Abstract
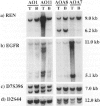
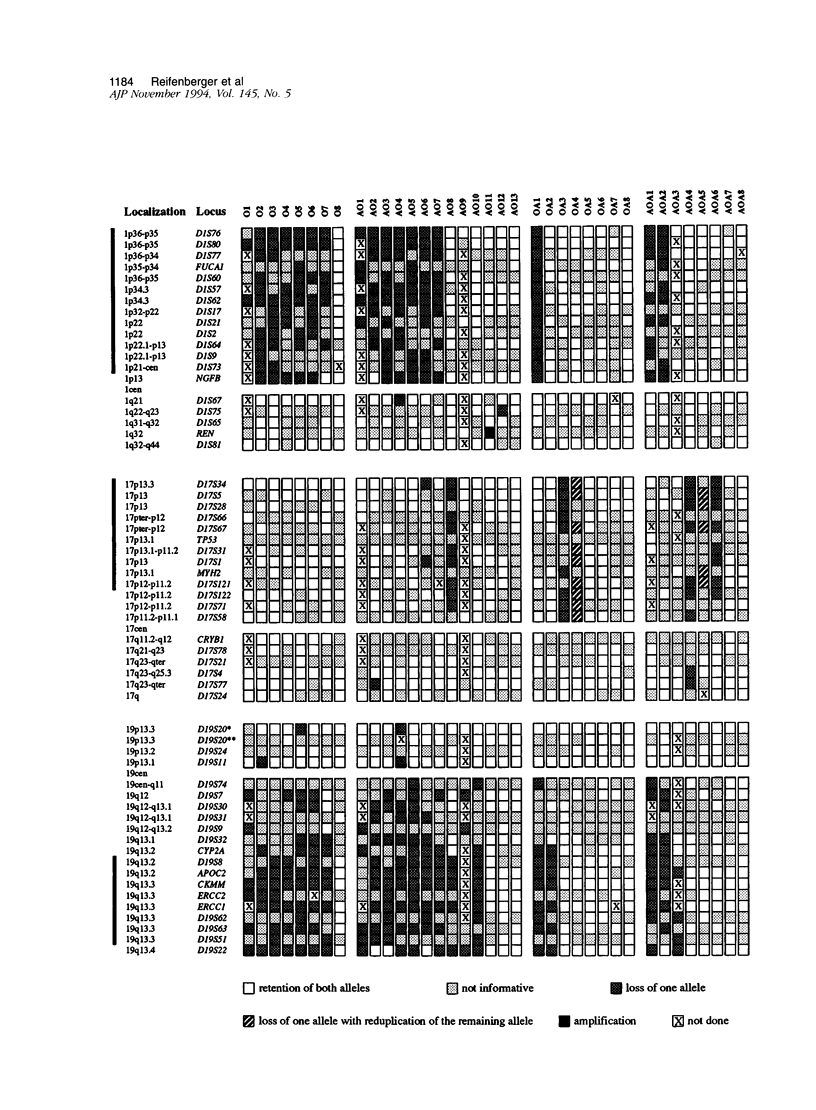
The molecular genetic alterations of oligodendroglial tumors and mixed gliomas of the central nervous system were studied in a series of 37 cases (8 oligodendrogliomas, 13 anaplastic oligodendrogliomas, 8 oligoastrocytomas, and 8 anaplastic oligoastrocytomas). A total of 180 polymorphic loci and 5 nonpolymorphic gene loci, distributed over all chromosomes, were examined by restriction fragment length polymorphism analysis. Loss of heterozygosity was most frequently observed for loci on 19q with a commonly deleted region at 19q13.2-q13.4 distal to the CYP2a gene and proximal to the D19S22 locus. The incidence of allelic loss on 19q was particularly high (81%) in oligodendroglial tumors and equal to 31% in mixed gliomas. More than 75% of the tumors with allelic deletions on 19q also showed loss of heterozygosity for loci on 1p with one tumor showing only loss of alleles distal to the NGFB gene (1p13-pter). Seven (19%) tumors had lost alleles from 17p with the deleted region including the TP53 tumor suppressor gene in all cases. Sequencing of the TP53 transcripts from exons 2 to 10, however, did not reveal mutations of the remaining allele in any of these tumors. Anaplastic oligodendrogliomas and anaplastic oligoastrocytomas demonstrated an increased incidence of additional allelic losses involving most frequently chromosomes 9p and 10. Gene amplification was detected in two anaplastic tumors, affecting the epidermal growth factor receptor gene in both cases, with additional amplification of the renin gene at 1q32 in one of these cases. In total our results indicate both differences and similarities between the molecular genetic alterations in tumors with oligodendroglial and astrocytic differentiation. The loss of genetic information from 19q and 1p as well as the rarity of TP53 mutations in oligodendroglial tumors suggests that the early events in their oncogenesis are distinct from those associated with astrocytic tumors. However, similarities are indicated by the allelic losses on 9p and 10 in the anaplastic tumors, suggesting the utilization of common pathways of progression.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardi G., Pandis N., Fenger C., Kronborg O., Bomme L., Heim S. Deletion of 1p36 as a primary chromosomal aberration in intestinal tumorigenesis. Cancer Res. 1993 Apr 15;53(8):1895–1898. [PubMed] [Google Scholar]
- Bergerheim U., Nordenskjöld M., Collins V. P. Deletion mapping in human renal cell carcinoma. Cancer Res. 1989 Mar 15;49(6):1390–1396. [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Bigner D. D. Cytogenetics of human brain tumors. Cancer Genet Cytogenet. 1990 Jul 15;47(2):141–154. doi: 10.1016/0165-4608(90)90024-5. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Burger P. C., Mahaley M. S., Jr, Bullard D. E., Muhlbaier L. H., Bigner D. D. Specific chromosomal abnormalities in malignant human gliomas. Cancer Res. 1988 Jan 15;48(2):405–411. [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Friedman H. S., Biegel J. A., Bigner D. D. Structural chromosomal abnormalities in human medulloblastoma. Cancer Genet Cytogenet. 1988 Jan;30(1):91–101. doi: 10.1016/0165-4608(88)90096-9. [DOI] [PubMed] [Google Scholar]
- Bièche I., Champème M. H., Matifas F., Cropp C. S., Callahan R., Lidereau R. Two distinct regions involved in 1p deletion in human primary breast cancer. Cancer Res. 1993 May 1;53(9):1990–1994. [PubMed] [Google Scholar]
- Brodeur G. M., Fong C. T. Molecular biology and genetics of human neuroblastoma. Cancer Genet Cytogenet. 1989 Sep;41(2):153–174. doi: 10.1016/0165-4608(89)90243-4. [DOI] [PubMed] [Google Scholar]
- Chen P., Ellmore N., Weissman B. E. Functional evidence for a second tumor suppressor gene on human chromosome 17. Mol Cell Biol. 1994 Jan;14(1):534–542. doi: 10.1128/mcb.14.1.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung R., Whaley J., Kley N., Anderson K., Louis D., Menon A., Hettlich C., Freiman R., Hedley-Whyte E. T., Martuza R. TP53 gene mutations and 17p deletions in human astrocytomas. Genes Chromosomes Cancer. 1991 Sep;3(5):323–331. doi: 10.1002/gcc.2870030502. [DOI] [PubMed] [Google Scholar]
- Coles C., Thompson A. M., Elder P. A., Cohen B. B., Mackenzie I. M., Cranston G., Chetty U., Mackay J., Macdonald M., Nakamura Y. Evidence implicating at least two genes on chromosome 17p in breast carcinogenesis. Lancet. 1990 Sep 29;336(8718):761–763. doi: 10.1016/0140-6736(90)93236-i. [DOI] [PubMed] [Google Scholar]
- Collins V. P. Amplified genes in human gliomas. Semin Cancer Biol. 1993 Feb;4(1):27–32. [PubMed] [Google Scholar]
- Collins V. P., James C. D. Gene and chromosomal alterations associated with the development of human gliomas. FASEB J. 1993 Jul;7(10):926–930. doi: 10.1096/fasebj.7.10.8344489. [DOI] [PubMed] [Google Scholar]
- Devilee P., van Vliet M., Bardoel A., Kievits T., Kuipers-Dijkshoorn N., Pearson P. L., Cornelisse C. J. Frequent somatic imbalance of marker alleles for chromosome 1 in human primary breast carcinoma. Cancer Res. 1991 Feb 1;51(3):1020–1025. [PubMed] [Google Scholar]
- Dracopoli N. C., Harnett P., Bale S. J., Stanger B. Z., Tucker M. A., Housman D. E., Kefford R. F. Loss of alleles from the distal short arm of chromosome 1 occurs late in melanoma tumor progression. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4614–4618. doi: 10.1073/pnas.86.12.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand A. J., James C. D., Cavenee W. K., Seliger B., Pettersson R. F., Collins V. P. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991 Apr 15;51(8):2164–2172. [PubMed] [Google Scholar]
- Frankel R. H., Bayona W., Koslow M., Newcomb E. W. p53 mutations in human malignant gliomas: comparison of loss of heterozygosity with mutation frequency. Cancer Res. 1992 Mar 15;52(6):1427–1433. [PubMed] [Google Scholar]
- Fults D., Brockmeyer D., Tullous M. W., Pedone C. A., Cawthon R. M. p53 mutation and loss of heterozygosity on chromosomes 17 and 10 during human astrocytoma progression. Cancer Res. 1992 Feb 1;52(3):674–679. [PubMed] [Google Scholar]
- Fults D., Tippets R. H., Thomas G. A., Nakamura Y., White R. Loss of heterozygosity for loci on chromosome 17p in human malignant astrocytoma. Cancer Res. 1989 Dec 1;49(23):6572–6577. [PubMed] [Google Scholar]
- Griffin C. A., Long P. P., Carson B. S., Brem H. Chromosome abnormalities in low-grade central nervous system tumors. Cancer Genet Cytogenet. 1992 May;60(1):67–73. doi: 10.1016/0165-4608(92)90235-z. [DOI] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Dumanski J. P., Hansen M., Nordenskjold M., Collins V. P., Cavenee W. K. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988 Oct 1;48(19):5546–5551. [PubMed] [Google Scholar]
- James C. D., Carlbom E., Nordenskjold M., Collins V. P., Cavenee W. K. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2858–2862. doi: 10.1073/pnas.86.8.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C. D., He J., Carlbom E., Nordenskjold M., Cavenee W. K., Collins V. P. Chromosome 9 deletion mapping reveals interferon alpha and interferon beta-1 gene deletions in human glial tumors. Cancer Res. 1991 Mar 15;51(6):1684–1688. [PubMed] [Google Scholar]
- Jenkins R. B., Kimmel D. W., Moertel C. A., Schultz C. G., Scheithauer B. W., Kelly P. J., Dewald G. W. A cytogenetic study of 53 human gliomas. Cancer Genet Cytogenet. 1989 Jun;39(2):253–279. doi: 10.1016/0165-4608(89)90192-1. [DOI] [PubMed] [Google Scholar]
- Leister I., Weith A., Brüderlein S., Cziepluch C., Kangwanpong D., Schlag P., Schwab M. Human colorectal cancer: high frequency of deletions at chromosome 1p35. Cancer Res. 1990 Nov 15;50(22):7232–7235. [PubMed] [Google Scholar]
- Lindström E., Salford L. G., Heim S., Mandahl N., Strömblad S., Brun A., Mitelman F. Trisomy 7 and sex chromosome loss need not be representative of tumor parenchyma cells in malignant glioma. Genes Chromosomes Cancer. 1991 Nov;3(6):474–479. doi: 10.1002/gcc.2870030610. [DOI] [PubMed] [Google Scholar]
- Ludwig C. L., Smith M. T., Godfrey A. D., Armbrustmacher V. W. A clinicopathological study of 323 patients with oligodendrogliomas. Ann Neurol. 1986 Jan;19(1):15–21. doi: 10.1002/ana.410190104. [DOI] [PubMed] [Google Scholar]
- Mørk S. J., Lindegaard K. F., Halvorsen T. B., Lehmann E. H., Solgaard T., Hatlevoll R., Harvei S., Ganz J. Oligodendroglioma: incidence and biological behavior in a defined population. J Neurosurg. 1985 Dec;63(6):881–889. doi: 10.3171/jns.1985.63.6.0881. [DOI] [PubMed] [Google Scholar]
- Nijjar T. S., Simpson W. J., Gadalla T., McCartney M. Oligodendroglioma. The Princess Margaret Hospital experience (1958-1984). Cancer. 1993 Jun 15;71(12):4002–4006. doi: 10.1002/1097-0142(19930615)71:12<4002::aid-cncr2820711234>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Ohgaki H., Eibl R. H., Wiestler O. D., Yasargil M. G., Newcomb E. W., Kleihues P. p53 mutations in nonastrocytic human brain tumors. Cancer Res. 1991 Nov 15;51(22):6202–6205. [PubMed] [Google Scholar]
- Ransom D. T., Ritland S. R., Kimmel D. W., Moertel C. A., Dahl R. J., Scheithauer B. W., Kelly P. J., Jenkins R. B. Cytogenetic and loss of heterozygosity studies in ependymomas, pilocytic astrocytomas, and oligodendrogliomas. Genes Chromosomes Cancer. 1992 Nov;5(4):348–356. doi: 10.1002/gcc.2870050411. [DOI] [PubMed] [Google Scholar]
- Ransom D. T., Ritland S. R., Moertel C. A., Dahl R. J., O'Fallon J. R., Scheithauer B. W., Kimmel D. W., Kelly P. J., Olopade O. I., Diaz M. O. Correlation of cytogenetic analysis and loss of heterozygosity studies in human diffuse astrocytomas and mixed oligo-astrocytomas. Genes Chromosomes Cancer. 1992 Nov;5(4):357–374. doi: 10.1002/gcc.2870050412. [DOI] [PubMed] [Google Scholar]
- Rey J. A., Bello M. J., de Campos J. M., Kusak M. E., Moreno S. Chromosomal composition of a series of 22 human low-grade gliomas. Cancer Genet Cytogenet. 1987 Dec;29(2):223–237. doi: 10.1016/0165-4608(87)90233-0. [DOI] [PubMed] [Google Scholar]
- Richards F. M., Latif F., Lerman M. I., Zbar B., Maher E. R. TaqI and PstI RFLPs in the von Hippel-Lindau disease gene (VHL). Hum Mol Genet. 1993 Oct;2(10):1750–1750. doi: 10.1093/hmg/2.10.1750. [DOI] [PubMed] [Google Scholar]
- Ropers H. H., Pericak-Vance M. A., Carrano A. V. Report of the Second International Workshop on Human Chromosome 19 mapping 1992. Cytogenet Cell Genet. 1992;60(2):87–95. doi: 10.1159/000133311. [DOI] [PubMed] [Google Scholar]
- Saylors R. L., 3rd, Sidransky D., Friedman H. S., Bigner S. H., Bigner D. D., Vogelstein B., Brodeur G. M. Infrequent p53 gene mutations in medulloblastomas. Cancer Res. 1991 Sep 1;51(17):4721–4723. [PubMed] [Google Scholar]
- Simon D., Knowles B. B., Weith A. Abnormalities of chromosome 1 and loss of heterozygosity on 1p in primary hepatomas. Oncogene. 1991 May;6(5):765–770. [PubMed] [Google Scholar]
- Sun Z. M., Genka S., Shitara N., Akanuma A., Takakura K. Factors possibly influencing the prognosis of oligodendroglioma. Neurosurgery. 1988 May;22(5):886–891. [PubMed] [Google Scholar]
- Thiel G., Losanowa T., Kintzel D., Nisch G., Martin H., Vorpahl K., Witkowski R. Karyotypes in 90 human gliomas. Cancer Genet Cytogenet. 1992 Feb;58(2):109–120. doi: 10.1016/0165-4608(92)90095-p. [DOI] [PubMed] [Google Scholar]
- Venter D. J., Bevan K. L., Ludwig R. L., Riley T. E., Jat P. S., Thomas D. G., Noble M. D. Retinoblastoma gene deletions in human glioblastomas. Oncogene. 1991 Mar;6(3):445–448. [PubMed] [Google Scholar]
- Venter D. J., Thomas D. G. Multiple sequential molecular abnormalities in the evolution of human gliomas. Br J Cancer. 1991 May;63(5):753–757. doi: 10.1038/bjc.1991.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. K., Folkerth R. D., Ye Z., Darras B. T. Aggressive oligodendroglioma predicted by chromosome 10 restriction fragment length polymorphism analysis. Case study. J Neurooncol. 1993 Jan;15(1):29–35. doi: 10.1007/BF01050260. [DOI] [PubMed] [Google Scholar]
- el-Azouzi M., Chung R. Y., Farmer G. E., Martuza R. L., Black P. M., Rouleau G. A., Hettlich C., Hedley-Whyte E. T., Zervas N. T., Panagopoulos K. Loss of distinct regions on the short arm of chromosome 17 associated with tumorigenesis of human astrocytomas. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7186–7190. doi: 10.1073/pnas.86.18.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Deimling A., Eibl R. H., Ohgaki H., Louis D. N., von Ammon K., Petersen I., Kleihues P., Chung R. Y., Wiestler O. D., Seizinger B. R. p53 mutations are associated with 17p allelic loss in grade II and grade III astrocytoma. Cancer Res. 1992 May 15;52(10):2987–2990. [PubMed] [Google Scholar]
- von Deimling A., Louis D. N., von Ammon K., Petersen I., Wiestler O. D., Seizinger B. R. Evidence for a tumor suppressor gene on chromosome 19q associated with human astrocytomas, oligodendrogliomas, and mixed gliomas. Cancer Res. 1992 Aug 1;52(15):4277–4279. [PubMed] [Google Scholar]