Abstract
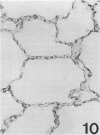
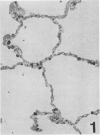
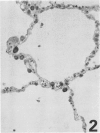
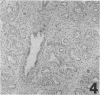
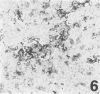
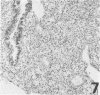
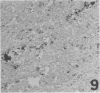
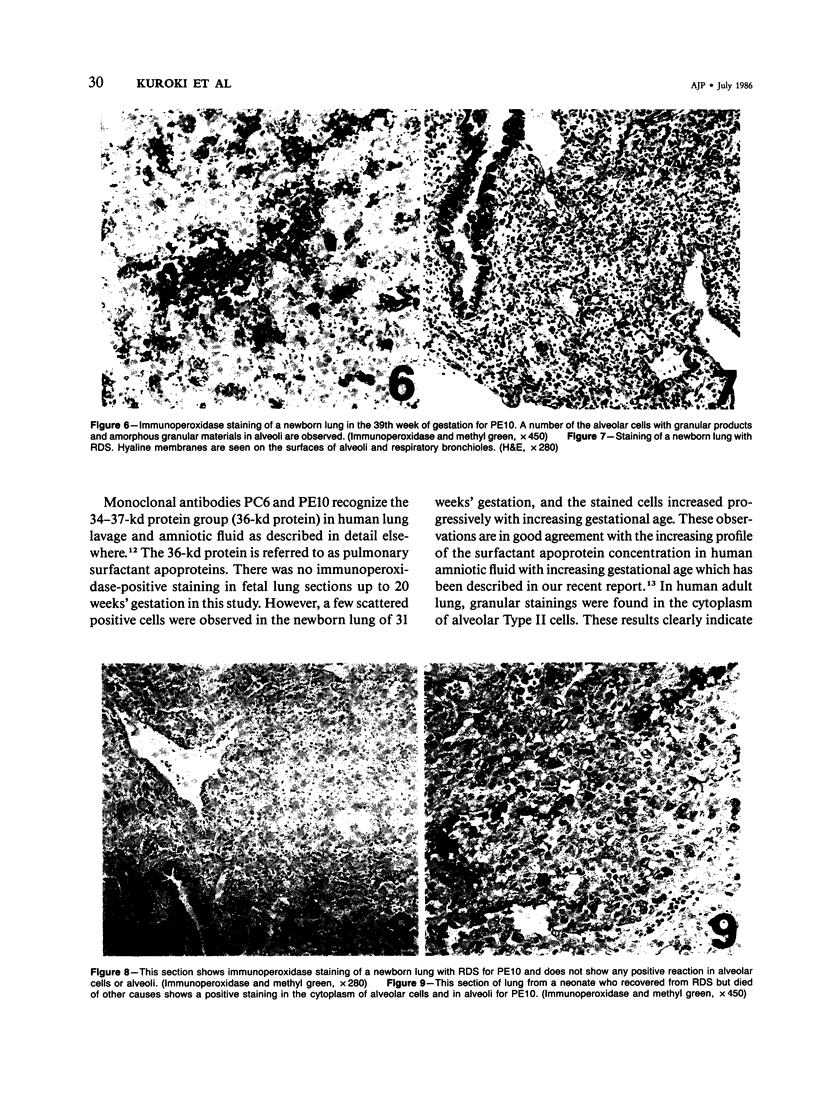
Three monoclonal antibodies, PC6, PE10, and PE12, were used for immunohistochemical studies of human lungs by immunoperoxidase staining. Monoclonal antibodies PC6 and PE10 against pulmonary surfactant apoproteins stained faint granules in the cytoplasm of some alveolar wall cells in adult lung. These stained cells appeared to be alveolar Type II cells. A fetal lung of 20 weeks' gestation had no any positive staining. However, a few scattered positive cells were observed in a newborn lung of 31 weeks' gestation, and the stained cells increased progressively with increasing gestational age. The positively stained cells were very few in the lungs of newborns who died of respiratory distress syndrome (RDS), but the lungs of newborns who died of other causes after recovery from RDS showed many positively stained cells. These results suggest that the immunohistochemical demonstration of the monoclonal antibodies PC6 and PE10 could be a good pathodiagnostic indicator reflecting the localization and development of pulmonary surfactant by alveolar Type II cells. On the other hand, monoclonal antibody PE12 was found to recognize the antigen that occurs on the surfaces of the alveoli of fetal, newborn, and adult lungs as one component of the alveolar lining layer, different from pulmonary surfactant.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Askin F. B., Kuhn C. The cellular origin of pulmonary surfactant. Lab Invest. 1971 Sep;25(3):260–268. [PubMed] [Google Scholar]
- Balis J. U., Delivoria M., Conen P. E. Maturation of postnatal human lung and the idiopathic respiratory distress syndrome. Lab Invest. 1966 Mar;15(3):530–546. [PubMed] [Google Scholar]
- Farrell P. M., Avery M. E. Hyaline membrane disease. Am Rev Respir Dis. 1975 May;111(5):657–688. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- Gandy G., Jacobson W., Gairdner D. Hyaline membrane disease. I. Cellular changes. Arch Dis Child. 1970 Jun;45(241):289–310. doi: 10.1136/adc.45.241.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerke J. Lung surfactant. Biochim Biophys Acta. 1974 Dec 16;344(3-4):241–261. doi: 10.1016/0304-4157(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Huang S. N., Minassian H., More J. D. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest. 1976 Oct;35(4):383–390. [PubMed] [Google Scholar]
- Katyal S. L., Singh G. An enzyme-linked immunoassay of surfactant apoproteins. Its application to the study of fetal lung development in the rat. Pediatr Res. 1983 Jun;17(6):439–443. doi: 10.1203/00006450-198306000-00001. [DOI] [PubMed] [Google Scholar]
- Katyal S. L., Singh G. An immunologic study of the apoproteins of rat lung surfactant. Lab Invest. 1979 May;40(5):562–567. [PubMed] [Google Scholar]
- King R. J., Klass D. J., Gikas E. G., Clements J. A. Isolation of apoproteins from canine surface active material. Am J Physiol. 1973 Apr;224(4):788–795. doi: 10.1152/ajplegacy.1973.224.4.788. [DOI] [PubMed] [Google Scholar]
- King R. J. The surfactant system of the lung. Fed Proc. 1974 Nov;33(11):2238–2247. [PubMed] [Google Scholar]
- Klass D. J. Immunochemical studies of the protein fraction of pulmonary surface active material. Am Rev Respir Dis. 1973 May;107(5):784–789. doi: 10.1164/arrd.1973.107.5.784. [DOI] [PubMed] [Google Scholar]
- Kuroki Y., Fukada Y., Takahashi H., Akino T. Monoclonal antibodies against human pulmonary surfactant apoproteins: specificity and application in immunoassay. Biochim Biophys Acta. 1985 Sep 11;836(2):201–209. [PubMed] [Google Scholar]
- Kuroki Y., Takahashi H., Fukada Y., Mikawa M., Inagawa A., Fujimoto S., Akino T. Two-site "simultaneous" immunoassay with monoclonal antibodies for the determination of surfactant apoproteins in human amniotic fluid. Pediatr Res. 1985 Oct;19(10):1017–1020. doi: 10.1203/00006450-198510000-00013. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M. "Hyaline membrane disease" in newborn infants. Macroscopic, radiographic, and light and electron microscopic studies. Hum Pathol. 1970 Jun;1(2):175–204. doi: 10.1016/s0046-8177(70)80033-8. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Dobbs L. G., Greenleaf R. D., Williams M. C. Alveolar type II cells. Fed Proc. 1977 Dec;36(13):2697–2702. [PubMed] [Google Scholar]
- Singh G., Katyal S. L. Surfactant apoprotein in nonmalignant pulmonary disorders. Am J Pathol. 1980 Oct;101(1):51–61. [PMC free article] [PubMed] [Google Scholar]
- Sueishi K., Tanaka K., Oda T. Immunoultrastructural study of surfactant system. Distribution of specific protein of surface active material in rabbit lung. Lab Invest. 1977 Aug;37(2):136–142. [PubMed] [Google Scholar]
- Williams M. C., Benson B. J. Immunocytochemical localization and identification of the major surfactant protein in adult rat lung. J Histochem Cytochem. 1981 Feb;29(2):291–305. doi: 10.1177/29.2.7019304. [DOI] [PubMed] [Google Scholar]