Abstract
Inflammation facilitates tumor progression including metastasis. Interleukin-8 (IL-8) is a chemokine that regulates polymorphonuclear neutrophil (PMN) mobilization and activity and we hypothesize this cytokine influences tumor behavior. We have demonstrated that IL-8 is crucial for PMN-mediated melanoma extravasation under flow conditions. In addition, IL-8 is up-regulated in PMNs upon co-culturing with melanoma cells. Melanoma cells induce IκB-α degradation in PMNs indicating that NF-κB signaling is active in PMNs. Furthermore, the production of IL-8 in PMNs is NF-κB dependent. We have further identified that IL-6 and IL-1β from PMN-melanoma co-cultures synergistically contribute to IκB-α degradation and IL-8 synthesis in PMNs. Taken together, these findings show that melanoma cells induce PMNs to secrete IL-8 through activation of NF-κB and suggest a model in which this interaction promotes a microenvironment that is favorable for metastasis.
Keywords: Cancer, Chemokines, Extravasation, Immunoediting, Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Nuclear factor-κB (NF-κB), Signaling
Introduction
The induction and release of cytokines and chemotactic cytokines (chemokines) that results from inflammation directly influences tumor metastasis, the course of cancer and associated diseases. Metastasis is a sequential process in which a tumor cell is required to transmigrate through the endothelium; an event that necessitates the tumor cell to undergo extensive interactions with host cells [1]. Human polymorphonuclear neutrophils (PMNs), which function as professional phagocytes and are important source of cytokines, actively participate in inflammatory responses and facilitate tumor cell extravasation. A recent study has indicated that melanoma cells by themselves cannot effectively adhere to and migrate through the endothelium under flow conditions. However, when PMNs are present, melanoma cell adhesion and migration are observed [2]. The PMN-facilitated melanoma transmigration correlates with an increase in soluble interleukin-8 (IL-8) production within the melanoma-PMN microenvironment and Mac-1 expression on PMNs [2]. The mechanisms by which melanoma cells and PMNs interact and communicate during metastasis have not been fully elucidated.
Chemokines are a group of peptides with a molecular mass of 8–14 kDa. In addition to their primary function of attracting leukocytes to sites of inflammation, chemokines regulate tumor growth, angiogenesis and metastasis [3–5]. IL-8 is of particular interest because of its ability to mediate PMN infiltration through CXC chemokine receptors 1 and 2 (CXCR1 and CXCR2) [5]. Although CXCR1 has not been shown to be expressed in melanoma cells [6], melanoma cells secrete IL-8, which alters adhesion molecule expression on PMNs. IL-8 regulates ligand binding activity of the β2 integrins of PMNs [7], which, through the binding of intracellular adhesion molecule-1 (ICAM-1) of melanoma cells, are important for PMN-mediated melanoma transmigration under flow conditions [2].
IL-8 mRNA expression and IL-8 protein production are triggered by various stimuli, such as lipopolysaccharide (LPS), tumor necrosis factor (TNF)-α and IL-1β [8, 9]. Accumulating evidence indicates that the transcription of IL-8 gene requires the activation of nuclear factor κB (NF-κB), NF-IL6 (C/EBPβ) or activator protein (AP)-1 [9–14]. Activation of NF-κB has been shown to be indispensable for IL-8 synthesis [13]. Upon activation, NF-κB inhibitor, IκB is proteolytic degraded and dissociates from NF-κB dimers, thereby allowing NF-κB translocation to the nucleus and binding to target genes [15]. Although a role for NF-κB in regulating IL-8 expression has been reported, whether NF-κB is required for melanoma-dependent IL-8 production from PMNs remains unclear. In addition to IL-8, IL-1β, IL-6, and GRO have been shown to regulate the immune response and modulate tumor behavior [4, 11, 16]. In this study, we have found that melanoma cells induce IL-8 synthesis from PMNs through activation of NF-κB. Furthermore, IL-8, IL-1β and IL-6 have been shown to participate in inducing IL-8 expression in PMNs.
Material and Methods
Cell culture and preparation
Human melanoma cell lines WM35 and WM9 were provided by Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA) and maintained in Roswell Park Memorial Institute 1640 medium (RPMI 1640; Biosource, Inc., Camarillo, CA) supplemented with 10 % fetal bovine serum (FBS; Biosource, Inc.) and 100 units/ml penicillin-streptomycin (Biosource, Inc.) at 37 °C under 5 % CO2. 1205Lu cells (a kind gift from Dr. Gavin P. Robertson, Penn State Hershey Medical Center, Hershey, PA) [17] and C8161 cells (obtained from Dr. Danny Welch, University of Alabama, Birmingham, AL) [18] were cultured respectively in Dulbecco’s Modified Eagle’s Medium (DMEM) and DMEM-F12 (Biosource, Inc.) supplemented with 10 % FBS in a standard cell culture condition (5 % CO2 / 37 °C). Correlation of tumor metastatic potentials with melanoma cell invasiveness, chemotactic migration and adhesiveness is shown in Table 1. Fibroblast L-cells that had been transfected to express human E-selectin and ICAM-1 (EI cells; provided by Dr. Scott Simon, University of California, Davis, CA) were maintained in culture as described elsewhere [19]. For experiments, culture medium were removed and fresh RPMI 1640 medium with 5 % FBS were added. The melanoma cells were cultured alone, Transwell (Sigma Chemical Co., St Louis, MO) cultured or contact cultured with human PMNs in 1: 3 ratio at 37 °C.
Table 1. Correlation of tumor metastatic potentials with melanoma cell invasiveness, chemotactic migration and adhesiveness.
Metastatic potential was qualitatively determined by references listed; chemotactic potential was determined by cell migration toward soluble CIV (100 μg/ml) using Boyden chamber; and adhesion molecules were measured by ICAM-1 expression on cells upon TNF-α stimulation (10 U/ml; 24 hours); which are represented by “−”, “+”, “++” and “+++”, for negative, moderate-positive, high-positive, and extremely high-positive, respectively.
| Cell line | Potentials | Reference | ||
|---|---|---|---|---|
| Metastatic | Chemotatic (CIV) | Adhesion (ICAM-1) | ||
| 1205 Lu | ++++ | +++ | +++ | (17) |
| C8161 | +++ | ++ | ++ | (18) |
| WM 9 | ++ | ++ | +++ | www.wistarupenn.edu/herlyn/melcell.htm |
| WM35 | − | + | + | www.wistarupenn.edu/herlyn/melcell.htm |
Isolation and culture of human PMNs
Fresh human blood was drawn from healthy donors under informed consent as approved by the Pennsylvania State University Institutional Review Board. PMNs were separated from blood using Ficoll-Hypaque (Histopaque; Sigma Chemical Co.). After centrifugation at 620 g for 30 min, PMNs were carefully isolated and suspended in Dulbecco’s phosphate-buffered saline (DPBS) with 0.1 % human serum albumin (HSA; Sigma Chemical Co.). Erythrocytes were lysed using ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA; [pH 7.4]) and removed. The cells were washed and resuspended in 0.1 % HSA/DPBS, and gently rocked until they were used in experiments. The cell preparations were 99.5 % pure PMNs, as analyzed by a Diff-Quick stain (Dade Behring Inc., Newark, DE). PMNs were resuspended in RPMI 1640 medium supplemented with 5 % FBS and cultured with or without melanoma cells. For PMN and melanoma cell co-culture, PMNs were added to the confluent melanoma cells, either directly or separated by a Transwell insert with 0.4 μm pore size. In some experiments, PMNs were stimulated with recombinant human IL-8 (12.5, 25 or 125 ng/ml), IL-1β (5, 10 or 25 ng/ml), IL-6 (50 or 100 ng/ml), GRO-α (100 or 200 ng/ml) (Biosource, Inc.) or a cocktail of IL-1β, IL-6 and GRO-α cytokines.
Pharmacological inhibitors and antibodies
PMNs were pretreated with 100 μM pyrrolidinedithiocarbamate (PDTC; Calbiochem, San Diego, CA) for one hour to inhibit NF-κB, and the cells were then washed and co-cultured with melanoma cells for six hours. For anti-human CXCR1 and anti-human CXCR2 (Sigma Chemical Co.) pretreatment, cells were incubated in culture medium containing 0.5 μg/ml anti-CXCR1 and 15 μg/ml anti-CXCR2 monoclonal antibodies (mAbs) for 30 minutes at 37 °C. In GRO-α, IL-1β and IL-6 neutralization studies, cells were exposed to culture medium containing 1 μg/ml anti-human GRO-α, IL-1β and IL-6 (R & D Systems, Inc.) for six hours at 37 °C. Cell viability was verified using trypan blue showing > 95 % of PMNs were alive at the end of these assays.
Western Blots
Cells were collected and washed with cold PBS, then whole cell extracts were prepared by resuspending cells in lysis buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA [pH 8.0], 2 mM Na3VO3, 10 mM NaF, 10 mM Na4P2O7, 1 % NP-40, 1 mM PMSF, 2 ng/ml pepstatin A). Lysates were incubated on ice for 30 minutes followed by a centrifugation at 16,000 g for 1 minute at 4 °C. The pellet was discarded and the supernatant was mixed with 2×SDS running buffer (0.2 % bromophenol blue, 4 % SDS, 100 mM Tris [pH 6.8], 200 mM DTT, 20 % glycerol) in 1 : 1 ratio. Samples were boiled for three minutes and 15 μl were loaded onto a 12 % or 15 % SDS-PAGE gel and proteins were transferred to a 0.2 μm PVDF membrane (Millipore Co., Billerica, MA) by electroblotting. Primary antibodies included rabbit anti-human IL-8 (Biosource, Inc.), anti-IκB-α (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and anti-β-actin IgG1 (Sigma Chemical Co.) Secondary antibodies were peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG. Proteins were detected using the Enhanced Chemiluminescence Detection System (Amersham Pharmacia Biotech, Arlington Heights, IL).
ELISA
At the end of assays, cell-free supernatants were collected by a centrifugation at 430 g for five minutes and stored at −80 °C until enzyme-linked immunosorbent assay (ELISA) was performed. ELISA detection of protein secretion was performed at the Pennsylvania State University NIH Cytokine Core Lab. Mouse anti-human capture antibody to specific target chemokine or cytokine was diluted to 2 μg/ml in coating buffer (0.1 M NaHCO3 [pH 8.2]) and 50 μl was added to each well of the 96-well ELISA plate for overnight incubation at 4°C. The plate was then washed four times with 20 % Tween 20 in phosphate-buffered saline (PBST) [pH 7.0] and blocked with 1 % BSA in PBS for two hours at room temperature.100 μl target chemokine or cytokine standards and samples were added to each well for overnight incubation at 4 °C. The plate was washed four times next day and 100 μl of 0.2 μg/ml biotinylated affinity purified goat anti-human polyclonal detection antibody was added to each well, followed by two hour incubation at room temperature. The plate was then washed six times and incubated with 10 μl streptavidin peroxidase (1 μg/ml, Sigma Chemical Co.) for 30 minutes at room temperature. 100 μl of 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (Sigma Chemical Co.)/peroxide substrate solution was then added for 1 hour in the dark. The plate was read using a microtiter plate reader (Packard, Downers Grove, IL) at a wavelength of 405 nm.
Flow-migration chamber assay
Flow-migration assay was carried out in a modified 48-well chemotactic Boyden chamber (Figure 1A and 1B) [20, 21]. In brief, the polycarbonate chamber consists of a top and bottom plate separated by a gasket. The bottom plate has 48 centered chemotactic wells and screws around the perimeter to affix the top plate. The top plate has an inlet and outlet allowing for circulating flow through the chamber. PVP-free polycarbonate filters (8 μm pore size; NeuroProbe, Gaithersburg, MD) were sterilized and coated with fibronectin (30 μg/ml, 3 hr) (Sigma Chemical Co.). Prior to each experiment, a monolayer of EI cells was grown nearly to 100 % confluence on prepared filters. The chamber’s center 12 wells were filled with soluble chemoattractant, collagen-type IV (CIV; 100 μg/ml in RPMI 1640/0.1 % BSA) (BD Biosciences, San Jose, CA) and control wells were filled with medium (RPMI 1640/0.1 % BSA). The flow loop was primed with warmed medium to eliminate bubbles in the system. An equal number of PMNs and melanoma cells (a total of 106 cells) were put in the chamber. Experiments were run for 4 hours in a 37 °C, 5 % CO2 incubator. At the end of the assay, the flow chamber was removed from the flow loop and disassembled, and the filter was stained with Protocol Brand Hema3 solution (Fisher Scientific, Pittsburgh, PA). Cells on the top of the filter were removed by scraping. In IL-8 neutralization study, 1 μg/ml anti-human IL-8 (R & D Systems, Inc.) was added into the flow loop during the entire flow migration assay.
Figure 1. PMN facilitates melanoma cell migration.

(A) Top view of the flow migration chamber. (B) Melanoma cells extravasate through a cell monolayer in response to chemotactic stimulation from bottom wells under flow conditions. (C) 1205Lu, WM9, C8161 and WM35 cell migration under static conditions. (* P < 0.01 with respect to 1205Lu, WM9, C8161 static cases). (D) PMN affected all four cell lines migration under flow conditions (0.4 dyn/cm2). (& P < 0.05 with respect to 1205Lu + PMN case; # P < 0.05 with respect to the WM9 + PMN case; * P < 0.04 with respect to C8161 + PMN case; ** P < 0.05 with respect to WM35 + PMN case; ## P < 0.02 with respect to 1205Lu + PMN, C8161 + PMN and WM9 + PMN cases). Values are mean ± S.E.M. for n ≥ 3.
Statistical Analysis
All results are shown using mean ± standard error of the mean (S.E.M.) unless otherwise stated. One-way ANOVA analysis was used for multiple comparisons and t-tests were used for comparisons between two groups. P < 0.05 was considered to be significant.
Results
PMN-facilitated melanoma cell extravasation under flow conditions
Correlation of tumor metastatic potentials with melanoma cell invasiveness, chemotactic migration and adhesiveness in term of ICAM-1 expression is shown in Table 1. Melanoma cell chemotactic migration through EI cells in response to CIV (100 μg/ml) was characterized using a flow-migration chamber under both static and flow conditions (Figure 1). EI cells were fibroblast L-cells transfected to constitutively express ICAM-1 and E-selectins used to mimic IL-1β-stimulated human endothelial cells. EI cells did not secrete any cytokine/chemokine (data not shown). As a negative control, non-metastatic NHEM (melanocytes) migration was first tested under static conditions toward CIV and found to be near or at a background level (data not shown). In comparison, 1205Lu, WM9 and C8161 were highly migratory under static conditions. A fourth melanoma cell line, WM35 had lower ICAM-1 expression (data not shown) and modest ability to migrate towards CIV under the static conditions, and the number of cells that migrated was significantly less when compared to the three other melanoma cell lines suggesting that WM35 had low adhesiveness and metastatic potential (Figure. 1C). When exposed to a shear flow (0.4 dyn/cm2), none of the cell lines were able to efficiently extravasate. Importantly, the ability of 1205Lu, WM9 and C8161 to migrate under shear stress were rescued by the addition of PMNs while WM35 had only a modest change (Figure 1D). These results indicate that PMNs significantly facilitate melanoma cell migration through the endothelium under flow conditions.
Co-culturing PMNs with melanoma cells induces IL-8
In order to determine the relationship between tumor metastatic potential and cytokine expression in melanoma-PMN co-cultures, four human melanoma cell lines, 1205Lu, WM9, C8161 and WM35, were tested for their abilities to induce cytokine and chemokines in melanoma-PMN co-cultures. These cell lines have a range of metastatic potentials as reported in the literature as well as determined by migration assays (Table 1), with 1205Lu being the most metastatic, WM9 and C8161 more intermediate in their ability to extravasate and WM35 having the lowest metastatic potential of all four lines. A correlation was found between metastatic potentials of melanoma cells and IL-8 secretion profiles (Figure 2A). PMNs co-cultured either in a Transwell or in direct-contact with 1205Lu, WM9 and C8161 increased IL-8 expression above the summed background level, and co-culturing PMNs with WM35 did not induce IL-8 production. In addition, co-culturing of 1205Lu and WM9 with PMNs induced IL-8 production in a time-dependent manner (Figure 2B). In contrast, the IL-8 production from WM35 and PMN co-culture did not change over time. The induction of IL-8 was comparable whether cells were cultured in direct-contact or separated in a Transwell culture system indicating that melanoma cells modified PMNs activity through a soluble factor.
Figure 2. Co-culturing PMN with melanoma cells induces IL-8 increase.

(A) Induction of IL-8 in PMN-melanoma cell (1205Lu, WM9 and C8161) co-cultures, (contact or Transwell). * P < 0.05 compared with the sum of IL-8 expression from PMN and melanoma only. (B) IL-8 increased after PMN co-culturing with 1205Lu and WM9 in a time-dependent manner. Co-culturing PMN with WM35 did not increase IL-8 secretion over time. Values are mean ± S.E.M. for n ≥ 6.
Melanoma cells induce IL-8 expression in PMNs
In order to identify which cell type contributed to the increased amount of IL-8 in PMN-melanoma co-cultures, cell lysates were prepared from PMNs and melanoma cells following Transwell co-culture, and levels of IL-8 were detected by Western blots. As shown in Figure 3, melanoma cells with higher metastatic potentials (1205Lu, WM9 and C8161) increased IL-8 production in PMNs, while WM35 did not induce IL-8 protein in PMNs. In contrast, IL-8 expression in melanoma cells did not dramatically change when co-cultured in the presence or absence of PMNs (data not shown).
Figure 3. Melanoma cells induce IL-8 production in PMNs.
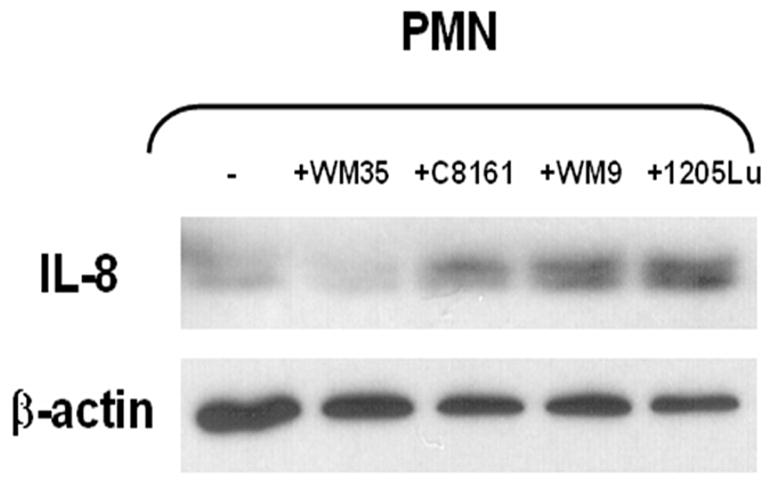
PMN lysates were analyzed by Western blotting with antibody against IL-8. The same blot was stripped and reprobed with β-actin antibody. C8161, WM9 and 1205Lu induced IL-8 production in PMNs, whereas WM35 did not. Results are representative of three experiments.
NF-κB activity is required in melanoma-induced IL-8 production in PMNs
To obtain insight into how melanoma cells signal and alter PMN function, we initially examined activation of NF-κB signaling following co-cultures. Levels of IκB-α in PMNs, which is degraded upon activation of NF-κB signaling to release NF-κB, were monitored by Western blots. As shown in Figure 4, IκB-α protein was degraded within 15 minutes upon co-culturing PMNs with 1205Lu. Similarly WM9 and C8161 induced dpegradation of IκB-α in PMNs in a time-dependent manner (data not shown). No changes in IκB-α were observed in PMNs cultured in the absence of metastatic melanoma cell lines or with WM35 cells (data not shown). These data would suggest that metastatic melanoma cells induce PMN NF-κB activation.
Figure 4. Melanoma cells induce IκB-α degradation in PMNs.

PMN lysates were analyzed by Western blotting with antibody against IκB-α. The same blot was stripped and then reprobed with antibody to β-actin. Time-dependent IκB-α degradation was observed in PMNs in response to melanoma cells. Results are a representative of three experiments.
In order to corroborate the role of NF-κB in regulating IL-8 production in PMNs, we pre-treated PMN with PDTC, an NF-κB inhibitor, for one hour at 37 °C. The amount of IL-8 protein in the presence of PDTC was reduced to summed background levels in the co-cultures (Figure 5A). Furthermore, PDTC suppressed IL-8 protein expression in PMNs following co-culturing with 1205Lu and WM9 as determined by Western blot for IL-8 (Figure 5B). Therefore, activity of NF-κB was required for IL-8 induction in PMNs.
Figure 5. Inhibition of NF-κB reduced IL-8 production in PMNs.

PMNs were pretreated with PDTC, an NF-κB inhibitor, for one hour. Production of IL-8 was inhibited in PDTC-treated PMNs shown by (A) ELISA and (B) Western blotting. ELISA data are mean ± S.E.M. for n ≥ 3. * P < 0.05 compared with the sum of IL-8 expression from PMN and 1205Lu only. Western results are a representative of three experiments.
Cytokines produced from PMN and melanoma cell cultures
Induction of IL-8 in PMNs after co-culturing with metastatic melanoma cells in a non-contact (Transwell) culture system implies that soluble factors play a critical role in the regulation of IL-8 production. To determine the potential factors in the system, a human cytokine array (Raybiotech, Inc., Norcross, GA) was performed to detect forty-two cytokines, chemokines and growth factors from supernatants obtained from cultures of individual cell types or co-cultures in either direct contact or separation using Transwells. Expressions of GRO, IL-1β, IL-6, IL-8 and MCP-1 were identified from the protein array screen (data not shown) and ELISAs were then used for quantitative profiling of the protein expressions. For all four melanoma cell lines tested, GRO-α (Figure 6A) and MCP-1 (data not shown) were constitutively expressed and co-culturing with PMNs did not change their expressions. Modest amount of IL-1β was produced by PMNs or melanoma cells cultured alone. It was slightly increased after 1205Lu, WM9 and C8161 were co-cultured with PMNs (Figure 6B). In addition, co-culture of PMNs with highly metastatic 1205Lu cells significantly induced IL-6 above the summed IL-6 from separate cultures (Figure 6C). These data suggest that the interaction between melanoma cell lines and primary PMNs altered the production of IL-1β and IL-6, in addition to IL-8.
Figure 6. Cytokine secretions from PMN and melanoma cell co-cultures.

(A) GRO-α, (B) IL-1β and (C) IL-6 from PMNs, melanoma cells or PMN co-cultured with melanoma cells were detected by ELISA. * P < 0.05 compared with the sum of IL-6 expression from PMN and 1205Lu only. Values are mean ± S.E.M. for n ≥ 6.
IL-1β and IL-6 induce IL-8 production in PMNs
To investigate the potential role of different cytokines/chemokines in the melanoma-PMN co-culture system on IL-8 production in PMNs, human recombinant proteins including GRO-α, IL-6, and IL-1β were used to stimulate PMNs. Results indicated that none of these cytokines or chemokines alone was sufficient to induce IL-8 production compared with untreated PMNs (Figure 7A). However, stimulating cells with the combination of IL-1β and IL-6 increased IL-8 production in PMNs (Figure 7A). Addition of GRO-α together with IL-1β and IL-6 combination did not further increase IL-8. We have also shown that IL-1β and IL-6 induced IκB degradation within 15 minutes upon stimulation (Figure 7B). Therefore, IL-1β and IL-6 act synergistically to increase IL-8 synthesis in PMNs in response to melanomas.
Figure 7. IL-8 production and IκB-α degradation in PMNs upon cytokine/chemokine stimulation.

(A) GRO-α, IL-6, and IL-1β were used to stimulate PMNs. * P < 0.05 compared with the sum of IL-8 expression from PMN only. Values are mean ± S.E.M. for n ≥ 3. (B) PMN lysates were analyzed by Western blotting with antibody against IκB-α. The same blot was stripped and then reprobed with antibody to β-actin. Time-dependent IκB-α degradation was observed in PMNs in response to combination of IL-1β and IL-6. Results are a representative of three experiments.
Paracrine stimulation of IL-8 in PMNs co-cultured with melanoma cells
In order to determine if IL-8 was responsible for the increase in IL-8 expression, human recombinant IL-8 at concentrations of 12.5, 25 and 125 ng/ml was added to the PMNs. After washing out the IL-8 used to stimulate PMNs, intracellular IL-8 was monitored in PMNs by Western blot. As shown in Figure 8A, addition of IL-8 to the cell culture medium was sufficient to significantly induce intracellular IL-8, compared with untreated PMNs.
Figure 8. Paracrine IL-8 production in PMNs co-cultured with melanoma cells.

(A) Human recombinant IL-8 with concentrations of 12.5, 25 or 125 ng/ml was added to the PMNs. PMN lysates were analyzed by Western blotting with antibody against IL-8. The same blot was stripped and then reprobed with antibody to β-actin. Addition of IL-8 to the cell culture medium significantly induced intracellular IL-8, compared with untreated PMNs. Results are a representative of three experiments. (B) Melanoma-induced IL-8 was reduced after blocking CXCR1 and CXCR2 receptors on PMN or with neutralizing antibodies against IL-1β and IL-6. Combination of anti-IL-1β and anti-IL-6 as well as anti-IL-8 totally inhibited IL-8 induction from co-cultures. * P < 0.05 compared with untreated PMN and melanoma co-culture case.
To confirm that endogenously produced IL-8 induces IL-8 in PMNs, PMNs were pre-treated with anti-CXCR1 and CXCR2 mAbs to block the IL-8 receptors. The amount of IL-8 protein found in the presence of anti-CXCR1 and CXCR2 was reduced compared with the untreated PMN and 1205Lu co-cultures (Figure 8B). In addition, we have also confirmed the roles of IL-1β and IL-6 in the melanoma-mediated IL-8 induction by showing that IL-8 was decreased in the presence of neutralizing antibody against IL-1β and IL-6 (Figure 8B). Mouse anti-IgG was used as a negative control showing there is no significant difference from co-culture case. Most importantly, melanoma cell-induced IL-8 was totally inhibited by anti-CXCR1 and CXCR2 as well as anti-IL-1β and IL-6 indicating the redundancy of signaling pathways that regulate IL-8 production (Figure 8B).
IL-8 is required in PMN-facilitated melanoma cell extravasation
To further elucidate the role of IL-8 produced by PMNs and melanoma cells in melanoma-PMN communication, melanoma cell migration was evaluated in the absence or presence of anti-IL-8 (1 μg/ml) and anti-CXCR1 plus CXCR2 under flow conditions. PMN-facilitated melanoma cell extravasation dramatically decreased under 0.4 dyn/cm2 shear stress in the presence of anti-IL-8 or anti-CXCR1 plus CXCR2 as shown in Figure 9. These results indicate that PMN-mediated melanoma migration is modulated by endogenously produced IL-8.
Figure 9. PMN-facilitated melanoma cell migration is mediated by IL-8.

1205Lu, WM9, C8161 and WM35 cell migration in the absence and presence of anti-IL-8 under flow conditions at 0.4 dyn/cm2. Mouse IgG antibody was used as a negative control. * P < 0.05 with respect to WM35 + PMN case; # P < 0.05 with respect to C8161 + PMN case; ** P < 0.05 with respect to WM9 + PMN case; ## P < 0.05 with respect to 1205Lu + PMN case. Values are mean ± S.E.M. for n ≥ 3.
Discussion
In this study, we have demonstrated that IL-8 is induced in human PMNs that have been co-cultured with metastatic melanoma cells. IL-8 plays an important role in modulating PMN-facilitated melanoma cell extravasation under flow conditions. In addition, melanoma cells induce degradation of IκB-α. A role for NF-κB in regulating IL-8 production has been demonstrated by using a pharmacological inhibitor. Cytokines IL-6 and IL-1β, as well as IL-8 from co-culture media, provide a co-stimulatory signal for the IL-8 induction in PMNs. Taken together, these findings suggest that the production of soluble mediators in response to PMN-melanoma interactions lead to the induction of IL-8 transcription by PMNs, creating a favorable microenviroment for metastasis.
IL-8 plays a crucial role in regulating cell function for host defense and natural immunity [9]. IL-8 is released by various cell types, including PMNs, monocytes, T lymphocytes and endothelial cells, upon exposure to inflammatory stimuli, such as TNF-α, IL-1 and LPS [8, 9, 22]. IL-8, in particular, is a major mediator of PMN activation and migration. IL-8 has been previously shown to activate Mac-1 up-regulation in PMNs which facilitates melanoma cell migration under flow conditions [21]. Consistent with this observation, we have also found that IL-8 neutralization leads to a reduction in melanoma transmigration.
Melanoma cells have been reported to express IL-8 and this influences their oncogenic properties [23], including their metastatic abilities. In the current study, we have shown that IL-8 is constitutively produced by melanoma cells regardless of their metastatic potentials. Furthermore, PMNs do not influence IL-8 expression in melanoma cells. However, tumor metastatic potentials were directly related to the ability of the melanoma cells to induce IL-8 production in PMNs. IL-8 has been shown to induce Mac-1 expression in PMNs [21]; therefore, melanoma cells with high metastatic potentials (1205Lu, WM9 and C8161) directly alter the microenvironment by modifying PMN function and expression of chemokines and integrins to promote extravasation through β2-ICAM-1 bindings under flow conditions, while there is no significant effect under static conditions [21].
Induction of IL-8 at the site of inflammation requires de novo biosynthesis which is in part regulated by transcriptional activation of the IL-8 gene promoter [13, 24]. In particular, NF-κB is induced upon inflammation to coordinate the expression of a number of cytokine and acute response genes. We used IκB-α as an alternative indicator for NF-κB activity since we had difficulty in getting quality nuclear extract from primary PMNs. Our data show that a melanoma-mediated IL-8 production in PMNs occurs through the activation of NF-κB, as we have observed that IκB-α in PMNs is rapidly degraded when co-cultured with melanoma cells. The activation of NF-κB occurs through the activation of IκB kinase leading to phosphorylation of IκB-α, its subsequent ubiquitnation-mediated degradation, and concurrent release and nuclear translocation of NF-κB [15]. The pathway appears to be sensitive to PDTC, since PDTC inhibits downstream IL-8 expression from PMNs. NF-κB also plays a crucial role in controlling IL-8 promoter activities in other cell types [10, 13]. In agreement with previous research, we have demonstrated that expression of IL-8 requires NF-κB. Inhibition of NF-κB has also been shown to suppress surface expression of the cell adhesion molecules such as Mac-1 on monocytic HL-60 cells [25]. Therefore, NF-κB might regulate other genes in PMNs that facilitate melanoma cell adhesion and migration.
The promoter for the IL-8 gene has been well characterized and shown to include two cis-regulatory elements, NF-κB and C/EBPβ binding sites, which have been shown to be necessary for IL-8 gene expression [10, 13]. NF-κB and C/EBPβ can be induced by IL-1 and IL-6 [10, 11, 13, 26]. Our data indicates that IL-1β, IL-6 (data not shown) or combination of IL-1β and IL-6 induces IκB degradation but for induction of IL-8 in PMNs both cytokines are required. Therefore, IL-8 synthesis may depend on the strength of NF-κB activation and cooperation with other transcription factors, such as C/EBPβ. Furthermore, we have confirmed the role of combination of IL-1β and IL-6 in the melanoma-mediated IL-8 induction by showing that IL-8 was reduced in the presence of neutralizing antibodies against IL-1β and IL-6, indicating that melanoma-derived IL-1β and IL-6 act synergistically to regulate IL-8 in PMNs in response to melanomas.
PMN-generated CXC chemokines can act through autocrine or paracrine mechanisms to amplify PMN inflammatory activities via suppression of apoptosis [27]. In the study, paracrine mechanisms are operative since melanoma-derived IL-8 is required for induction of IL-8 in PMNs. IL-8 binds with high affinity to two distinct receptors, CXCR1 and CXCR2. Blocking these two receptors on PMNs significantly reduced IL-8 induction after PMN and melanoma cells are co-cultured, but did not reduce the prodcution of IL-8 to the summed background level of the PMN and melanoma cells indicating that there are cytokines other than IL-8, such as IL-1β and IL-6, which are responsible for the increase of IL-8 secretion after co-culture.
In summary, we have demostrated a melanoma cell and cytokine-induced IL-8 production in PMNs though a NF-κB-dependent pathway. The functional significance of IL-8 is that it facilitates the ability of PMNs to assist melanoma cell extravasation. A better understanding in PMN inflammatory activation in response to tumor cells may provide insights into potenital cancer therapies.
Acknowledgments
Authors thank Dr. Avery August (Penn State University, Department of Veterinary and Biomedical Sciences) for helpful discussions. The Authors are grateful for the support of the Penn State General Clinical Research Center (GCRC) staff who provided nursing care. This work was supported by NSF-BES0138474, NIH-CA 097306, NIH-AI 062467, Grace Woodward Collaborative Research Fund and Johnson & Johnson Innovative Technology Research Fund, and the PA Dept. of Health Research Fund (SAP #41000-26343).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lichtenstein A. Stimulation of the respiratory burst of murine peritoneal inflammatory neutrophils by conjugation with tumor cells. Cancer Res. 1987;47:2211–2217. [PubMed] [Google Scholar]
- 2.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol. 2005;288:C831–839. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frederick MJ, Clayman GL. Chemokines in cancer. Expert Rev Mol Med. 2001;3:1–18. doi: 10.1017/S1462399401003301. [DOI] [PubMed] [Google Scholar]
- 5.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Chan SY, Roczniak S, Shanafelt AB. The Functional Role of the ELR Motif in CXC Chemokine-mediated Angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 6.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 7.Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 8.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immuno. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 9.Strieter RM, Kasahara K, Allen RM, Standiford TJ, Rolfe MW, Becker FS, Chensue SW, Kunkel SL. Cytokine-induced neutrophil-derived interleukin-8. Am J Pathol. 1992;141:397–407. [PMC free article] [PubMed] [Google Scholar]
- 10.Akira S, Kishimoto T. NF-IL6 and NF-kappa B in cytokine gene regulation. Adv Immuno2l. 1997;65:1–46. [PubMed] [Google Scholar]
- 11.Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 12.Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–558. [PubMed] [Google Scholar]
- 14.Stein B, Baldwin AS. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol Cell Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finco TS, Baldwin AS. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 16.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD, Dong C, Robertson GP. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–8209. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welch DR, Bisi JE, Miller BE, Conaway D, Seftor EA, Yohem KH, Gilmore LB, Seftor RE, Nakajima M, Hendrix MJ. Characterization of a highly invasive and spontaneously metastatic human malignant melanoma cell line. Int J Cancer. 1991;47:227–237. doi: 10.1002/ijc.2910470211. [DOI] [PubMed] [Google Scholar]
- 19.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil Tethering on E-Selectin Activates {beta}2 Integrin Binding to ICAM-1 Through a Mitogen-Activated Protein Kinase Signal Transduction Pathway. J Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 20.Dong C, Slattery MJ, Rank BM, You J. In vitro characterization and micromechanics of tumor cell chemotactic protrusion, locomotion, and extravasation. Ann Biomed Eng. 2002;30:713–722. doi: 10.1114/1.1468889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slattery MJ, Dong C. Neutrophils influence melanoma adhesion and migration under flow conditions. Int J Cancer. 2003;106:713–722. doi: 10.1002/ijc.11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marie C, Roman-Roman S, Rawadi G. Involvement of Mitogen-Activated Protein Kinase Pathways in Interleukin-8 Production by Human Monocytes and Polymorphonuclear Cells Stimulated with Lipopolysaccharide or Mycoplasma fermentans Membrane Lipoproteins. Infect Immun. 1999;67:688–693. doi: 10.1128/iai.67.2.688-693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 24.Baggiolini M, Dewald B, Moser B. HUMAN CHEMOKINES:An Update. Annual Review of Immunology. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 25.Lee DH, Tam SS, Wang E, Taylor GR, Plante RK, Lau CY. The NF-kappa B inhibitor, tepoxalin, suppresses surface expression of the cell adhesion molecules CD62E, CD11b/CD18 and CD106. Immunol Lett. 1996;53:109–113. doi: 10.1016/s0165-2478(96)02619-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. IL-6 Induces NF-{kappa}B Activation in the Intestinal Epithelia. J Immunol. 2003;171:3194–3201. doi: 10.4049/jimmunol.171.6.3194. [DOI] [PubMed] [Google Scholar]
- 27.Grutkoski PS, Graeber CT, Ayala A, Simms HH. Paracrine suppression of apoptosis by cytokine-stimulated neutrophils involves divergent regulation of NF-kappaB, Bcl-X(L), and Bak. Shock. 2002;17:47–54. doi: 10.1097/00024382-200201000-00009. [DOI] [PubMed] [Google Scholar]


