analyses may be confounded by differences in randomization criteria between component trials, and individual study effects may be obscured. However, the phase III trials used for this analysis were all of similar design and used BR as a randomization criterion. Furthermore, a key strength of this study was that all patients were treated with an accelerated care pathway practiced at some specialized centers to facilitate GI recovery.7,8 Indeed, the length of hospital stay for the placebo group in this analysis was 5 days less than the national average.1 Thus, alvimopan provided benefits beyond those achieved by the accelerated care pathway alone.
CONCLUSION
In the pooled analysis of the phase III efficacy studies, alvimopan accelerated GI recovery and reduced postoperative morbidity and readmission after BR surgery.
ACKNOWLEDGMENTS
We would like to thank Amy Rachfal, PhD; ProEd Communications, Inc.; for her medical editorial assistance with this manuscript.
The following investigators and institutions participated in the Alvimopan Postoperative Ileus Study Group for studies 14CL302, 14CL308, and 14CL313: Herand Abcarian, MD, University of Illinois at Chicago, Chicago, IL; Shireen Ahmad, MD, Northwestern University Medical School, Chicago, IL; H. Randolph Bailey, MD, Baylor College of Medicine, Houston, TX; Paul Baron, MD, Discovery Alliance, Inc., Mt. Pleasant, SC; James Barone, MD, The Stamford Hospital, Stamford, CT; Joel Bauer, MD, Mount Sinai School of Medicine, New York, NY; P. Sue Beckwith, MD, The Iowa Clinic, Des Moines, IA; Maria Bell, MD, South Dakota Health Research Foundation, Sioux Falls, SD; Eric Bieber, MD, Geisinger Medical Center, Danville, PA; Sander R. Binderow, MD, Atlanta Colon and Rectal Surgeons, Atlanta, GA; Casey Blitt, MD, Tucson Medican Center, Tucson, AZ; Christine Brody, MD, The Damluji Research Center Medical Center, Oceanside, CA; Lance Bruck, MD, Jacobi Medical Center, Bronx, NY; W. Donald Buie, MD, University of Calgary, Calgary, AB; Thomas Cataldo, MD, Cooper Health System/University Medical Center, Camden, NJ; Michael A. Choti, MD, Johns Hopkins Hospital, Baltimore, MD; Colm Cole, MD, St. Paul's Hospital, Vancouver, BC; Robert L. Coleman, MD, Parkland Health and Hospital System, Dallas, TX; Gene Coppa, MD, Staten Island University Hospital, Staten Island, NY; Daniel Dempsey, MD, Temple University Hospital, Philadelphia, PA; Daniel Dent, MD, University of Texas Health Science Center at San Antonio, San Antonio, TX; Robert Donoway, MD, Horizon Institute for Clinical Research, Hollywood, FL; Richard C. Earnhardt, MD, Surgical Associates of Fredericksburg, Fredericksburg, VA; Warren E. Enker, MD, Beth Israel Medical Center, New York, NY; Edward J. Eyring, II, MD, Wasatch Clinical Research, Salt Lake City, UT; Samir Fakhry, MD, INOVA Fairfax Hospital, Falls Church, VA; Linda Farkas, MD, UPMC Cancer Pavilion, Pittsburgh, PA; Michael Ferrante, MD, UCLA Medical Center, Los Angeles, CA; Stephanie Fine, MD, West Jordan, UT; James W. Fleshman, MD, Washington University School of Medicine, St. Louis, MO; Philip Fleshner, MD, Cedars-Sinai Medical Center, Los Angeles, CA; Robert D. Fry, MD, Hospital of the University of Pennsylvania, Philadelphia, PA; Gerard J. Fulda, MD, Christiana Care Health System, Newark, DE; Susan Galandiuk, MD, University of Louisville Hospital, Louisville, KY; Gary Gecelter, MD, North Shore – Long Island Jewish Health System, New Hyde Park, NY; Todd M. Gerkin, MD, Central Carolina Surgery, PA, Greensboro, NC; Bimal Ghosh, MD, New York Harbor Healthcare System – Brooklyn Campus, Brooklyn, NY; Stephen Gordon, MD, Comprehensive NeuroScience, Inc., Atlanta, GA; Richard H. Greenberg, MD, Albert Einstein Medical Center, Philadelphia, PA; Roy Greengrass, MD, Mayo Clinic Jacksonville, Jacksonville, FL; Francis J. Harford, MD, Loyola University Medical Center, Maywood, IL; William E. Haun, MD, Exempla Saint Joseph Hospital, Denver, CO; Michael D. Holzman, MD, Vanderbilt University Hospital, Nashville, TN; Neil H. Hyman, MD, Fletcher Allen Health Care, Burlington, VT; Matthew Indeck, MD, Geisinger Medical Center, Danville, PA; Gaby A. P. Iskander, MD, Marshfield Clinic, Saint Joseph Hospital, Marshfield, WI; Ivan Kangrga, MD, Washington University Medical Center, St. Louis, MO; Donald G. Kim, MD, Michigan Medical PC/Ferguson Clinic, Grand Rapids, MI; Andrea Kurz, MD, Washington University Medical Center, St. Louis, MO; Edward Lee, MD, Albany Medical College, Albany, NY; Warren Lichliter, MD, Sammons Cancer Center, Dallas, TX; Daniel G. Lorch, Jr., MD, PAB Clinical Research, Brandon, FL; Kirk Ludwig, MD, Duke University Medical Center, Durham, NC; Robert D. Madoff, MD, Abbott Northwestern Hospital, Minneapolis, MN; Robert Martindale, MD, Medical College of Georgia, Augusta, GA; Martin McCarter, MD, Denver, CO; Ronald Medak, MD, The Damluji Research Center Medical Center, Oceanside, CA; Andrew Menzin, MD, North Shore University Hospital, Manhasset, NY; Fabrizio Michelassi, MD, University of Chicago Medical Center, Chicago, IL; Edward Michna, MD, Brigham & Women's Hospital, Boston, MA; Brent Miedema, MD, Columbia, MO; Benjie B. Mills, MD, Center for Women's Medicine, Greenville, SC; Deborah Nagle, MD, Graduate Hospital, Philadelphia, PA; Wade Naziri, MD, Carolina Research Center, Greenville, NC; Lars Newsome, MD, Scripps Memorial Hospital, La Jolla, CA; Bruce Orkin, MD, George Washington University Hospital, Washington, DC; Craig A. Paterson, MD, UMASS Medical Center, Worcester, MA; C. Richard Patterson, MD, Baptist Memorial Hospital, Memphis, TN; Michael Pearl, MD, State University of New York at Stony Brook, Stony Brook, NY; Mark Pello, MD, Cooper Health System, Camden, NJ; William B. Perry, MD, Wilford Hall Medical Center, Lackland AFB, TX; Alexander Pue, MD, Sharp Mary Birch Hospital for Women, San Diego, CA; Janice Rafferty, MD, University of Cincinnati College of Medicine, Cincinnati, OH; Mikhail I. Rakhmanine, MD, Lehigh Valley Hospital, Allentown, PA; Jebadurai Ratnaraj, MD, Washington University Medical Center, St. Louis, MO; Thanjuvar Ravikumar, MD, Montefiore Medical Center, Bronx, NY; Harry Reynolds, MD, University Hospitals of Cleveland, Cleveland, OH; Miguel A. Rodriguez-Bigas, MD, MD Anderson Cancer Center, Houston, TX; Rabih Salloum, MD, University of Rochester Medical Center, Rochester, NY; Armando Sardi, MD, St. Agnes HealthCare, Baltimore, MD; Steven Schechter, MD, Rhode Island Colorectal Clinic, Providence, RI; Paul W. Schroeder, MD, Medford Women's Clinic, LLP, Medford, OR; James V. Sitzmann, MD, University of Rochester Medical Center, Rochester, NY; Ronald P. Spencer, MD, Renstar Medical Research, Ocala, FL; Thomas Stahl, MD, Washington Hospital Center, Washington, DC; Raymond J. Staniunas, MD, Scott & White Memorial Hospital & Clinic, Temple, TX; John Daniel Stanley, MD, University of TN College of Medicine, Chattanooga, TN; Richard A. Steinbrook, MD, Beth Israel Deaconess Medical Center, Boston, MA; Kurt Stockamp, MD, Pensacola, FL; Theresa M. Terem, MD, Mt. Ranier Surgical Associates, Tacoma, WA; Michael L. Twede, MD, Salt Lake Women's Center, Salt Lake City, UT; Stephen Unger, MD, Mt. Sinai Medical Center, Miami Beach, FL; Anthony Vernava, MD, Colon/Rectal Surgery, Cleveland Clinic, Naples, FL; Eugene Viscusi, MD, Thomas Jefferson University Hospitals, Philadelphia, PA; Brian Warriner, MD, Vancouver Hospital, Vancouver, BC; Harry Joseph Wasvary, MD, Oakland Colon and Rectal Associates, Royal Oak, MI; James L. Weese, MD, UMDNJ-School of Osteopathic Medicine, Stratford, NJ; Carl J. Wescott, MD, Wake Forest University School of Medicine, Winston-Salem, NC; Steven Wexner, MD, Cleveland Clinic, Weston, FL; Barth L. Wilsey, MD, University of California Davis Medical Center, Davis, CA; Bruce G. Wolff, MD, Mayo Clinic, Rochester Methodist Hospital, Rochester, MN; Sherry Wren, MD, Palo Alto Veterans Health Care System, Palo Alto, CA; Richard T. Zera, MD, PhD, Hennepin County Medical Center, Minneapolis, MN.
Footnotes
Reprints: Conor P. Delaney, MD, PhD, FRCSI, FACS, Division of Colorectal Surgery, Department of Surgery, Case Western Reserve University, University Hospitals of Cleveland, 11100 Euclid Avenue, Cleveland, OH 44106-5047. E-mail: conor.delaney@uhhs.com.
REFERENCES
- 1.Healthcare Costs and Utilization Project (HCUP). 2002 National Statistics. Available at: http://www.ahrq.gov/HCUPnet. Accessed March 18, 2005.
- 2.Livingston EH, Passaro EP Jr. Postoperative ileus. Dig Dis Sci. 1990;35:121–132. [DOI] [PubMed] [Google Scholar]
- 3.Behm B, Stollman N. Postoperative ileus: etiologies and interventions. Clin Gastroenterol Hepatol. 2003;1:71–80. [DOI] [PubMed] [Google Scholar]
- 4.Resnick J, Greenwald DA, Brandt LJ. Delayed gastric emptying and postoperative ileus after nongastric abdominal surgery: part II. Am J Gastroenterol. 1997;92:934–940. [PubMed] [Google Scholar]
- 5.Schmitt SL, Cohen SM, Wexner SD, et al. Does laparoscopic-assisted ileal pouch anal anastomosis reduce the length of hospitalization? Int J Colorectal Dis. 1994;9:134–137. [DOI] [PubMed] [Google Scholar]
- 6.Delaney CP, Fazio VW, Senagore AJ, et al. ‘Fast track’ postoperative management protocol for patients with high co-morbidity undergoing complex abdominal and pelvic colorectal surgery. Br J Surg. 2001;88:1533–1538. [DOI] [PubMed] [Google Scholar]
- 7.Miedema BW, Johnson JO. Methods for decreasing postoperative gut dysmotility. Lancet Oncol. 2003;4:365–372. [DOI] [PubMed] [Google Scholar]
- 8.Correia MI, da Silva RG. The impact of early nutrition on metabolic response and postoperative ileus. Curr Opin Clin Nutr Metab Care. 2004;7:577–583. [DOI] [PubMed] [Google Scholar]
- 9.Basse L, Thorbol JE, Lossl K, et al. Colonic surgery with accelerated rehabilitation or conventional care [published correction appears in: Dis Colon Rectum. 2004;47:951]. Dis Colon Rectum. 2004;47:271–277. [DOI] [PubMed] [Google Scholar]
- 10.Basse L, Hjort Jakobsen D, Billesbolle P, et al. A clinical pathway to accelerate recovery after colonic resection. Ann Surg. 2000;232:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiran RP, Delaney CP, Senagore AJ, et al. Outcomes and prediction of hospital readmission after intestinal surgery. J Am Coll Surg. 2004;198:877–883. [DOI] [PubMed] [Google Scholar]
- 12.Holte K, Kehlet H. Postoperative ileus: progress towards effective management. Drugs. 2002;62:2603–2615. [DOI] [PubMed] [Google Scholar]
- 13.Luckey A, Livingston E, Taché Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2003;138:206–214. [DOI] [PubMed] [Google Scholar]
- 14.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671. [DOI] [PubMed] [Google Scholar]
- 15.Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000;87:1480–1493. [DOI] [PubMed] [Google Scholar]
- 16.Austrup ML, Korean G. Analgesic agents for the postoperative period. Opioids. Surg Clin North Am. 1999;79:253–273. [DOI] [PubMed] [Google Scholar]
- 17.Wolff BG, Michelassi F, Gerkin TM, et al. Alvimopan, a novel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg. 2004;240:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaney CP, Weese JL, Hyman NH, et al. Phase III trial of alvimopan, a novel, peripherally acting, mu opioid antagonist, for postoperative ileus after major abdominal surgery. Dis Colon Rectum. 2005;48:1114–1125. [DOI] [PubMed] [Google Scholar]
- 19.Viscusi ER, Goldstein S, Witkowski T, et al. Double-blind, randomized, placebo-controlled, phase III clinical trial of alvimopan for the management of postoperative ileus (POI) following major abdominal surgery (Study 14CL308) [Abstract No. S075]. Presented at the Society of American Gastrointestinal and Endoscopic Surgeons, Hollywood, FL, April 14–17, 2005.
- 20.Bungard TJ, Kale-Pradhan PB. Prokinetic agents for the treatment of postoperative ileus in adults: a review of the literature. Pharmacotherapy. 1999;19:416–423. [DOI] [PubMed] [Google Scholar]
- 21.Kehlet H, Wilmore DW. Fast-track surgery. Br J Surg. 2005;92:3–4. [DOI] [PubMed] [Google Scholar]
- 22.Hjort Jakobsen D, Sonne E, Basse L, et al. Convalescence after colonic resection with fast-track versus conventional care. Scan J Surg. 2004;93:24–28. [DOI] [PubMed] [Google Scholar]
- 23.Cepeda MS, Carr DB, Miranda N, et al. Comparison of morphine, ketorolac, and their combination for postoperative pain: results from a large, randomized, double-blind trial. Anesthesiology. 2005;103:1225–1232. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen H, Wetterslev J, Moiniche S, et al. Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev. 2001;CD001893. [DOI] [PubMed] [Google Scholar]
- 25.Werawatganon T, Charuluxanuun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev. 2005;Issue 1:CD004088. [DOI] [PubMed]
- 26.Delaney CP, Zutshi M, Senagore AJ, et al. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum. 2003;46:851–859. [DOI] [PubMed] [Google Scholar]
- 27.Kehlet H, Büchler MW, Beart RW, et al. Care after colonic operation-is it evidence-based? Results from a multinational survey in Europe and the United States. J Am Coll Surg. 2006;202:45–54. [DOI] [PubMed] [Google Scholar]







