Summary
Rho-family GTPases are activated by the exchange of bound GDP for GTP, a process that is catalyzed by Dbl-family guanine nucleotide exchange factors (GEFs). The catalytic unit of Dbl-family GEFs consists of a Dbl-homology (DH) domain followed almost invariantly by a pleckstrin-homology (PH) domain. The majority of the catalytic interface forms between the switch regions of the GTPase and the DH domain, but full catalytic activity often requires the associated PH domain. Although PH domains are usually characterized as lipid binding regions, they also participate in protein-protein interactions. For example, the DH-associated PH domain of Dbs must contact its cognate GTPases for efficient exchange. Similarly, the N-terminal DH/PH fragment of Trio, which catalyzes exchange on both Rac1 and RhoG, is four-fold more active in vitro than the isolated DH domain. Given continued uncertainty regarding functional roles of DH-associated PH domains, we have undertaken structural and functional analyses of the N-terminal DH/PH cassette of Trio. The crystal structure of this fragment of Trio bound to nucleotide-depleted Rac1 highlights the engagement of the PH domain with Rac1 and substitution of residues involved in this interface substantially diminishes activation of Rac1 and RhoG. Also, these mutations significantly reduce the ability of full-length Trio to induce neurite outgrowth dependent on RhoG activation in PC-12 cells. Overall, these studies substantiate a general role for DH-associated PH domains in directly engaging Rho GTPases for efficient guanine nucleotide exchange and support a parsimonious explanation for the essentially invariant linkage between DH and PH domains.
Keywords: Trio, Dbl-family GEF, pleckstrin-homology (PH) domain, Rac1, RhoG
Introduction
Rho GTPases act as molecular switches that cycle between inactive GDP-bound states and active GTP-bound states.1-3 Once activated, Rho GTPases bind to their effectors to elicit a variety of downstream signaling responses, including: cytoskeletal reorganization, gene expression, cell cycle progression, membrane trafficking, cell adhesion, and cell migration.3; 4 Given their involvement in many critical cellular processes, it is not surprising that aberrant regulation of Rho GTPases contributes to various diseases such as cancer,5 hypertension,6 and mental retardation.7 It is generally appreciated that delineating the mechanisms involved in the activation of Rho GTPases might guide treatment regimens for a variety of human diseases.
The activity of Rho GTPases is regulated mainly by three classes of proteins: 1) GTPase activating proteins (GAPs) accelerate the hydrolysis of bound GTP; 2) guanine nucleotide dissociation inhibitors (GDIs) sequester prenyl groups added post-translationally to Rho GTPases and thus stabilize cytosolic, inactive forms of the GTPases; and 3) Dbl-family guanine nucleotide exchange factors (GEFs) catalyze the exchange of bound GDP for GTP, thereby activating the GTPases.1; 8-10
Not including splice variants, the human genome encodes 69 Dbl-family GEFs10 of varying domain architecture, size, and GTPase specificity.1; 10 All Dbl-family members contain a conserved Dbl-homology (DH) domain, followed almost invariably by a tandem pleckstrin-homology (PH) domain.2; 10 The DH domain forms the majority of the interface with the GTPase and is often sufficient to catalyze nucleotide exchange10-12 and dictate GTPase specificity.10; 11; 13 The PH domain is necessary for regulating exchange in vivo,14-18 and in many cases, in vitro,13; 19-22 but its exact functions remain unclear.
DH-associated PH domains, like most PH domains, are traditionally characterized as phosphoinositide binding modules; but the ability of phosphoinositides to allosterically regulate the exchange activities of Dbl-family GEFs is, at best, controversial.16; 18; 23-25 In addition, in several instances, DH-associated PH domains are not necessary for recruiting GEFs to cellular membranes where they normally operate on membrane-resident GTPases. This is true even though PH domain mutations abrogating phosphoinositide binding diminish the ability of Dbl-family GEFs to activate their cognate GTPases in vivo.16; 18; 26; 27 Another possibility that finds growing support is that DH-associated PH domains facilitate exchange through direct interactions with cognate GTPases.13; 19; 20; 26; 28 For example, structures of Dbs DH/PH in complex with nucleotide-depleted Cdc42 or RhoA (henceforth, Dbs•Cdc42 or Dbs•RhoA, respectively) show direct contacts between the β3/β4 loop of the PH domain and its cognate GTPases.13; 28 Mutational analysis has confirmed the catalytic importance of this interface both in vitro and in vivo and has identified His814, Gln834, and Tyr889 of Dbs as functionally significant for guanine nucleotide exchange.18; 28 Given the highly conserved position of PH domains directly adjacent to DH domains, it is likely that other Dbl-family GEFs also use their DH-associated PH domains to directly engage their cognate GTPases to facilitate guanine nucleotide exchange. In particular, published data on the Dbl-family GEF Trio22; 29 and the C. elegans ortholog of Trio, UNC-73,26 provide strong circumstantial support for this hypothesis.
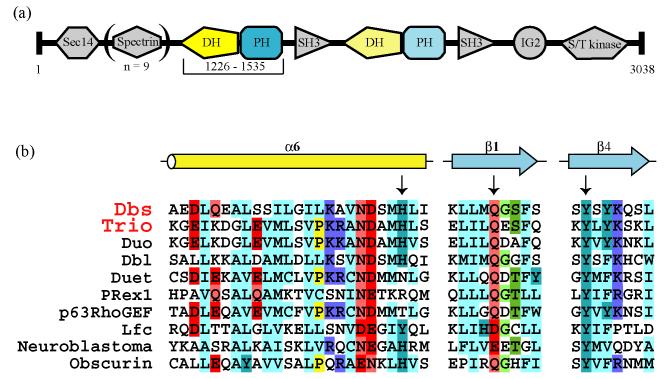
Trio is a large, evolutionarily conserved Dbl-family GEF that is best characterized for its role in regulating neurite outgrowth.30-37 Unlike most Dbl-family GEFs, Trio has two DH/PH cassettes (Figure 1(a)); the first DH/PH cassette catalyzes exchange on Rac1 and RhoG,38-41 while the second DH/PH cassette is specific for RhoA.38; 39 Physiological functions of the C-terminal DH/PH cassette remain relatively unclear, but the N-terminal DH/PH cassette is critical and often sufficient for regulating neuronal development through activation of RhoG, and possibly Rac1.31; 35; 36 Interestingly, although there is some discrepancy over the relative rates of exchange, the isolated N-terminal DH domain of Trio does not exchange as effectively as the corresponding DH/PH cassette in vitro.22; 29 Furthermore, deletion of the PH domain from the N-terminal DH/PH cassette of full-length Trio reduces its ability to induce neurite outgrowth in PC-12 cells through activation of RhoG.29 Finally, residues in the PH domain of Dbs that are functionally significant for exchange on its cognate GTPases are conserved in Trio (Figure 1(b)). Together, these data strongly suggest that the N-terminal DH/PH cassette of Trio might use its PH domain similarly to Dbs when catalyzing exchange on its cognate GTPases Rac1 and RhoG.
Figure 1.
Trio-related GEFs.
(a) Crystallized fragment of Trio is highlighted within the domain architecture of full-length Trio. (b) Residues of Dbs (arrows) that mediate contacts between its N-terminal PH domain and cognate GTPases are conserved in other Dbl-family GEFs, including Trio. The relative position of these residues in context of the DH/PH cassette is indicated in Figure 3.
Thus, to better understand the possible functional interplay between the N-terminal DH and PH domains of Trio specifically, and between DH and PH domains in general, we determined the crystal structure of the N-terminal DH/PH cassette of Trio in complex with nucleotide-depleted Rac1 (henceforth, Trio•Rac1). The complex recapitulates many of the interactions involving the PH domain previously seen in the Dbs / GTPase structures that are required for efficient guanine nucleotide exchange by Dbs. Mutation of this interface in Trio confirms the necessary involvement of the N-terminal PH domain of Trio in directly engaging Rac1 and RhoG for their efficient activation in vitro and in vivo. These studies further support the general capacity of DH-associated PH domains to be active participants in the exchange process and suggest a coherent model of guanine nucleotide exchange catalyzed by Dbl-family proteins that requires the direct and cooperative engagement of DH domains, as well as their associated PH domains, by Rho GTPases for their efficient activation.
Results
Structure of Trio in complex with nucleotide-depleted Rac1
A complex between the N-terminal DH/PH cassette of Trio (residues 1226 – 1536) and residues 1-189 (C189S) of Rac1 yielded poorly diffracting crystals. Since several structures of Rho GTPases indicate that the C-terminal polybasic tail is not typically well-structured, nor directly involved in binding GEFs,13; 19; 28; 42 a truncated version of Rac1 lacking this region and ending at residue 177 (Rac177) was crystallized with the N-terminal DH/PH cassette of Trio and used for structure determination of the Trio•Rac1 complex. Previous work from this lab has shown that full-length, unprenylated Rac1 and Rac177 exhibited identical capacity to bind guanine nucleotides and to be activated by the DH/PH cassette of Tiam1 in vitro.12 Phases were determined for a native data set collected at the SER-CAT beamline (ID-22, Advanced Photon Source) using Trio DH/PH43 and Rac112 as a model for molecular replacement. The final structure has an Rwork = 22.3% and an Rfree = 24.9% and incorporates data from 19.4Å– 2.0 Å (see Table 1 for data collection and refinement statistics).
Table 1.
Data collection and refinement statistics.
| Data collection | |
| Wavelength (Å) | 1.0712 |
| Resolution (Å) | 19.4 – 2.0 |
| Total observations | 213,443 |
| Unique reflections | 39,416 |
| Completeness1 (%) | 99.6 (99.8) |
| I/σ1,2 | 38.1 (3.5) |
| Rsym1,3 (%) | 6.9 (52.4) |
| Refinement statistics | |
| Resolution (Å) | 19.4 – 2.0 |
| Reflections (working/test) | 36932/1968 |
| Rwork4 (%) | 22.3 |
| Rfree5 (%) | 24.9 |
| R.m.s. deviations | |
| Bond distances (Å) | 0.009 |
| Bond angles (°) | 1.138 |
| Average B-factor (Å2) | |
| Molecule | 61.1 |
| Rac1 | 39.4 |
| DH domain | 39.1 |
| PH domain | 143.6 |
| Waters | 51.4 |
| Ramachandran Statistics | |
| Favorable (%) | 99.8 |
| Allowed (%) | 0.2 |
| Disallowed (%) | 0.00 |
Values for the highest resolution shell are given in parentheses.
I/σ is the mean signal to noise ratio, where I is the integrated intensity for a measured reflection and σ is the estimated error in the measurement.
Rsym = 100 × ∑|I – <I>|/ ∑I, where I is the integrated intensity for a measured reflection.
Rwork = ∑|Fo – Fc|/∑Fo, where Fo and Fc are the observed and calculated structure factor amplitudes, respectively
Rfree is calculated similarly to Rwork using test set reflections.
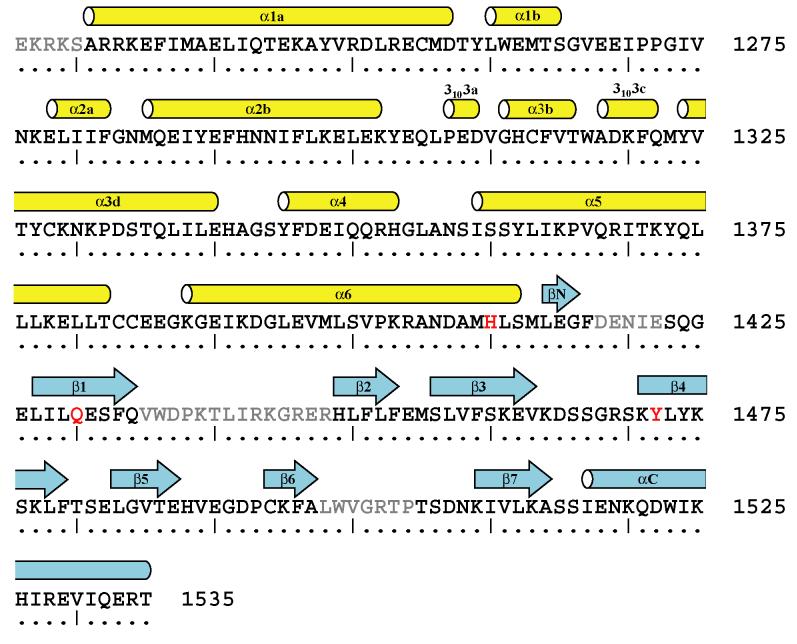
The overall domain architecture of the Trio•Rac1 structure is similar to that seen in other complexes of Dbl-family GEFs and their GTPases; the DH domain of Trio consists of a bundle of six α-helices while the PH domain consists of a core seven strand β-sandwich with three inter-strand loops and a capping C-terminal helix10; 13; 28; 42; 44-46 (Figure 2(a) and 3). The majority of the interface between Rac1 and Trio, which buries ∼2500 Å2 of surface area, is mediated by the DH domain. However, the complex also shows significant interactions between Rac1 and the PH domain of Trio that occur predominantly through residues in the β3/β4 loop and His1410 (Figure 2(a) and (b)), similar to the interactions seen between Dbs and its cognate GTPases.13; 28
Figure 2.
Crystal structure of the DH/PH fragment of Trio bound to nucleotide-free Rac1.
(a) The N-terminal DH (yellow) and PH (blue) domains of Trio are bound to nucleotide-depleted Rac1 (green with switch regions in red). Disordered regions are indicated with dotted lines. (b) Atomic details of the interface between Rac1 and the PH domain of Trio. Hydrogen bonds (2.6 – 4.0 Å) are indicated with dotted lines (c) A simulated annealing omit map (left) contoured at 1.0σ and a 2Fo-Fc map (right) contoured at 1.2σ generated using the final coordinates highlight the electron density at the interface between Rac1 and the PH domain. (d) The anisotropic motion of each atom is displayed as a thermal ellipse (left). An identical image without the thermal ellipses is shown as a reference (right). The interface between Rac1 and the PH domain of Trio, also depicted in Figure 2(b) and 2(c), is highlighted by the box.
Figure 3.
Secondary structure of the DH/PH fragment of Trio.
The crystal structure of the Trio fragment bound to Rac1 was used to define α-helices and β-strands according to nomenclature standardized for DH/PH cassettes.12 Secondary structure assignments were made using the program DSSP,73 coupled with visual assessment. Residues highlighted in red contribute to the interface between the PH domain of Trio and Rac1. Residues in gray are disordered and were not modeled.
There is excellent electron density for Rac1, the DH domain, and the parts of the PH domain that contact Rac1 (Figure 2(c) and Table 1). In contrast, electron density is poor for the remainder of the PH domain, especially the β1/β2 and β6/β7 loops, which have not been modeled. The unusually high average B-factor for the PH domain (Table 1) suggests that it is inherently mobile. To illustrate the predicted motion of the PH domain, we have displayed the thermal ellipses for each atom of the Trio•Rac1 structure (Figure 2(d)). These ellipses represent the anisotropic B-values obtained after TLS refinement47; 48 and indicate that a portion of the PH domain is highly mobile. This observation is consistent with several reports that indicate many DH-associated PH domains have significant conformational mobility and are often difficult to model when crystallized.28; 42; 44 One notable exception to this generalization is the structure of Trio crystallized without bound GTPase.43 However, in this case, the PH domain is locked into place by several crystal contacts.
Interestingly, the anisotropic B-values and the corresponding thermal ellipses associated with residues of the PH domain that mediate the interface with Rac1, including those in the β3/β4 loop, are comparable to those for atoms in the DH domain and Rac1 (Figure 2(d) and data not shown). While the majority of the PH domain has few stabilizing interactions, the interaction of the β3/β4 loop with Rac1 appears to restrict its motion. Furthermore, a simulated annealing omit map generated from the final model after omitting residues in the interface between the PH domain and Rac1 and the final 2Fo-Fc map both show clear density for residues mediating this interface (Figure 2(c)). Thus, the interactions between the DH-associated PH domain of Trio and Rac1 illustrated in Figure 2(b) are not modeling artifacts
Comparison of Trio•Rac1 to Dbs•Cdc42
Superimposition of the structures of Trio•Rac1 with the corresponding DH/PH portion of Trio in isolation indicates that an ∼10° rotation of the PH domain with respect to the DH domain (toward Rac1) occurs upon complex formation. This shift mimics a similar rearrangement within the DH/PH portion of Dbs upon engagement of Cdc42 or RhoA13; 28 (Figure 4(a) and (b)). Consistent with sequence conservation between Dbs and Trio, the molecular interactions between the PH domain of Trio and Rac1 are analogous to those that mediate the interface between the PH domain of Dbs and Cdc42 (Figure 4(c) and (d)). Specifically, the interactions between Asp65 (Rac1), His1410 (Trio, DH domain) and Tyr1472 (Trio, PH domain) mimic the interactions between Asp65 (Cdc42), His814 (Dbs, DH domain), and Tyr889 (Dbs, PH domain). In addition, interactions between Asp65 (Rac1), Gln1430 (Trio, PH domain), and a bridging water molecule are recapitulated in the Dbs•Cdc42 complex. Other similar interactions also occur within both structures, i.e., Ser1470 within the PH domain of Trio interacts with His103 of Rac1 while Lys885 of the PH domain of Dbs interacts with His103 of Cdc42.
Figure 4.
The PH domains of Trio and Dbs interact similarly with their cognate GTPases.
DH/PH fragments of Trio (a) and Dbs (b) in complex with their cognate GTPases have been superimposed upon equivalent, unbound fragments (gray) using the DH domains. (c and d) Lower panels highlight conserved interactions found in both GEF/GTPase complexes that require specific residues involving the PH domains. Color scheme is maintained from Figure 2.
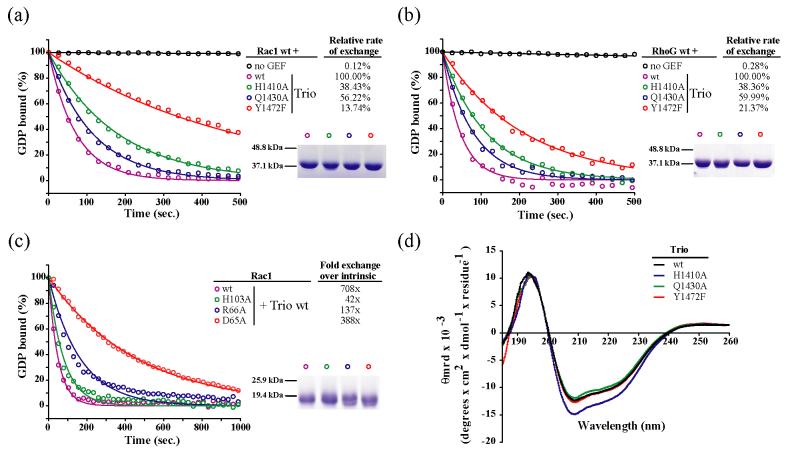
Functional analysis of the interface between the PH domain of Trio and Rac1
To test the functional significance of the interactions between Rac1 and the PH domain of Trio, mutations designed to disrupt these interactions were introduced into the DH/PH fragment used for crystallization (Figure 5) and purified mutant proteins were assayed for exchange activity on soluble wild-type Rac1 (residues 1-189, c189s) (Figure 5(a)). Relative to the equivalent wild-type DH/PH fragment of Trio, mutation of either His1410 to Ala, Gln1430 to Ala, or Tyr1472 to Phe significantly decreases exchange activity. Similar to Dbs,28 mutation of Tyr1472 to Phe produces the largest reduction in exchange activity. Substitution of Tyr 1472 to Phe was previously assessed under similar conditions43 and the two sets of measurements are consistent. Unsurprisingly, complementary mutations within Rac1 designed to disrupt interaction with the PH domain of Trio reduced exchange by the wild-type fragment of Trio (Figure 5(c)), further confirming the functional importance of engaging Rac1 through the PH domain for catalyzed exchange. The D65A and R66A mutations introduced in Rac1 lie within the switch region responsible for binding nucleotide. However, rates of spontaneous exchange for these mutant forms of Rac1 were no more than 2-fold higher than wild-type Rac1 (data not shown) and these small differences cannot account for the relatively large decrement in the capacity of Trio to activate them. The spontaneous rate of exchange for Rac1 H103A was essentially identical to wild-type Rac1.
Figure 5.
Mutations that disrupt the interface between the PH domain of Trio and Rac1 diminish nucleotide exchange.
Residues within, or supported by, the PH domain of Trio that form the interface with Rac1 were mutated and the exchange activities of the mutants were measured on both Rac1 (a) and RhoG (b). (c) Residues of Rac1 that interact directly with the PH domain of Trio were also analyzed. Exchange assays (n=2) were carried out as described in Methods. Exchange rates are reported as a percentage of the exchange rate of wild-type Trio (a and b) or as fold exchange over the intrinsic exchange rate of the respective mutant of Rac1 (c). Proteins (5 μg) were subjected to SDS-PAGE and stained with Coomassie Blue (insets) to verify purity and concentration. (d) Circular dichroism spectroscopy confirmed the proper folding of Trio DH/PH fragments.
In this study, Trio was crystallized in complex with nucleotide-depleted Rac1, but Trio alsoexchanges on RhoG both in vitro29; 43 and in vivo,29; 31; 40; 41 and does so approximately three times more efficiently in vitro.43 RhoG is 80% homologous to Rac1, 65% identical, and the residues in nucleotide-depleted Rac1 that are buried in the interface with Trio are 100% identical in RhoG (data not shown). Consequently, mutants of Trio that have reduced exchange activity on Rac1 were also tested for activity on soluble, wild-type RhoG (Figure 5(b)). Predictably, mutations within the PH domain of Trio also reduced its exchange activity on RhoG, with Trio Y1472F exchanging the least efficiently. These data strongly suggest that the interface between Trio and RhoG is highly similar, if not identical, to its interface with Rac1. Circular dichroism spectroscopy (Figure 5(d)) and gel filtration chromatography (data not shown) confirmed that all mutant forms of Trio were properly folded and monodisperse.
Wild-type Trio robustly activates RhoG through its N-terminal DH/PH cassette to promote neurite outgrowth in PC-12 cells.31 Furthermore, mutant forms of Trio lacking the N-terminal PH domain exhibit a reduced capacity to both activate RhoG in vitro and induce neurite formation in PC-12 cells.29; 31 While these studies indicate the importance of the N-terminal PH domain of Trio for guanine nucleotide exchange of its cognate GTPases and its attendant morphological consequences, they do not address the underlying mechanistic causes for these results. Therefore, to test the effects of disrupting specific interactions between the N-terminal PH domain of Trio and its cognate GTPases, the single substitutions described above were introduced into full-length Trio (3038 amino acids) and the capacity of these substituted forms of Trio to induce neurite outgrowth of PC-12 cells was quantified (Figure 6). Consistent with the inability of these mutant forms of Trio to activate Rac1 and RhoG efficiently in vitro, these singly-substituted forms of full-length Trio are also significantly compromised in their capacity to induce neurite outgrowth in PC-12 cells. Since these substitutions do not perturb the overall fold of the isolated DH/PH fragment of Trio, it is highly unlikely that they do so within the context of full-length Trio.
Figure 6.
Mutations within, or supported by, the PH domain of Trio that reduce GTPase activation in vitro also reduce the capacity of full-length Trio to induce neurite outgrowth in PC-12 cells.
(a) Neurite outgrowth in transfected PC-12 cells was assessed as described in Methods (*; p-values < 0.05 in comparison to wild-type Trio using student's T-test). (b) Representative images of transfected PC-12 cells show both GFP fluorescence (left) and filamentouse actin stained with Alexa Fluor 546 phalloidin (right). All constructs were GFP-tagged at the N-terminus.
Discussion
The work presented here describes specific interactions between the N-terminal PH domain of Trio and Rac1 or RhoG that are essential for productive activation of these GTPases. These interactions strikingly mimic the coordinate engagement of RhoA or Cdc42 by the DH and PH domains of Dbs. Moreover, PH domain-mediated interactions between both Trio and Dbs and their cognate GTPases are required for efficient guanine nucleotide exchange in vitro and in vivo and indicate that the coordinate engagement of cognate GTPases by DH and PH domains necessary for efficient guanine nucleotide exchange might be more wide-spread than currently appreciated.
For example, as predicted by sequence homology (Figure 1(b)), the residues in the PH domain of Trio that mediate its interface with Rac1 are identical to the residues in the PH domain of Dbs that mediate its interface with Cdc42 and RhoA.28 Interestingly, these residues are conserved in other GEFs, including: Duo, Dbl, neuroblastoma, and obscurin (Figure 1(b)); and research has shown that Dbl also requires its DH-associated PH domain to catalyze exchange with maximal efficiency.49 Therefore, while it is not conclusive that these GEFs also require direct engagement of their cognate GTPases by their DH-associated PH domains for full exchange potential, sequence conservation shared among this set of GEFs strongly suggests this possibility.
While the residues of Dbs and Trio that mediate the interface between their PH domains and cognate GTPase are conserved only in a subset of Dbl-family GEFs, recent data shows that more distantly related Dbl-family GEFs also use their DH-associated PH domain to engage their cognate GTPases, albeit through different interactions. For example, the DH domains of both LARG and PDZ-RhoGEF exchange less efficiently on RhoA than their respective DH/PH cassette.19; 20 Not surprisingly, a comparison of the structure of LARG DH/PH to the structure of LARG DH/PH bound to nucleotide free RhoA (henceforth LARG•RhoA) reveals an ∼30° rotation of the PH domain, allowing contact between the PH domain and the GTPase.19 This rotation is similar to that seen with both Trio and Dbs upon engaging the nucleotide-free GTPase.44 However, instead of allowing contact between the cognate GTPases and the β3/β4 loop of the respective PH domains, the LARG•RhoA structure and the structure of PDZ-RhoGEF bound to RhoA both reveal contacts between RhoA and residues in the αC helix of the PH domains19; 20 In the case of LARG•RhoA, mutational analysis has shown that these contacts are functionally significant. The structure of LARG•RhoA and accompanying biochemical analyses also reveal functionally significant contacts between the GTPase and the β1 strand of the PH domain which are mediated through Arg 986 in the αN helix of the PH domain.19 The αN helix extends from the C-terminus of the α6 helix of the DH domain,19 and interestingly, similar contacts are seen between the GTPase and C-terminus of the α6 helix of Trio, Dbs, and PDZ-RhoGEF.20; 28 In the cases of PDZ-RhoGEF and LARG, the in vivo relevance of contacts between the PH domain and the GTPase has not been pursued. LARG and PDZ-RhoGEF belong to a subset of Dbl-family GEFs that include Lfc and p114-RhoGEF; further analysis is needed to determine if other members of this subset also require their DH-associated PH domains for maximal guanine nucleotide exchange.
In contrast to Dbs, Trio, LARG, and PDZ-RhoGEF, crystals structures of the DH/PH cassettes of the Dbl-family GEFs Tiam1,12 intersectin,13 and collybistin,42 in complex with their cognate GTPases show little or no contact between their PH domains and their cognate GTPase. While these structures might indicate that not all Dbl-family GEFs use their DH-associated PH domain to engage cognate GTPases for effective exchange, it has been suggested that these structures might not precisely reflect the molecular details of the exchange process in vivo.16-18; 28 Specifically, Dbl-family GEFs operate on membrane-resident GTPases and it has been proposed that biological membranes impose additional constraints on the conformational flexibility of DH/PH cassettes such that specific conformers are favored that could promote direct interactions between GTPases and DH-associated PH domains necessary for full exchange activity.16; 18; 28 Indeed, many DH-associated PH domains have been shown to bind various phosphoinositides with micromolar affinities,23; 50 and while these protein-lipid interactions are not normally considered sufficient to drive subcellular re-localization,45 they might provide points of membrane attachment that would favor specific conformers or orientations of DH/PH cassettes that stabilize interactions between DH-associated PH domains and GTPases necessary for full catalytic exchange. Studies of Tiam116 and Dbs18; 27 provide substantial support for this scenario.
There is evidence that due to the conformational flexibility within the DH/PH cassettes, structural studies provide only a partial description of the exchange process. For example, there are four independent copies of the DH/PH cassette of Dbs in the crystal structure of this fragment without bound GTPase.44 In each of the four molecules, the position of the PH domain is different relative to the DH domain. Much of this conformational flexibility is lost when Dbs engages a cognate GTPase as evidenced in the crystal structures of Dbs bound to either RhoA13 or Cdc42.28 Similarly, the structure of collybistin in complex with Cdc42 shows two conformers of the DH/PH cassette within the asymmetric unit that differ by an ∼35 degree rotation of the PH domain with respect to the DH domain.42 Sos presents an extreme example. In this case, the PH domain occludes the GTPase-binding site on the DH domain,51; 52 and the DH/PH fragment alone is incapable of activating GTPases in vitro (S. Soisson, personal communication). However, Sos-1 does activate Rac in vivo,14 and for this to occur, the PH domain must undergo a dramatic rearrangement from its position relative to the DH domain to allow binding and activation of Rac. Some evidence suggests that the E3b1/Eps8 complex activates Sos-1 downstream of Ras and PI3K,53; 54 but the molecular details of the Sos DH/PH rearrangement remain unclear. In the case of Trio, we show here that portions of the PH domain that are not in direct contact with Rac1 are highly mobile in the crystal structure of the Trio•Rac1 complex. This mobility does not manifest in the crystal structure of the DH/PH fragment of Trio in isolation. However, in this case, lattice contacts within the crystal clearly limit possible motion.
The studies presented here support earlier indications that the N-terminal PH domain of Trio is required for the activation of its cognate GTPases, and together with previous studies of Dbs,13; 28 provide detailed mechanistic information regarding roles of DH-associated PH domains in directly engaging GTPases for their activation in vivo. PH domains associated with DH domains might function in a variety of contexts to support activation of GTPases. However, an attractive and parsimonious model posits that both of the two domains must engage cognate GTPases for their effective and regulated activation in vivo. The inherent flexibility between DH and PH domains would be restricted under controlled cellular conditions, i.e., at membranes and upon the binding of specific phosphoinositides to the PH domain, which would favor productive engagement of both portions of the cassette with cognate GTPases. Under extreme conditions, such as Sos, the PH domain would move off the surface of the DH domain to allow access by GTPases51; 52 and this movement might also be controlled by membranes and phosphoinositide binding to the DH-associated PH domain. Alternatively, the conformation of the DH/PH cassette might be altered by interaction with currently unknown protein activators. In all cases though, the DH and PH domains would act cooperatively to integrate various cellular inputs leading to Rho GTPases activation; the PH domain would not be a simple membrane localization device, but more properly thought of as an intrinsic component of the exchange process carried out by Dbl-family proteins reacting to various cellular conditions.
Materials and Methods
Sequence alignment
The sequences of Dbs (NP 079255), Trio (NP 009049), Duo (NP 003938), Dbl (NP 005360), Duet (NP 008995), PRex1 (NP 065871), p63RhoGEF (NP 891992), Lfc (NP 004714), neuroblastoma (NP 005263), and obscurin (NP 443075) shown in Figure 1(b) were aligned using Clustal X.55 The NCBI accession numbers are given in parentheses.
Protein preparation for guanine nucleotide exchange assays
Human Trio DH/PH (residues 1226 – 1535) (kindly provided by Dr. Yi Zheng, Cincinnati Children's Hospital Medical Center), was encoded as a fusion with an N-terminal His6-tag in pET15a (Novagen), expressed in the BL21 (DE3) E. coli strain, and purified similarly to published protocols.28; 56 Briefly, transformed cells were grown in LB media containing 0.1 mg/ml ampicillin at 37°C to an OD600 of 0.7 (mid-log phase) and induced with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at 27°C for 5 hours. Harvested cells were resuspended in 50 mM NaH2PO4, pH 8, 300 mM NaCl (buffer A), and 5mM imidazole and lysed with an Emulsi-Flex C5 (Avestin). Lysate was clarified by centrifugation at > 125,000 × g for 30 minutes prior to loading the supernatant onto a Ni2+-Sepharose affinity column (GE Healthcare) equilibrated in buffer A containing 5 mM imidazole. The column was washed with buffer A containing 55 mM imidazole and Trio eluted with buffer A containing 400 mM imidazole. The protein was further purified using a 26/60 Sephacryl-200 size exclusion column (GE Healthcare) equilibrated with 50 mM Tris, pH 8, 2 mM DTT, 2mM EDTA, 150 mM NaCl, and 5% glycerol. Fractions containing Trio were pooled, concentrated, and stored at −80°C.
Human Rac1 (residues 1-189, C189S) was expressed from pET21a (Novagen) in the BL21 (DE3) E. coli strain. Transformed cells were grown and induced similarly to cells expressing Trio. Harvested cells were resuspended in 10 mM MES, pH 6, 2 mM DTT, 10% glycerol, 1 mM MgCl2 (buffer B) and 10 mM NaCl prior to lysis and clarification as described above. Supernatant was loaded onto an SP-Sepharose Fast Flow 26/10 column (GE Healthcare). Rac1 was eluted from the column using buffer B with an increasing gradient of NaCl and further purified using a 26/60 Sephacryl-200 size exclusion column (GE Healthcare) equilibrated with 50 mM Tris, pH 8, 2 mM DTT, 2mM MgCl2, 150 mM NaCl, and 5% glycerol. Fractions containing Rac1 were pooled, concentrated, and stored at −80°C.
Human RhoG (residues 1-188, C188S) was expressed from the pGEX4TEV2 vector57 with an N-terminal GST-tag in the BL21 (DE3) E. coli strain. Transformed cells were grown in LB media containing 0.1 mg/ml ampicillin at 37°C to an OD600 of 0.7 (mid-log phase) and induced with 1 mM IPTG at 20°C for 16 hours to 18 hours. Cells were resuspended in buffer C (150 mM NaCl, 20 mM Tris, pH 8, 2 mM DTT, 1 mM MgCl2, 10 μM GDP, and 5% glycerol) prior to lysis and clarification as described above. Supernatant was loaded onto a GST-Sepharose affinity column (GE Healthcare) equilibrated in buffer C; the column was washed with buffer C, and RhoG eluted with buffer C containing 10 mM glutathione (reduced). The GST tag was removed by cutting with TEV while dialyzing against buffer C overnight. The protein was further dialyzed against buffer D (10 mM MES, pH 6, 2 mM DTT, 2 mM MgCl2, 10 μM GDP, and 5% glycerol) and applied to a Source-S 16/10 column (GE Healthcare) equilibrated in buffer D. RhoG was eluted with buffer D containing an increasing concentration of NaCl. The fractions containing RhoG were then loaded onto a GST-Sepharose affinity column equilibrated in buffer C to remove residual amounts of GST-tagged protein. The flow-through from the GST column was buffer exchanged into buffer C, concentrated, and stored at −80°C.
Mutations in Trio DH/PH and Rac1 were made using the Quikchange site directed mutagenesis kit (Stratagene) following manufacturer's instructions. Sequences were verified using automated sequencing. Mutants of Trio and Rac1 were purified as described above for wild-type Trio and Rac1, respectively.
Guanine nucleotide exchange assays
Nucleotide exchange was measured using a fluorescence based assay, similar to published protocols,28; 58 in which N-methylanthraniloyl (mant)-GTP was loaded onto the GTPase. Spectroscopic analysis was carried out using a Perkin-Elmer LS 55 spectrometer at 20°C. The exchange assay mixture containing 20 mM Tris pH 7.5, 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, 100 uM mant-GTP, and 2 uM GTPase was allowed to equilibrate with constant stirring. Trio was then added at 50 or 400 nM for exchange assays with RhoG or Rac1, respectively, and nucleotide exchange was measured by monitoring the decrease in intrinsic tryptophan fluorescence (λex=295 nm, λem=335 nm) of the GTPase due to the binding of mant-GTP. The data were fit to one phase exponential decay curves using the program GraphPad Prism™ in order to determine the rate of nucleotide exchange.
Protein preparation for formation of Trio/Rac1 complex
Human Trio DH/PH (residues 1226 – 1536) was cloned in a pPROEX-HTa vector (Invitrogen) using NcoI and XhoI cleavage sites, expressed as a fusion protein with an N-terminal His6-tag in BL21 (DE3)s E. coli strain, and eluted from a Ni2+-Sepharose affinity column (GE Healthcare) as described above. The His6-tag was removed by cleavage with TEV while dialyzing (16 hrs) against a buffer containing 50 mM Tris, pH 8, 2 mM DTT, 2mM EDTA, 150 mM NaCl, and 5% glycerol. The removal of the His6-tag was confirmed using SDS-PAGE. The protein was then stored at 4°C until it was used to form a complex with Rac1.
Rac177 was expressed from the pET15b vector (Novagen) in BL21 (DE3)s E .coli strain and protein was purified according to published protocols.12 Minor variations include that the 45% ammonium sulfate pellet was resuspended in 20 mM Tris, pH 8, 2 mM DTT, 1 mM MgCl2, and 10 μM GDP and dialyzed extensively against buffer E (20 mM Tris pH 8, 10 mM NaCl, 2 mM DTT, 1 mM MgCl2, and 10 μM GDP) to remove the ammonium sulfate and increase the pH. The resuspended pellet was loaded onto a Q-Sepharose Fast Flow 26/10 column (GE Healthcare) instead of a size-exclusion column. Rac177 was eluted from the column using buffer E with increasing amounts NaCl. The fractions containing Rac177 were pooled together and stored at 4°C before being used to form a complex with Trio.
Crystallization of the Trio/Rac177 complex
The Trio/Rac177 complex was formed in the presence of an excess of nucleotide-free Rac177 in 20 mM Tris, pH 8, 2 mM DTT, 4mM EDTA, 200 mM NaCl, and 5% glycerol. The complex was purified on a 26/60 Sephacryl-200 size exclusion column equilibrated with 20 mM Tris, pH 8, 2 mM DTT, 4 mM EDTA, 200 mM NaCl, and 5% glycerol. Fractions containing purified complex were pooled together, dialyzed into a buffer containing 50 mM NaCl, 10 mM Tris, 2 mM EDTA, and 2 mM DTT, concentrated to ∼21 mg/ml, and stored at −80°C.
Trio/Rac177 crystals were obtained by vapor diffusion at 18 °C. Drops were formed by combining equal volumes of protein complex and reservoir solution (100 mM sodium cacodylate pH 5.5 – 6.5, 14 – 18% (w/v) PEG 8000 (FLUKA), and 300-500 mM calcium acetate). Crystals typically appeared after 2 – 3 days and grew to final dimensions of 0.1 × 0.1 × 0.05 mm after several days. Crystals were cryoprotected by increasing the glycerol concentration of the drop to 21% (v/v) in 3% increments. Cryoprotected crystals were then suspended in a rayon loop (Hampton Research) and snap frozen in liquid nitrogen. The crystals belong to the space group P21212 with unit cell parameters a = 97.492 Å, b = 108.558 Å, and c = 53.416 Å.
Data collection and structure determination
A native data set was collected using a single frozen crystal at the SER-CAT beamline (ID-22, Advanced Photon Source). Data were integrated and scaled using DENZO and SCALEPACK59. Phases were calculated by molecular replacement using the Trio DH/PH43 and Rac112 structures. The calculations were performed using both AMORE60 and PHASER61 from the CCP4 suite of programs,62 which yielded identical solutions.
Model building and structure refinement
Electron density maps were calculated using CNS.63 The interactive graphics program O was used for the majority of the model building,64 but the molecular graphics program COOT65 was also used to add water molecules. Most of the subsequent refinement was performed using CNS;63 however, due the conformational mobility of the PH domain, CNS refinement was unable to produce clear density for this region of the molecule. Thus, TLS refinement from the CCP4 program Refmac547; 48 was used to improve the quality of the electron density maps. Using Rac1, the DH domain, the PH domain, and the waters as TLS “groups” and employing tight geometrical restraints (matrix diagonal weighing term = 0.06), a final model with Rwork = 22.3% and Rfree = 24.9% was produced. In addition, the torsion angles for every non-glycine residue are within the most favored or allowed regions of the Ramachandran diagram, as determined by PROCHECK.66 Please refer to Table 1 for refinement statistics.
The simulated annealing omit map used to validate the interactions seen at the interface between Rac1 and the PH domain of Trio was generated from the final coordinates after omitting residues involved in this interface (residues 64 – 68, and 102 – 104 from Rac1 and residues 1405 – 1411, 1429 – 1431, and 1469 – 1473 from Trio).
All images of protein structures, with the exception of Figure 2(d), were generated using Pymol.67 The images in Figure 2(d) displaying the theoretical anisotropic motion of the atoms68 were generated using CCP4MG.69; 70 Pymol67 was also used to calculate buried surface areas.
Protein Data Bank accession codes
The atomic coordinates and structure factors (code 2NZ8) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ.
Cell culture and transfection
To introduce point mutations (H1410A, Q1430A, and Y1472F) into EGFP-Trio FL wild-type (human) (generously donated by Dr. Anne Debant, Centre de Recherche en Biochimie Macromoléculaire, Montpellier, France), a section of Trio containing the N-terminal DH/PH cassette (Trio DH/PH-long) was subcloned into the pMCSG7 vector.71 Mutations were introduced using the Quikchange site directed mutagenesis kit (Stratagene) following manufacturer's instructions and verified by automated sequencing. The DH/PH cassette of Trio was then digested from Trio DH/PH-long and subcloned into EGFP-Trio FL.
PC-12 cells were cultured in DMEM containing 10% horse serum (HS), 5% fetal bovine serum (FBS), and 1x penicillin/streptomycin (100 units/ml penicillin; 0.1 mg/ml streptomycin) at 37°C in the presence of 5% CO2. For transfection, cells were seeded at a density of 500,000 cells per well on glass coverslips coated with rat-tail collagen type I (BD biosciences) in 6-well dishes. Cells were cultured for 18 hrs and then transfected with 1 μg of EGFP-C2 vector (Clonetech), EGFP-RhoG wild-type (human) (generously donated by Dr. Keith Burridge, UNC-Chapel Hill), or EGFP-Trio FL (wild-type and mutant forms) per well using Lipofectamine plus (Invitrogen) according to the manufacturer's instructions. Cells were fixed 48 to 72 hours post transfection for 10 minutes with 3.7% (v/v) paraformaldehyde in PBS, permeabilized with 0.3% Triton X-100, stained for actin with Alexa Fluor 546 phalloidan (Invitrogen), washed, and mounted using Fluorsave Reagent (Calbiochem) according the manufacturer's instructions.
Image collection and data processing for neurite outgrowth assays
Fixed cells were observed using an Olympus Fluoview 300 laser scanning confocal microscope with a 60X PL APO oil immersion objective when scoring for neurite positive cells. Neurite outgrowth activity was determined by positively scoring transfected cells displaying one or more neurites greater than one cell body in length. Cells found in large clumps (> 10 cells) were excluded from analysis. Displayed results represent the average of 3 independent experiments in which at least 100 cells per condition were counted for each experiment. In addition, the studies were blinded to conceal which slides contained cells that had been transfected with wild-type and mutant versions of Trio.
Images were obtained using an Olympus Fluoview 1000 laser scanning confocal microscope with 60× PL APO oil immersion objective in 20 – 30 micron XYZ stacks. Final images were processed in ImageJ72 and represent a standard deviation projection of the compiled stacks.
Acknowledgments
We would like to acknowledge Dr. Ashutosh Tripathy at the UNC-Chapel Hill Macromolecular Interactions Facility for his assistance with circular dichroism spectroscopy. We would also like to acknowledge Dr. Alex Singer for his technical assistance with model building and refinement. This work was supported by National Institutes of Health Grant GM62299 and GM65533.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Dvorsky R, Ahmadian MR. Always look on the bright site of Rho: structural implications for a conserved intermolecular interface. EMBO Rep. 2004;5:1130–6. doi: 10.1038/sj.embor.7400293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman GR, Cerione RA. Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett. 2002;513:85–91. doi: 10.1016/s0014-5793(01)03310-5. [DOI] [PubMed] [Google Scholar]
- 3.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci. 2000;25:388–91. doi: 10.1016/s0968-0004(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 5.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 6.Laufs U, Liao JK. Targeting Rho in cardiovascular disease. Circ Res. 2000;87:526–8. doi: 10.1161/01.res.87.7.526. [DOI] [PubMed] [Google Scholar]
- 7.Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- 8.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 9.Boettner B, Van Aelst L. The role of Rho GTPases in disease development. Gene. 2002;286:155–74. doi: 10.1016/s0378-1119(02)00426-2. [DOI] [PubMed] [Google Scholar]
- 10.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 11.Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–32. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- 12.Worthylake DK, Rossman KL, Sondek J. Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature. 2000;408:682–8. doi: 10.1038/35047014. [DOI] [PubMed] [Google Scholar]
- 13.Snyder JT, Worthylake DK, Rossman KL, Betts L, Pruitt WM, Siderovski DP, Der CJ, Sondek J. Structural basis for the selective activation of Rho GTPases by Dbl exchange factors. Nat Struct Biol. 2002;9:468–75. doi: 10.1038/nsb796. [DOI] [PubMed] [Google Scholar]
- 14.Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–3. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead IP, Campbell S, Rossman KL, Der CJ. Dbl family proteins. Biochim Biophys Acta. 1997;1332:F1–23. doi: 10.1016/s0304-419x(96)00040-6. [DOI] [PubMed] [Google Scholar]
- 16.Baumeister MA, Martinu L, Rossman KL, Sondek J, Lemmon MA, Chou MM. Loss of phosphatidylinositol 3-phosphate binding by the C-terminal Tiam-1 pleckstrin homology domain prevents in vivo Rac1 activation without affecting membrane targeting. J Biol Chem. 2003;278:11457–64. doi: 10.1074/jbc.M211901200. [DOI] [PubMed] [Google Scholar]
- 17.Pruitt WM, Karnoub AE, Rakauskas AC, Guipponi M, Antonarakis SE, Kurakin A, Kay BK, Sondek J, Siderovski DP, Der CJ. Role of the pleckstrin homology domain in intersectin-L Dbl homology domain activation of Cdc42 and signaling. Biochim Biophys Acta. 2003;1640:61–8. doi: 10.1016/s0167-4889(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 18.Rossman KL, Cheng L, Mahon GM, Rojas RJ, Snyder JT, Whitehead IP, Sondek J. Multifunctional roles for the PH domain of Dbs in regulating Rho GTPase activation. J Biol Chem. 2003;278:18393–400. doi: 10.1074/jbc.M300127200. [DOI] [PubMed] [Google Scholar]
- 19.Kristelly R, Gao G, Tesmer JJ. Structural determinants of RhoA binding and nucleotide exchange in leukemia-associated Rho guanine-nucleotide exchange factor. J Biol Chem. 2004;279:47352–62. doi: 10.1074/jbc.M406056200. [DOI] [PubMed] [Google Scholar]
- 20.Derewenda U, Oleksy A, Stevenson AS, Korczynska J, Dauter Z, Somlyo AP, Otlewski J, Somlyo AV, Derewenda ZS. The crystal structure of RhoA in complex with the DH/PH fragment of PDZRhoGEF, an activator of the Ca(2+) sensitization pathway in smooth muscle. Structure (Camb) 2004;12:1955–65. doi: 10.1016/j.str.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Rossman KL, Worthylake DK, Snyder JT, Cheng L, Whitehead IP, Sondek J. Functional analysis of cdc42 residues required for Guanine nucleotide exchange. J Biol Chem. 2002;277:50893–8. doi: 10.1074/jbc.M208580200. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Wang H, Eberstadt M, Schnuchel A, Olejniczak ET, Meadows RP, Schkeryantz JM, Janowick DA, Harlan JE, Harris EA, Staunton DE, Fesik SW. NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell. 1998;95:269–77. doi: 10.1016/s0092-8674(00)81757-2. [DOI] [PubMed] [Google Scholar]
- 23.Snyder JT, Rossman KL, Baumeister MA, Pruitt WM, Siderovski DP, Der CJ, Lemmon MA, Sondek J. Quantitative analysis of the effect of phosphoinositide interactions on the function of Dbl family proteins. J Biol Chem. 2001;276:45868–75. doi: 10.1074/jbc.M106731200. [DOI] [PubMed] [Google Scholar]
- 24.Fuentes EJ, Karnoub AE, Booden MA, Der CJ, Campbell SL. Critical role of the pleckstrin homology domain in Dbs signaling and growth regulation. J Biol Chem. 2003;278:21188–96. doi: 10.1074/jbc.M211792200. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–60. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 26.Kubiseski TJ, Culotti J, Pawson T. Functional analysis of the Caenorhabditis elegans UNC-73B PH domain demonstrates a role in activation of the Rac GTPase in vitro and axon guidance in vivo. Mol Cell Biol. 2003;23:6823–35. doi: 10.1128/MCB.23.19.6823-6835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumeister MA, Rossman KL, Sondek J, Lemmon MA. The Dbs PH domain contributes independently to membrane targeting and regulation of guanine nucleotide exchange activity. Biochem J. 2006 doi: 10.1042/BJ20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossman KL, Worthylake DK, Snyder JT, Siderovski DP, Campbell SL, Sondek J. A crystallographic view of interactions between Dbs and Cdc42: PH domain-assisted guanine nucleotide exchange. Embo J. 2002;21:1315–26. doi: 10.1093/emboj/21.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellanger JM, Estrach S, Schmidt S, Briancon-Marjollet A, Zugasti O, Fromont S, Debant A. Different regulation of the Trio Dbl-Homology domains by their associated PH domains. Biol Cell. 2003;95:625–34. doi: 10.1016/j.biolcel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Bateman J, Shu H, Van Vactor D. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron. 2000;26:93–106. doi: 10.1016/s0896-6273(00)81141-1. [DOI] [PubMed] [Google Scholar]
- 31.Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, Debant A. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr Biol. 2002;12:307–12. doi: 10.1016/s0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 32.Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development. 2005;132:1983–94. doi: 10.1242/dev.01736. [DOI] [PubMed] [Google Scholar]
- 33.McPherson CE, Eipper BA, Mains RE. Multiple novel isoforms of Trio are expressed in the developing rat brain. Gene. 2005;347:125–35. doi: 10.1016/j.gene.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 34.Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, Dickson BJ. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–94. doi: 10.1016/s0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 35.Portales-Casamar E, Briancon-Marjollet A, Fromont S, Triboulet R, Debant A. Identification of novel neuronal isoforms of the Rho-GEF Trio. Biol Cell. 2006;98:183–93. doi: 10.1042/BC20050009. [DOI] [PubMed] [Google Scholar]
- 36.Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz Morales A, Hogue CW, Pawson T, Culotti J. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92:785–95. doi: 10.1016/s0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc Natl Acad Sci U S A. 2000;97:12074–8. doi: 10.1073/pnas.97.22.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellanger JM, Lazaro JB, Diriong S, Fernandez A, Lamb N, Debant A. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene. 1998;16:147–52. doi: 10.1038/sj.onc.1201532. [DOI] [PubMed] [Google Scholar]
- 39.Debant A, Serra-Pages C, Seipel K, O'Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–71. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat Cell Biol. 2000;2:888–92. doi: 10.1038/35046533. [DOI] [PubMed] [Google Scholar]
- 41.Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouviere C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci. 2000;113(Pt 4):729–39. doi: 10.1242/jcs.113.4.729. [DOI] [PubMed] [Google Scholar]
- 42.Xiang S, Kim EY, Connelly JJ, Nassar N, Kirsch J, Winking J, Schwarz G, Schindelin H. The crystal structure of Cdc42 in complex with collybistin II, a gephyrin-interacting guanine nucleotide exchange factor. J Mol Biol. 2006;359:35–46. doi: 10.1016/j.jmb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 43.Skowronek KR, Guo F, Zheng Y, Nassar N. The C-terminal basic tail of RhoG assists the guanine nucleotide exchange factor Trio in binding to phospholipids. J Biol Chem. 2004 doi: 10.1074/jbc.M312677200. [DOI] [PubMed] [Google Scholar]
- 44.Worthylake DK, Rossman KL, Sondek J. Crystal structure of the DH/PH fragment of Dbs without bound GTPase. Structure (Camb) 2004;12:1078–86. doi: 10.1016/j.str.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 45.Lemmon MA. Pleckstrin homology domains: not just for phosphoinositides. Biochem Soc Trans. 2004;32:707–11. doi: 10.1042/BST0320707. [DOI] [PubMed] [Google Scholar]
- 46.Lemmon MA, Ferguson KM, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–4. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 47.Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr D Biol Crystallogr. 2001;57:122–33. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 48.Pannu NS, Murshudov GN, Dodson EJ, Read RJ. Incorporation of prior phase information strengthens maximum-likelihood structure refinement. Acta Crystallogr D Biol Crystallogr. 1998;54:1285–94. doi: 10.1107/s0907444998004119. [DOI] [PubMed] [Google Scholar]
- 49.Hart MJ, Eva A, Zangrilli D, Aaronson SA, Evans T, Cerione RA, Zheng Y. Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J Biol Chem. 1994;269:62–5. [PubMed] [Google Scholar]
- 50.Fleming IN, Gray A, Downes CP. Regulation of the Rac1-specific exchange factor Tiam1 involves both phosphoinositide 3-kinase-dependent and -independent components. Biochem J. 2000;351:173–82. doi: 10.1042/0264-6021:3510173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sondermann H, Soisson SM, Boykevisch S, Yang SS, Bar-Sagi D, Kuriyan J. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell. 2004;119:393–405. doi: 10.1016/j.cell.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Soisson SM, Nimnual AS, Uy M, Bar-Sagi D, Kuriyan J. Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein. Cell. 1998;95:259–68. doi: 10.1016/s0092-8674(00)81756-0. [DOI] [PubMed] [Google Scholar]
- 53.Scita G, Tenca P, Areces LB, Tocchetti A, Frittoli E, Giardina G, Ponzanelli I, Sini P, Innocenti M, Di Fiore PP. An effector region in Eps8 is responsible for the activation of the Rac-specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J Cell Biol. 2001;154:1031–44. doi: 10.1083/jcb.200103146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegard M, Betsholtz C, Di Fiore PP. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–3. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 55.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skowronek K, Ghumman M, Zheng Y, Nassar N. Crystallization and initial crystal characterization of the N-terminal DH/PH domain of Trio. Acta Crystallogr D Biol Crystallogr. 2003;59:1273–5. doi: 10.1107/s0907444903009442. [DOI] [PubMed] [Google Scholar]
- 57.Kimple RJ, Willard FS, Hains MD, Jones MB, Nweke GK, Siderovski DP. Guanine nucleotide dissociation inhibitor activity of the triple GoLoco motif protein G18: alanine-to-aspartate mutation restores function to an inactive second GoLoco motif. Biochem J. 2004;378:801–8. doi: 10.1042/BJ20031686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rojas RJ, Kimple RJ, Rossman KL, Siderovski DP, Sondek J. Established and emerging fluorescence-based assays for G-protein function: Rassuperfamily GTPases. Comb Chem High Throughput Screen. 2003;6:409–18. doi: 10.2174/138620703106298509. [DOI] [PubMed] [Google Scholar]
- 59.Otwinowski Z. Maximum Likelihood Refinement of Heavy Atom Parameters. Daresbury Laboratory; Warrington, UK: 1991. [Google Scholar]
- 60.Navaza J. Implementation of molecular replacement in AMoRe. Acta Crystallogr D Biol Crystallogr. 2001;57:1367–72. doi: 10.1107/s0907444901012422. [DOI] [PubMed] [Google Scholar]
- 61.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallogr D Biol Crystallogr. 2005;61:458–64. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 62.The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–3. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 63.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 5):905–21. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 64.Jones TA, Zou JY, Cowan SW, Kjeldgaard Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47(Pt 2):110–9. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 65.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 66.Laskowski RA, W. MM, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993;26:283–291. [Google Scholar]
- 67.DeLano WL. The Pymol User's Manual. Delano Scientific; San Carlos, CA: 2002. [Google Scholar]
- 68.Howlin B, Butler SA, Moss DS, Harris GW, Driessen HPC. TLSANL: TLS parameter analysis program for segmented anisotropic refinement of macromolecular structures. Journal of Applied Crystallography. 1993;26:622–624. [Google Scholar]
- 69.Potterton E, McNicholas S, Krissinel E, Cowtan K, Noble M. The CCP4 molecular-graphics project. Acta Crystallogr D Biol Crystallogr. 2002;58:1955–7. doi: 10.1107/s0907444902015391. [DOI] [PubMed] [Google Scholar]
- 70.Potterton L, McNicholas S, Krissinel E, Gruber J, Cowtan K, Emsley P, Murshudov GN, Cohen S, Perrakis A, Noble M. Developments in the CCP4 molecular-graphics project. Acta Crystallogr D Biol Crystallogr. 2004;60:2288–94. doi: 10.1107/S0907444904023716. [DOI] [PubMed] [Google Scholar]
- 71.Stols L, Gu M, Dieckman L, Raffen R, Collart FR, Donnelly MI. A new vector for high-throughput, ligation-independent cloning encoding a tobacco etch virus protease cleavage site. Protein Expr Purif. 2002;25:8–15. doi: 10.1006/prep.2001.1603. [DOI] [PubMed] [Google Scholar]
- 72.Abramoff M, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 73.Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]