Abstract
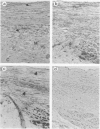
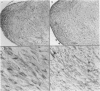
Association of group A streptococci with acute rheumatic fever and valvular heart disease is well established; however the basis of valve injury remains unclear. In this study, anti-streptococcal monoclonal antibodies (MAbs) cross-reactive with myocardium were reacted with sections from 22 rheumatic valves, nine normal, five endocarditic, one 'floppy,' and one Marfan valve. In immunohistochemical studies, MAb reactivity was observed with cardiac myocytes, smooth muscle cells, cell surface and cytoplasm of endothelial cells lining valves, and valvular interstitial cells. Endothelial basement membrane and elastin fibrils reacted with the MAbs, whereas collagen was unreactive. Similar reactivity was seen with sera from acute rheumatic fever patients. The anti-streptococcal MAbs reacted with intravalvular myosin and vimentin in Western blots, and purified elastin competitively inhibited the binding of the anti-streptococcal MAbs to whole group A streptococci. The data show that human heart valves have numerous sites of immunoreactivity with anti-streptococcal MAbs and acute rheumatic fever sera of potential importance in the pathogenesis of rheumatic valvular injury.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André-Schwartz J., Datta S. K., Shoenfeld Y., Isenberg D. A., Stollar B. D., Schwartz R. S. Binding of cytoskeletal proteins by monoclonal anti-DNA lupus autoantibodies. Clin Immunol Immunopathol. 1984 May;31(2):261–271. doi: 10.1016/0090-1229(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Clark W. A., Jr, Chizzonite R. A., Everett A. W., Rabinowitz M., Zak R. Species correlations between cardiac isomyosins. A comparison of electrophoretic and immunological properties. J Biol Chem. 1982 May 25;257(10):5449–5454. [PubMed] [Google Scholar]
- Cunningham M. W., Hall N. K., Krisher K. K., Spanier A. M. A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J Immunol. 1986 Jan;136(1):293–298. [PubMed] [Google Scholar]
- Cunningham M. W., Krisher K., Graves D. C. Murine monoclonal antibodies reactive with human heart and group A streptococcal membrane antigens. Infect Immun. 1984 Oct;46(1):34–41. doi: 10.1128/iai.46.1.34-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., McCormack J. M., Fenderson P. G., Ho M. K., Beachey E. H., Dale J. B. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J Immunol. 1989 Oct 15;143(8):2677–2683. [PubMed] [Google Scholar]
- Cunningham M. W., McCormack J. M., Talaber L. R., Harley J. B., Ayoub E. M., Muneer R. S., Chun L. T., Reddy D. V. Human monoclonal antibodies reactive with antigens of the group A Streptococcus and human heart. J Immunol. 1988 Oct 15;141(8):2760–2766. [PubMed] [Google Scholar]
- Cunningham M. W., Russell S. M. Study of heart-reactive antibody in antisera and hybridoma culture fluids against group A streptococci. Infect Immun. 1983 Nov;42(2):531–538. doi: 10.1128/iai.42.2.531-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., Swerlick R. A. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J Exp Med. 1986 Oct 1;164(4):998–1012. doi: 10.1084/jem.164.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Epitopes of streptococcal M proteins shared with cardiac myosin. J Exp Med. 1985 Aug 1;162(2):583–591. doi: 10.1084/jem.162.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Protective antigenic determinant of streptococcal M protein shared with sarcolemmal membrane protein of human heart. J Exp Med. 1982 Oct 1;156(4):1165–1176. doi: 10.1084/jem.156.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Sequence of myosin-crossreactive epitopes of streptococcal M protein. J Exp Med. 1986 Nov 1;164(5):1785–1790. doi: 10.1084/jem.164.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenderson P. G., Fischetti V. A., Cunningham M. W. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989 Apr 1;142(7):2475–2481. [PubMed] [Google Scholar]
- Filip D. A., Radu A., Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 1986 Sep;59(3):310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Giro M. G., Davidson J. M. Elastin. Methods Enzymol. 1988;163:656–673. [PubMed] [Google Scholar]
- Gross L., Kugel M. A. Topographic Anatomy and Histology of the Valves in the Human Heart. Am J Pathol. 1931 Sep;7(5):445–474.7. [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- KAPLAN M. H., BOLANDE R., RAKITA L., BLAIR J. PRESENCE OF BOUND IMMUNOGLOBULINS AND COMPLEMENT IN THE MYOCARDIUM IN ACUTE RHEUMATIC FEVER. ASSOCIATION WITH CARDIAC FAILURE. N Engl J Med. 1964 Sep 24;271:637–645. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- KAPLAN M. H. IMMUNOLOGIC RELATION OF STREPTOCOCCAL AND TISSUE ANTIGENS. I. PROPERTIES OF AN ANTIGEN IN CERTAIN STRAINS OF GROUP A STREPTOCOCCI EXHIBITING AN IMMUNOLOGIC CROSS-REACTION WITH HUMAN HEART TISSUE. J Immunol. 1963 Apr;90:595–606. [PubMed] [Google Scholar]
- Kraus W., Ohyama K., Snyder D. S., Beachey E. H. Autoimmune sequence of streptococcal M protein shared with the intermediate filament protein, vimentin. J Exp Med. 1989 Feb 1;169(2):481–492. doi: 10.1084/jem.169.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus W., Seyer J. M., Beachey E. H. Vimentin-cross-reactive epitope of type 12 streptococcal M protein. Infect Immun. 1989 Aug;57(8):2457–2461. doi: 10.1128/iai.57.8.2457-2461.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisher K., Cunningham M. W. Myosin: a link between streptococci and heart. Science. 1985 Jan 25;227(4685):413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder J., Ye Y. L., Harrington D. S., Armitage J. O., Weisenburger D. D. Monoclonal antibodies marking T lymphocytes in paraffin-embedded tissue. Am J Pathol. 1987 Apr;127(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Sequence homology of group A streptococcal Pep M5 protein with other coiled-coil proteins. Biochem Biophys Res Commun. 1986 Oct 30;140(2):684–690. doi: 10.1016/0006-291x(86)90786-2. [DOI] [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980 Mar 1;151(3):695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Trus B. L., Fischetti V. A. Presence of two distinct regions in the coiled-coil structure of the streptococcal Pep M5 protein: relationship to mammalian coiled-coil proteins and implications to its biological properties. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1064–1068. doi: 10.1073/pnas.82.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu N., Rose N. R., Beisel K. W., Herskowitz A., Gurri-Glass G., Craig S. W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- PARTRIDGE S. M., DAVIS H. F., ADAIR G. S. The chemistry of connective tissues. 2. Soluble proteins derived from partial hydrolysis of elastin. Biochem J. 1955 Sep;61(1):11–21. doi: 10.1042/bj0610011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle J. M., Wang C. H., Magilligan D. J., Jr, Stein P. D. Scanning electron microscopy of surgically excised human mitral valves in patients over 45 years of age. Am J Cardiol. 1989 Feb 15;63(7):471–477. doi: 10.1016/0002-9149(89)90322-6. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Goldman R. D. Isolation and preliminary characterization of 10-nm filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2422–2426. doi: 10.1073/pnas.74.6.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Rosenfelder G., Wieslander J., Avila J. L., Rojas M., Szarfman A., Esser K., Nowack H., Timpl R. Circulating antibodies to mouse laminin in Chagas disease, American cutaneous leishmaniasis, and normal individuals recognize terminal galactosyl(alpha 1-3)-galactose epitopes. J Exp Med. 1987 Aug 1;166(2):419–432. doi: 10.1084/jem.166.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Varon D., Linder S., Gembom E., Guedg L., Langbeheim H., Berrebi A., Eshhar Z. Human monoclonal antibody derived from an autoimmune thrombocytopenic purpura patient, recognizing an intermediate filament's determinant common to vimentin and desmin. Clin Immunol Immunopathol. 1990 Mar;54(3):454–468. doi: 10.1016/0090-1229(90)90058-x. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Ostlund R. E., Pastan I. Myosin is a component of the cell surface of cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4144–4148. doi: 10.1073/pnas.71.10.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. Endothelial cell monolayer integrity. I. Characterization of dense peripheral band of microfilaments. Arteriosclerosis. 1986 Mar-Apr;6(2):212–219. doi: 10.1161/01.atv.6.2.212. [DOI] [PubMed] [Google Scholar]
- Zabriskie J. B., Freimer E. H. An immunological relationship between the group. A streptococcus and mammalian muscle. J Exp Med. 1966 Oct 1;124(4):661–678. doi: 10.1084/jem.124.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B. The relationship of streptococcal cross-reactive antigens to rheumatic fever. Transplant Proc. 1969 Dec;1(4):968–975. [PubMed] [Google Scholar]