Abstract
Chromatin remodelling factors and histone chaperones were previously shown to cooperatively affect nucleosome assembly and disassembly processes in vitro. Here, we show that Schizosaccharomyces pombe CHD remodellers, the Hrp1 and Hrp3 paralogs physically interact with the histone chaperone Nap1. Genome-wide analysis of Hrp1, Hrp3 and Nap1 occupancy, combined with nucleosome density measurements revealed that the CHD factors and Nap1 colocalized in particular to promoter regions where they remove nucleosomes near the transcriptional start site. Hrp1 and Hrp3 also regulate nucleosome density in coding regions, where they have redundant roles to stimulate transcription. Previously, DNA replication-dependent and -independent nucleosome disassembly processes have been described. We found that nucleosome density increased in the hrp1 mutant in the absence of DNA replication. Finally, regions where nucleosome density increased in hrp1, hrp3 and nap1 mutants also showed nucleosome density and histone modification changes in HDAC and HAT mutants. Thus, this study revealed an important in vivo role for CHD remodellers and Nap1 in nucleosome disassembly at promoters and coding regions, which are linked to changes in histone acetylation.
Keywords: CHD, chromatin, fission yeast, gene regulation, nucleosome remodelling
Introduction
In eukaryotes, the basic structural unit of chromatin is the nucleosome. It consists of 146 base pairs of DNA wrapped around a histone octamer that contains two H2A/H2B dimers and one H3/H4 histone tetramer (Luger et al, 1997). The regulation of chromatin structure is central to many cellular processes such as transcription, DNA replication, DNA repair, as well as centromere and telomere function (Ehrenhofer-Murray, 2004; Pidoux and Allshire, 2004). Silent heterochromatin regions and active euchromatin regions are both highly dynamic with respect to the composition of nucleosomes during all phases of the cell cycle (Kimura and Cook, 2001; Chen et al, 2005). Genome-wide studies indicate that reduced histone density in promoter regions correlates well with increased transcriptional activity in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe (Wyrick et al, 1999; Bernstein et al, 2004; Lee et al, 2004; Wiren et al, 2005).
ATP-dependent chromatin remodelling factors of the SNF2 superfamily and histone chaperones play a key role in regulating chromatin assembly (Tyler, 2002; Akey and Luger, 2003; Haushalter and Kadonaga, 2003; Loyola and Almouzni, 2004). The Drosophila Chd1 remodelling factor (dChd1) was recently shown to promote assembly and nucleosome spacing in a catalytic manner by transferring histones from Nap1 onto the DNA template in vitro (Lusser et al, 2005). Conversely, the budding yeast RSC (scRSC) remodelling factor complex facilitated nucleosome disassembly by transferring histones in a stepwise manner to Nap1 in vitro (Lorch et al, 2006). Remodelling factors and chaperones may also affect histone exchange in an independent manner. Both S. cerevisiae and human Nap1 chaperones have a remodelling factor-independent activity in histone H2A.Bdb/H2B and H2A.Z variant exchange in vitro (Okuwaki et al, 2005; Park et al, 2005). Remodelling factors such as scSWI/SNF, hSWI/SNF and scRSC possess nucleosome turnover activity independent of histone chaperones in vitro (Owen-Hughes and Workman, 1996; Lorch et al, 1999; Phelan et al, 2000).
Nap1 binds to all four core histones, but co-purifies mainly with H2A/H2B histones (Ishimi et al, 1984; Ishimi et al, 1987; Ito et al, 1996). The Drosophila, human and plant Nap1 homologs all shuttle to the nucleus during the onset of S-phase (Ito et al, 1996; Rodriguez et al, 2000; Dong et al, 2005). dNap1 and scNap1 interact with the H2A/H2B importin karyopherin kap114, indicative for a function in replication-dependent chromatin assembly (Mosammaparast et al, 2002; Kobor et al, 2004). In S. cerevisiae, Nap1 has a role in transcriptional regulation manifested as a >2-fold change in expression for about 10% of the genes in nap1-deficient cells (Ohkuni et al, 2003). The role in transcription involves effects on elongation through chromatin, since Nap1 in vitro remove histones in front of elongating RNA polymerase (Levchenko and Jackson, 2004), a role reminiscent of that of the FACT complex ‘facilitates transcriptional elongation' (Orphanides et al, 1998). Thus, Nap1 has dual functions both in DNA replication-dependent and -independent histone exchange during transcription.
In general, nucleosome exchange is closely correlated to changes of histone modifications during replication and transcription. Chromatin assembly during replication involves acetylation of newly synthesized histones (Ruiz-Carrillo et al, 1975; Jackson et al, 1976; Allis et al, 1985). Kinetic studies in budding yeast show that transcriptional induction of heat-shock and PHO5 genes is followed by histone hyperacetylation at the promoters resulting in a decreased nucleosome density (Reinke and Horz, 2003; Zhao et al, 2005). The reduction of histones at the Pho5 promoter was later shown to be removed in-trans and not slided along DNA (Boeger et al, 2004). Our previous genome-wide studies in S. pombe revealed a function of histone deacetylases (HDACs) in the regulation of global histone density (Wiren et al, 2005). ACF is required for p300-mediated histone acetylation, leading to a transfer of H2A/H2B histones from a DNA template to Nap1 (Ito et al, 2000). Thus, Nap1 has a role in p300 histone acetylation-dependent nucleosome disassembly, which stimulates transcription (Asahara et al, 2002).
The CHD (chromo-helicase/ATPase DNA binding) remodelling factors are highly conserved and distinguished from other remodelling factors by their double chromodomains (Woodage et al, 1997). The localization of Drosophila Chd1 to interbands of polytene chromosomes first suggested a role in activation of transcription (Stokes et al, 1996). Subsequently, scChd1 was detected at promoters and actively transcribed coding regions (Tran et al, 2000; Alen et al, 2002; Simic et al, 2003). Both mouse and S. cerevisiae Chd1 proteins interact with elongation factors such as FACT components, CkII, Spt5 and Rtf1 (Kelley et al, 1999; Krogan et al, 2002). In addition, the S. cerevisiae chd1 mutant also interacts genetically with elongation factor mutants (Costa and Arndt, 2000; Simic et al, 2003). In both S. pombe and S. cerevisiae, CHD factors are also needed for correct transcriptional termination (Alen et al, 2002).
Here, we have focused on obtaining a genome-wide view of the CHD paralogs Hrp1 and Hrp3 in fission yeast. Our approach has been to combine characterization of CHD interacting proteins with microarray analysis. We performed affinity purifications of Hrp1-associated proteins, cDNA expression profiling and nucleosome density measurements in hrp1 and hrp3 mutants and Hrp1 and Hrp3 occupancy mapping by ChIP–CHIP. Collectively, our data clearly suggest that the main in vivo function of CHD remodelling factors is to promote nucleosome disassembly, which at gene promoters involves the function of the histone chaperone Nap1.
Results
The CHD chromatin remodelling factors Hrp1 and Hrp3 interact with each other and with the histone chaperone Nap1
To identify novel proteins interacting with Hrp1, we performed affinity purifications using Hrp1-FLAG epitope-tagged yeast strains, followed by tryptic in gel digestion and analysis by MALDI-TOF mass spectrometry. Affinity purifications of Hrp1-FLAG extracts yielded co-purification of Hrp3, Cdc68, the translation initiation factor subunits Tif33, eIF3b and eIF3i, heat-shock protein 70, the WD repeat protein SPCC330.09, the probable 26S proteasome regulatory subunit and the Nucleosome assembly protein 1 (Nap1). Hrp3 co-purified with Hrp1-FLAG in near stoichiometric amounts (Figure 1A). Interestingly, our affinity purification of Nap1-TAP identified Nap1.2 (SPBC2D10.11c), an Nap1 paralog, and the casein kinase II alpha subunit as co-purifying proteins (see Discussion). However, Hrp1 was not detected, probably since it was masked by a prominent band representing the fatty acid synthetase beta subunit (see Supplementary Figure 1). Control purifications using cell extracts without the epitope tag were performed using identical conditions and these ruled out unspecific interactions of any of these proteins with the beads (Figure 1B). Next, we performed co-immunoprecipitation (co-IP) experiments using Nap1-TAP Hrp3-myc double tagged strains and this revealed a physical interaction between Hrp3 and Nap1 (Figure 1C). In addition, detectable levels of Hrp1-myc co-immunoprecipitated with Nap1, thus independently confirming the Nap1–Hrp1 interaction. Hence, the Hrp1-FLAG purification analysis provided evidence for a novel physical interaction between CHD remodelling factors and the histone chaperone Nap1. Co-IP experiments confirmed that Nap1, Hrp1 and Hrp3 physically interact in vivo.
Figure 1.

Hrp1, Hrp3 and Nap1 physically interact and bind to the same chromosomal regions. (A, B) NuPAGE Coomassie-stained, gradient gels showing proteins in affinity-purified epitope-tagged Hrp1-FLAG material from the strain Hu1237. The untagged control strain was Hu303. The protein bands from the gel on the left were identified by trypsin digestion and MALDI-TOF mass spectrometry analysis (see text for details). (C) Western blot of co-IP experiments performed using IgG beads on protein extracts from strains with double epitope-tagged Nap1-TAP/Hrp1-myc (strain Hu1439), Nap1-TAP/Hrp3-myc (Hu1440) and the untagged control strain. The co-immunoprecipitated Hrp1-myc and Hrp3-myc proteins were visualized using á-myc antibody (9E10, Sigma). (D, E) Venn diagrams showing comparisons of genome-wide Hrp1-myc (strain Hu764), Hrp3-myc (strain Hu118) and Nap1-myc (Hu1285) IGR and ORF occupancy determined by the percentile ranking method. Significant hypergeometric distribution P-values are indicated.
Overlapping genome wide occupancy of Hrp1, Hrp3 and Nap1 in promoters and coding regions
To determine the in vivo genomic targets for Hrp1, Hrp3 and Nap1, we performed Chip–CHIP according to Wiren et al (2005). Combined intergenic region (IGR) and coding region (ORF) S. pombe microarrays (Eurogentec SA) were used. The IGR probes on this microarray represent all promoter regions (and certain non-coding regions) in the S. pombe genome. The resulting lists of genomic binding targets for Hrp1, Hrp3 and Nap1 were compared using hypergeometric probability tests (See Materials and methods). The similarity in occupancy of Hrp1, Hrp3 and Nap1 was remarkable for IGR (promoter) targets, with hypergeometric P-values ranging from 4.0E-96 to 3.7E-180 (Figure 1D). Although the similarity of ORF targets was less significant as for IGR targets, substantial overlaps (P-values ranging from 4.4E-29 to 5.6E-127 between Hrp1, Hrp3 and Nap1) were obtained (Figure 1E). Similar overlaps in binding were also observed using Affymetrix tiling microarrays (Supplementary Figure 1B). Thus, the genome-wide occupancy profiles strongly supported the physical interactions of Hrp1, Hrp3 and Nap1 and suggested that the physical interactions also occurred at common genomic targets in particular at promoter (IGR) regions.
Hrp1, Hrp3 and Nap1 genomic targets display an increased nucleosome density in hrp1, hrp3 and nap1 mutants
The histone chaperone Nap1 has previously been demonstrated to promote both nucleosome assembly and disassembly in concert with remodelling factors in vitro (Lusser et al, 2005; Lorch et al, 2006). To investigate the genome-wide role of hrp1, hrp3 and nap1 in regulation of nucleosome dynamics in vivo, we determined the histone H3 density of mutant and wild-type (wt) strains using ChIP–CHIP on Eurogentec microarrays. The mutant/wt histone H3 density ratios indicated substantial changes in nucleosome density for all three mutants. To determine the direct effects on histone density changes, we compared the histone density ratio data in each mutant with the binding data. Strikingly, lists of Hrp1 and Hrp3 IGR binding targets almost exclusively overlapped with increased histone H3 density in the hrp1- and hrp3-deficient cells (P-values 6.8E-156 and 2.0E-73, respectively) (Figure 2A and C). A less pronounced but highly significant correlation (P-values 1.8E-81 and 2.8E-25, respectively) was observed for the Hrp1 and Hrp3 ORF targets (Figure 2B and D). None or very few Hrp1 and Hrp3 targets showed low H3 density. Binding of Nap1 to promoter regions correlated with increased H3 density in the nap1 mutant (P-value 4.5E-5), but in coding regions the changes were not statistically significant (Figure 2E and F). Hence, from these results it was evident that Hrp1, Hrp3 and Nap1 occupancy in vivo generally correlated with increased nucleosome densities in the corresponding mutants, and that this effect was most pronounced in promoter regions.
Figure 2.

Hrp1, Hrp3 and Nap1 binding targets show increased histone H3 density in hrp1, hrp3 and nap1 mutants. (A–F) Venn diagrams illustrating Hrp1-myc (Hu764), Hrp3-myc (Hu118) and Nap1-myc (Hu1285) occupancy in IGR and ORF regions as compared with regions with >2-fold changed histone H3 density in hrp1Δ (Hu808), hrp3Δ (Hu807) and nap1Δ (Hu1436) mutants, as indicated. Significant hypergeometric distribution P-values are indicated.
To address if the Hrp remodellers and Nap1 share common binding targets, which also showed increased histone density in the respective mutant, that is, ‘affected targets', we compared the lists of affected targets from the Eurogentec platform for the three proteins (Figure 3A and B). The lists of Hrp1 and Hrp3 affected IGR targets were strikingly similar to each other (hypergeometric probability P=4.0E-243) and to the list of Nap1 affected IGR targets (P-values 1.1E-47 and 1.8E-34, respectively). Regarding the ORF, affected targets Hrp1 targets showed significant similarity to Hrp3 and Nap1 (Hrp1 versus Hrp3, P=7.6E-61; Hrp1 versus Nap1, P=9.3E-7). This suggested that the primary function of Nap1 in the context of Hrp1 and Hrp3 is to promote disassembly of nucleosomes. The functional relationships of Nap1 and Hrp1/3 were further investigated by analyzing their mutual dependencies at genomic binding sites using the Affymetrix GeneChip® S. pombe tiling microarray platform (See Materials and methods). The nucleosome density at Hrp1/3 binding sites was significantly increased in nap1Δ (Supplementary Figure 2; hypergeometric P-value=4.1E-122). This suggested that Nap1 is involved in nucleosome disassembly at Hrp1/3 targets. The reciprocal correlation was less clear (P=0.014) suggesting that Hrp1/3 may recruit Nap1 and not vice versa.
Figure 3.

The direct effects of Hrp remodellers and Nap1 on nucleosome assembly occur mainly in promoter regions and near the transcriptional start site. (A, B) Venn diagrams showing the overlap between Hrp1-, Hrp3- and Nap1-affceted targets with increased histone H3 density. Left: promoter (IGR) and right: (ORF) coding regions. Significant hypergeometric distribution P-values are indicated. (C, D) Moving average plots of Eurogentec microarray datasets, which illustrate the genome wide tendencies in for increased nucleosome density as a function of for Hrp1, Hrp3 and Nap1 binding (moving average window size=100, step=1) (C) The mutant/wt histone H3 ratio at promoter (IGR) regions (moving average window size=100, step=1). (D) The mutant/wt histone H3 ratio at coding (ORF) regions (window size=100, step=1). (E) A graph representing Affymetrix tiling microarray data, showing the effects of hrp mutations on nucleosome density. The distribution of median values for hrp1Δ and hrp3Δ tiling microarray histone H3 ratios are shown across promoter and ORF regions. Only genes with enriched Hrp1 (red) and Hrp3 binding (blue) were selected (ratio of tiling Affymetrix microarray ChIP signals/input signals >1). A region of 2000 bp upstream and downstream of the ATG start codon is shown. (F) Top panels: graphs representing Affymetrix microarray data for histone H3 densities in hrp1, hrp3 and nap1 mutants across representative loci. Bottom panel: graphs representing Affymetrix microarray data for occupancy of Hrp1, Hrp3 and Nap1 across representative loci (as indicated).
Next, we examined the genome-wide roles of Hrp1, Hrp3 and Nap1 by performing moving average plots of mutant/wt histone H3 ratios as a function of IGR and ORF binding (using the Eurogentec platform). The plots revealed a clear tendency for an increased hrp1Δ/wt histone H3 density ratio with increased Hrp1 IGR binding (Figure 3C). The Hrp3 plot showed a similar but less pronounced tendency and Nap1 data gave a weaker positive trend. Regarding the ORF regions, the plots indicated positive tendencies for Hrp1 and Hrp3 but not for Nap1 (Figure 3D). Furthermore, the lists of Hrp1 affected IGR targets almost exclusively overlapped with lists of IGR targets with a high H3 density in wt cells (P=1.4E-92), whereas a similar bias for was not seen for Hrp1 affected ORF targets (Supplementary Figure 2). A similar situation was observed for Hrp3 (P=2.0E-52, data now shown). Taken together, these results suggest that CHD remodellers and Nap1 primarily affect promoter regions where they act to disassemble nucleosomes. In addition, Hrp1 and Hrp3 show a clear preference for nucleosome dense promoters.
The roles of Hrp1 and Hrp3 at the transcriptional start site
The Eurogentec microarray platform has a 500 bp probe fragment for each IGR and ORF region. Therefore, to determine the effect of Hrp1 and Hrp3 on nucleosome density with higher resolution, we also used the Affymetrix GeneChip® S. pombe tiling array for H3cter ChIP–CHIP. The median histone H3 chromatin immunoprecipitation (ChIP) ratios in hrp1/wt and hrp3/wt were calculated for Hrp1 and Hrp3 gene targets defined by ChIP using the same Affymetrix tiling microarray platform. Strikingly, the analysis revealed a distinct peak (a peak at 1.35 around −200) of the mutant/wt histone H3 ratios at the transcriptional start site (TSS) in both hrp1 and hrp3 mutants (Figure 3E). This suggested a direct in vivo function for both CHD factors in removing nucleosomes in particular from a region around the TSS. In addition, consistent with earlier studies (Walfridsson et al, 2005), the silent regions, centromere, telomere and mating type regions were affected by Hrp1, Hrp3 and Nap1 (Supplementary Figures 4 and 5). Regarding the TSS, the median values show clear tendencies for increased H3 densities near the TSS for nap1 and hrp1 mutants. However, this does not rule out effects also in the 3′ regions. By examination of specific regions we found several examples of specific loci showing 3′ binding and H3 density increase in nap1, hrp1 and hrp3 (Figure 3F; SPBC1539.02c). We also have evidence for 5′ promoter and 3' binding of Hrp1, Hrp3 and Nap1 and H3 density increase in the mutants (Figure 3F; cps1+). Thus, although we see prominent genome-wide effects in the promoter regions, our data are also consistent with the role described earlier for Hrp1 in transcriptional termination at 3′ ends (Alen et al, 2002). Interestingly, this function also involves the histone chaperone Nap1.
Hrp1-mediated nucleosome disassembly is independent of DNA replication
Nucleosome assembly and disassembly can occur via the DNA replication-dependent pathway, defined as nucleosome dynamics coupled to DNA synthesis, or via the DNA replication-independent pathway representing nucleosome dynamics outside S-phase. In order to determine if Hrp1-mediated nucleosome disassembly occurred via the replication-dependent pathways, we performed a replication-independent histone assembly assay according to Choi et al (2005). The cells were arrested before S-phase using hydroxyurea (HU), which inhibits ribonucleotide reductase and thereby depletes the cellular dNTP pools. In this situation, hemagglutinin (HA) epitope-tagged histone H3 (H3–HA) was ectopically expressed from a plasmid carrying the inducible inv1 promoter (pInv1-H3–HA). The cells were subjected to ChIP and the HA-tagged H3, as well as the endogenous histone H3 levels were quantified in wt and hrp1 cells. This assay demonstrated a ∼8-fold increase of endogenous H3 at the brr2+ promoter (compared with 4.0–6.9 in the ChIP–CHIP data), and a ∼5-fold increase at the SPAC11E3.14 promoter (compared with 2.0–4.7 by ChIP–CHIP). In addition, ectopically expressed H3-HA revealed a ∼4-fold increase in the hrp1 mutant at both the brr2+ and SPAC11E3.14 promoter regions (Figure 4A). The brr2+ and SPAC11E3.14 genes were selected for the assay, since these promoters displayed more than a two-fold increase in histone density in the hrp1, hrp3 and nap1 mutants and binding. The brr2 gene is in addition 1.7-fold downregulated in the hrp1 hrp3 double mutant. A Western blot of induced and non-induced control protein extracts confirmed the specific induction of the pInv1-H3–HA expression (Figure 4B). Thus, these results suggested that Hrp1 acts independently of DNA replication, since the nucleosome density increases in hrp1 mutants occurred in HU-arrested cells.
Figure 4.
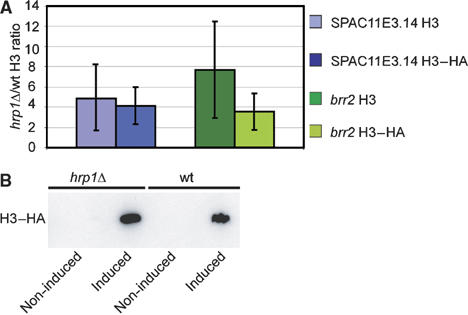
Hrp1 mediates histone disassembly independently of DNA replication. (A) The bar diagram represents the mean ratios and standard deviation (hrp1Δ/wt) of histone H3–HA ChIP using ectopically expressed (pInv1-H3–HA) and endogenous histone H3. The ChIP levels at brr2+ and SPAC11E14 promoter regions were assayed by real-time PCR (as indicated). Three independent measurements were recorded. The cells were treated with HU to arrest cells before S-phase. Each experiment was performed in duplicate and the average ChIP enrichment is indicated. (B) Western blot of non-induced and induced pInv1-H3–HA expression from HU-treated hrp1 cells (Hu1535) and wt control cells (Hu1539). The histone H3–HA fusion proteins were visualized using á-HA antibodies (12CA5, Roche).
Hrp1 and Hrp3 have redundant roles in regulation of gene expression
The above results pointed out a role for Hrp1 and Hrp3 in DNA replication-independent nucleosome disassembly in particular at promoter regions, which are likely linked to regulation of transcriptional initiation. Therefore, we wished to compare effects on gene expression with the nucleosome density changes in hrp1 and hrp3 mutants. To this end, we performed expression profiling experiments using Eurogentec ORF microarrays according to Xue et al (2004). Only a handful of genes were more than two-fold up or downregulated in hrp1 and hrp3 mutants (data not shown). Given the close similarity of Hrp1 and Hrp3 (65% identical amino-acid sequences), it is possible that they perform redundant functions. Therefore, an hrp1 hrp3 double mutant was used for expression profiling. In sharp contrast to the single mutants, the double mutant caused substantially more genes to be differentially regulated (Figure 5). A total of 580 genes was upregulated and 238 genes were two-fold down regulated, providing support for the notion that Hrp1 and Hrp3 normally function jointly both as repressors and activators of transcription.
Figure 5.

Gene expression in coding regions directly affected by Hrp1 is significantly downregulated genes in an hrp1 hrp3 double mutant. (A, B) Venn diagrams representing the overlap between downregulated and upregulated genes in the hrp1 hrp3 double mutant (Hu879) and Hrp1-bound targets with high H3 density in hrp1- or Hrp3-bound targets with high H3 density in hrp3. Significant hypergeometric distribution P-values are indicated (A) IGR regions (B) ORF regions.
To determine direct effects in gene regulation, we compared Hrp1- and Hrp3-affected targets with up- and downregulated genes in the hrp1 hrp3 double mutant. Analysis of these data sets revealed a significant overlap (P=1.69E-2) between nucleosome density-affected Hrp1 or Hrp3 ORF targets and downregulated genes in the hrp1 hrp3 double mutant (Figure 5B). Although the corresponding IGR data comparison was not statistically significant, 9.2% of the downregulated genes showed Hrp1 binding and increased H3 density in hrp1 or Hrp3 binding and increased H3 density in hrp3 (Figure 5A). Thus, Hrp1 and Hrp3 ORF targets were significantly downregulated in the hrp1 hrp3 double mutant, suggesting that nucleosome disassembly stimulates transcription. No other statistically significant links were revealed by these comparisons, indicating that additional factors also contribute to transcriptional regulation at a majority of the Hrp1 and Hrp3 IGR targets.
A link between histone acetylation and regulation of nucleosome density
Histone acetylation has been reported to mobilize nucleosomes or stimulate histone exchange (Reinke and Horz, 2003; Boeger et al, 2004; Zhao et al, 2005). Our previous work indicated that Sir2 and Hos2 HDACs have important genome-wide roles in regulation of nucleosome density in fission yeast (Wiren et al, 2005).
Therefore, we first compared the lists from Eurogentec data of Hrp1-, Hrp3- and Nap1-affected targets with lists of Sir2 and Clr3 targets, which showed nucleosome density changes in respective HDAC mutant (Figure 6A). It was clear that Hrp1-affected IGR targets correlated with Clr3 targets showing increased (P=1.2E-18) and decreased (P=3.9E-19) densities. The list of Hrp1-affected IGR targets was significantly similar to those of Sir2 IGR targets with increased (P=1.6E-16) and decreased (P=4.1E-14) densities. Hrp1-affected ORF targets were also significantly similar to those of Clr3 and Sir2 (Figure 6B). For Hrp3, the affected IGR and ORF targets correlated well with those of Clr3 and Sir2. Regarding the Nap1 targets, the only significant comparison was that to Sir2 and Clr3 IGR targets that showed increased density. Thus, these comparisons suggested that HDACs contribute to nucleosome density regulation in Hrp1-, Hrp3- and Nap1-affected regions.
Figure 6.

Histone H3 density changes in HDAC and HAT mutants at Hrp1-and Hrp3-ffected targets and a comparison with histone methylation. (A, B) The tables show comparisons of Clr3, Sir2 promoter (IGR) and ORF targets,with increased and decreased histone H3 density in the respective mutant, compared with corresponding Hrp1, Hrp3 and Nap1 data sets (Eurogentec platform). The P-values were calculated using hypergeometric distribution tests. The number of targets in each data set and the number of targets in the intersection between the datasets are shown within the brackets. (C) The image represents a gene cluster analysis of histone H3 density using the ‘Pearson' correlation method. The different mutant datasets (as indicated) were used to compare histone H3 density changes mutant/wt at the Hrp1-bound promoter (IGR) regions (n=429). The color scale represents relative changes in histone H3 density. (D) The table shows a comparison of gene lists for Hrp1-, Hrp3- and Nap10-affected targets, with lists for genes with high or low wt histone methylation levels (data H3K9me2 and H3K4me2 using the Eurogentec platform; Opel et al, 2007). The P-values were calculated using hypergeometric distribution tests.
To further investigate the relationship between histone acetylation and nucleosome density regulation by Hrp1 and Hrp3, we examined the effect of mst2 and gcn5 HAT mutants on genome-wide nucleosome density by ChIP–CHIP (see Materials and methods), using the Eurogentec microarrays. We focused this analysis on IGR regions, since they were generally more affected by hrp1and hrp3 (Figures 2 and 3). We employed ‘Pearson' correlation clustering analysis of hrp1, hrp3, nap1, mst2, gcn5, clr3, clr6, sir2 and hos2 mutant/wt ratio H3 density data sets (filtered for Hrp1 IGR and ORF binding targets). In the generated cluster tree, hrp1, hrp3 mutants grouped closely together in one branch (Figure 6C). Moreover, the mst2 and gcn5 HAT mutants grouped most closely to nap1, and consequently displayed similar general trends in H3 density changes. The clr3, clr6, hos2 and sir2 HDAC mutants clustered together. Interestingly, HDAC mutants showed both agonistic and antagonistic effects on nucleosome density at Hrp1- and Hrp3-affected IGR targets, whereas HAT mutants and Nap1 predominantly showed agonistic effects, indicating that the HAT enzymes and the Nap1 chaperone primarily promote disassembly of nucleosomes by Hrp1 and Hrp3. In addition, a comparison of Hrp1-, Hrp3- and Nap1-affected targets with histone H3K9me2 and H3K4me2 methylation in wt cells (Opel et al, 2007) revealed a high correlation with high levels of H3K9me2 at IGR regions, indicating that the chromodomains of Hrp1 and Hrp3 might target the remodelling factors to these regions. Interestingly, the chromodomains of Hrp1 and Hrp3 lack the conserved aromatic pocket residues essential for beta-strand formation in chromo-barrel domains (Nielsen et al, 2005), and are thus ‘typical' chromodomains similar to that of Swi6 that binds H3K9me2 (Nielsen et al, 2002).
Discussion
Hrp1, Hrp3 and Nap1 interacting proteins
The affinity purifications and co-IP experiments revealed physical interactions between the CHD remodelling factors Hrp1, Hrp3 and the histone chaperone Nap1. Our previous genetic studies showed functional interactions between hrp1 and hrp3 in centromere silencing and chromosome segregation (Jae Yoo et al, 2002; Walfridsson et al, 2005). The physical interaction between Hrp1 and Hrp3 observed here confirms a shared role for these remodellers, as was suggested by our previous genetic experiments. Furthermore, the ChIP–CHIP binding data using both Eurogentec and Affymetrix tiling microarrays demonstrated that Nap1, Hrp1 and Hrp3 share in vivo binding targets to a remarkable extent. In addition to these intimate interactions between the CHD proteins and Nap1, we also detected other interacting proteins. For example, the FACT component Cdc68 was detected in Hrp1-FLAG affinity-purified material. FACT components have previously been reported to interact with CHD1 homologues both in S. cerevisiae and mammalian cells (Kelley et al, 1999; Krogan et al, 2002). This suggests that interactions between CHD proteins and FACT are evolutionarily conserved. It is also interesting to note that the Nap1-TAP-purified material revealed the presence of the CkII alpha subunit of casein kinase, in nearly stoichiometric amounts. Casein kinase was earlier demonstrated to phosphorylate human Nap1 and Nap2, and to have a role in cell cycle-dependent localization of human Nap2 (Rodriguez et al, 2000). Furthermore, in S. cerevisiae, CkII kinase co-purifies with scChd1 (Krogan et al, 2002), and in Drosophila, CkII phosphorylation regulates ATPase activity and nucleosome affinity of the CHD containing remodelling complex dMi2, (Bouazoune and Brehm, 2005). Thus, our finding raises the possibility for similar functional links between Hrp1, Hrp3 and casein kinase in S. pombe.
Cooperation between CHD remodellers and Nap1 in nucleosome disassembly
Earlier experiments indicate that SNF2 superfamily of remodellers and Nap1 can act both in disassembly and in assembly of nucleosomes in vitro. The Drosophila Chd1 remodeller stimulates transfer of histones from Nap1 onto DNA (Lusser et al, 2005), whereas the RSC remodeller together with Nap1 act cooperatively in nucleosome disassembly in vitro (Lorch et al, 2006). At this moment, it is not clear if all remodellers can stimulate both assembly and disassembly dependent on the experimental conditions used, or if these contrasting in vitro results reflect genuine differences between different classes of remodellers. In any case, our study provides convincing in vivo evidence for a shared function for members of the CHD subclass SNF2 superfamily of remodellers and histone chaperones in promoting histone mobilization from DNA. First, from the genome-wide nucleosome density (ChIP–CHIP) experiments it was remarkably clear that the main role of Hrp1 and Hrp3 remodellers in vivo is to disassemble nucleosomes. Second, although the nucleosome density both increased and decreased at Nap1 binding sites in the nap1 mutant, only increased density correlated with hrp1 and hrp3 data. Thus, in vivo Nap1 alone or together with other cofactors is likely to have a function in nucleosome assembly. However, together with the Hrp remodellers, Nap1 primarily stimulates histone removal from promoter regions. Interestingly, it was also clear from the comparisons made in Figure 3A and B that the overlaps between Hrp3 and Nap1 affected targets completely depend on Hrp1 , that is, no targets were shared exclusively between Hrp3 and Nap1, suggesting that Hrp1 mediates the interaction between Nap1 and Hrp3.
Direct effects in histone density changes overlaps with histone acetylation mutants
The extensive overlap between genome-wide data sets from the remodeller mutants hrp1, hrp3, the chaperone mutant nap1, the HAT mutants mst2 gcn5, and HDAC mutants clr3, sir2 in histone density changes, suggested that histone modifications, remodelling factors and histone chaperones are mechanistically coupled in histone assembly and disassembly. In addition, we found a substantial correlation between Hrp1-, Hrp3- and Nap1-affected targets and high levels of H3K9me2 in wt cells at IGR regions, suggesting that the chromodomains of Hrp1 and Hrp3 target the remodelling factors to these regions. Thus, once the Hrp remodelling factors are recruited to these regions, there are at least three distinct possible models: First, acetylation patterns generated by HATs and HDACs enzymes could be functioning as a marks for CHD and Nap1 proteins to remove specific subsets of histones. Such a regulatory mechanism would be in agreement with some kinetic studies in budding yeast, since the induction of heat-shock genes and PHO5 is governed by histone acetylation and subsequently nucleosome eviction at the promoters (Reinke and Horz, 2003; Zhao et al, 2005). Consistent with this first model, there is precedence for histone disassembly of acetylated nucleosomes, which involves Nap1 (Ito et al, 2000). A second possibility is that the Hrp and Nap1 proteins would be involved in stimulating histone acetylation and/or deacetylation, which subsequently leads to nucleosome disassembly. This idea is supported by the finding that scChd1 interaction to methylated histones is required for histone acetylation by SLIK (Pray-Grant et al, 2005). A third possibility is that remodelling would be a requirement for histone acetylation or deacetylation. In human cells, CHD proteins have been shown to be components of the Mi2 HDAC complex, which mediates histone deacetylation (Tong et al, 1998; Zhang et al, 1998). Our experiments do not distinguish between these possibilities. Nevertheless, our findings are likely to represent a novel in vivo interplay between HDACs, HATs and histone chaperones that together with remodelling factors are contributing to nucleosome disassembly.
Replication-independent nucleosome disassembly by Hrp1
The first demonstration that nucleosome depletion affects gene expression were the studies in S. cerevisiae in which histone expression was turned off from a regulatable promoter, leading to changed expression of certain genes (Han and Grunstein, 1988; Han et al, 1988). More recently, the consequences of histone depletion in S. cerevisiae were investigated by genome-wide methods (Wyrick et al, 1999). In these studies, 10% of all genes showed reduced expression and 15% showed increased expression upon H4 depletion. In general, regulated changes in nucleosome density resulting in differentiated gene expression could either be the consequence of replication-dependent or replication-independent pathways. Here, we show that nucleosomes are disassembled from promoters of which bind Hrp1, Hrp3 and Nap1 proteins and that this takes place without DNA replication. This is likely to stimulate gene expression, since the same genes were found to be downregulated in the hrp1 hrp3 double mutant. In addition, we also show that histone density increases in the coding regions correlate with downregulation of gene expression in the hrp1 hrp3 double mutant genome wide. It is possible that the effects that we observe in S. pombe coding regions are related to what was recently observed for the S. cerevisiae Chd1 enzyme. Chd1 stimulates transcriptional elongation by RNA pol II through nucleosome barriers in vitro (Carey et al, 2006). Thus, it is clear that Hrp1, Hrp3 and Nap1 proteins in S. pombe act in vivo to promote nucleosome disassembly, which stimulates different parts of the transcription cycle: initiation and elongation by RNA pol II.
Materials and methods
Strains and media
The relevant genotypes of the strains used in this study are listed in Table I. Genetic techniques were performed according to Moreno et al (1991). The endogenously C-terminally epitope-tagged hrp1-FLAG and nap1-TAP strains were generated using a PCR-based approach described in Bahler et al (1998). The hrp1-FLAG-tagged strain was constructed using the modified pFA6-2 × FLAG-kanMX6 plasmid (Linder and Gustafsson, 2004).
Table 1.
Fission yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| Fy367 | h- leu1-32 ura4-D18 ade6-M210 | |
| Hu118 | hrp3-myc::kanMX6 leu1-32 ura4-D18 ade6-M210 | Walfridsson et al (2005) |
| Hu303 | 972 h- | |
| Hu764 | hrp1-myc::kanMX6 leu1-32 ade6-M216 | Walfridsson et al (2005) |
| Hu807 | h- hrp3:: leu2 leu1-32 | This study |
| Hu808 | h- hrp1::leu2 leu1-32 | This study |
| Hu879 | h- hrp1:: leu2 hrp3:: leu2 leu1-32 ura4-D18 ade6-M210 | This study |
| Hu984 | h- gcn5::kanMX6 leu1-32 ura4-D18 ade6-M210 | A Wright |
| Hu988 | h- mst2::kanMX6 leu1-32 ura4-D18 ade6-M210 | A Wright |
| Hu 1237 | hrp1-FLAG::kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| Hu1285 | nap1-myc::kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| Hu1286 | nap1-TAP::kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| Hu1436 | h- nap1::kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| Hu1439 | nap1-TAP::kanMX6 hrp1-myc::kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| Hu1440 | nap1-TAP::kanMX6 hrp3-myc::kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| Hu1535 | hrp1::ura pInv1-H3-HA::leu2 leu1-32 ura4DS/E adeM216 | This study |
| Hu1539 | pInv1-H3-HA leu1-32 ura4DS/E adeM216 | This study |
Replication-dependent/independent histone H3 density assay
The replication-dependent/independent histone H3 assay was performed as described in Choi et al (2005), with the following modifications: after sucrose induction, the Hu1539 (wt) and Hu1535 (hrp1Δ) cells harboring the pInv1-H3–HA plasmids were subjected to ChIP, as described in Walfridsson et al (2005). Proteins crosslinked to DNA were immunoprecipitated using 1:100 dilutions of anti-HA antibody (12CA5, Roche) and the anti H3 C-terminal antibody (AB1781, Abcam). Immunoprecipitated and input DNA were quantified using real-time PCR (Applied Biosystems). Ratios of chromatin immunoprecipitated histone H3 levels were calculated using the formula: (immunoprecipitated DNA/Input)hrp1Δ/(immunoprecipitated DNA/Input)wt and background from beads was subtracted from the ChIP signals.
Protein purification
TAP purifications were carried out according to Spahr et al (2003). A 15 l volume of cells was grown to mid-log phase in YEA media (5 g/l yeast extract, 2 g/l casamino acids and 20 g/l glucose) and harvested at 8000 r.p.m. at 4°C for 10 min using a Beckman JA-10 rotor. Cell pellets were washed in ice-cold PBS, resuspended in 3 × lysis buffer (0.45 M Hepes–KOH (pH 7.6), 30% (v/v) glycerol, 3 mM EDTA, 0.15 M KCl, 3 mM DTT, protease inhibitors (Roche)). Cells were disrupted using a Bead beater (Stratech) in liquid nitrogen, for 25 cycles, 30 s of beating and 90 s of rest. Cell debris was pelleted by centrifugation as above, resuspended in 1/9 volume of 3 M potassium chloride and stirred for 15 min at 4°C. Protein extracts were centrifuged using the Beckman 45-Ti rotor 42 000 rpm at 4°C for 30 min. Next, the protein extracts were incubated with IgG-agarose beads (Sigma) for 1 h at 4°C. The beads were transferred to a column and washed with 30 ml of IgG buffer (10 mM Tris–HCl pH 8.0 150 mM NaCl, protease inhibitors), followed by 20 ml wash using TEV buffer (10 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.1% NP-40). The TAP tag was cleaved of by incubation of 200 U of TEV protease for 2 h at 16°C, eluted and separated on 4–12% NuPage gradient gels (Invitrogen).
In the Hrp1-FLAG purifications, cells were grown and disrupted in the same way as described for the TAP purifications. After ultracentrifugation, the protein was incubated with anti-FLAG beads (Sigma), washed two times in 50 ml IgG buffer (10 mM Tris–HCl pH 8.0, 150 mM NACl, 0.5 mM DTT) for 15 min, at 4°C, transferred to 1.5 ml tubes and washed five times with TEV binding buffer (10 mM Tris–HCl, 150 mM NACl, 0.1% (v/v) NP-40, 0.5 mM DTT, 10% (v/v) glycerol) at 4°C, for 5 min. Next, protein was eluted using FLAG peptides (Sigma) by incubation for 15 min. Finally, the protein extract was separated as described for TAP purification.
Co-IP
For the co-IP the protein extracts were prepared similarly as the TAP purifications, with some exceptions. Cells were grown in 500 ml YEA media, harvested and resuspended using the same conditions as in the TAP purification. The cells were lysed using a ‘Fast Prep FP120' bead beater (ThermoSavant), five times for 20 s, with 2 min rest, at 4°C. Protein extracts were incubated with IgG-agarose beads for (Sigma) for 1 h at 4°C and washed five times in IgG buffer, followed by two washes using TEV buffer. Finally, the samples were subjected to Western blotting. Dilutions (1:5000) of primary antibody 9E10 α-myc (Sigma), or 1:1000 of the α-TAP antibody (CAB1001, Open Biosystems) and 1:2000 of α-mouse (Sigma) or α-rabbit horseradish peroxidase-conjugated secondary antibodies (Sigma), together with ECL (Amersham Pharmacia), were used to visualize the proteins.
Protein analysis with mass spectrometry
Matrix-assisted laser desorption ionization (MALDI) time-of-flight (TOF) analysis of in-gel digested proteins was carried out with an Ultraflex II TOF/TOF mass spectrometer from Bruker (Billerica, MA). The samples were prepared as described previously (Spahr et al, 2001). Mass spectra were obtained in the positive ion mode at an acceleration voltage of 25 kV and a pulsed ion extraction time of 80 ns. A total of 300 shots were used to combine one spectrum. Database searches were performed with the MS BIOTOOLS software (Bruker) by using the Mascot search engine (Matrix Science, London, UK).
Expression profiling and ChIP on CHIP microarray
Expression profiling experiments were performed and quantified according to Xue et al (2004). Data were normalized using Lowess per spot per chip intensity-dependent Gene Spring v 7.2 (Silicon Genetics). IGR and ORF DNA microarrays (Eurogentec) and GeneChip® S. pombe Tiling Array (Affymetrix) were used to create ChIP on CHIP protein binding and histone H3 density maps. Cells cultures for both microarray platforms were grown to mid-log phase, harvested, subjected to formaldehyde fixation, lysed and chromatin immunoprecipitated, as described in Sinha et al (2006). Antibodies specific for α-myc (9E10, Sigma) and anti-H3 C-terminal antibody (AB1791, Abcam) were used for the ChIP.
For the Eurogentec ChIP on CHIP DNA microarrays, 1.0 μg of input DNA and ChIP DNA was labelled with either Cy3 or Cy5 fluorescent dyes, respectively, and both labelled samples were together hybridized and quantified according to Wiren et al (2005). All protein binding experiments and the histone H3 density experiments were performed in duplicate or triplicate, generating four to six data points, since each microarray slide contains duplicate spots for each fragment. In addition, we performed Cy3 and Cy5 dye swaps for each experiment to correct for dye bias. ChIP–CHIP data for HDACs (Wiren et al, 2005) were used to investigate direct effects on nucleosome density changes in the same way as described above for the Hrp1, Hrp3 and Nap1.
For the Affymetrix tiling microarrays, ChIP DNA was extracted as described above and amplified to 5.0 μg. Next, the DNA was fragmented to ∼100 bp using DNAse I, followed by biotin labelling and hybridized according to Lengronne et al (2004). The Affymetrix tiling microarray experiments to determine H3 density throughout genes at and binding of Hrp1, Hrp3 and Nap1 were performed in duplicates.
Data analysis
Normalizations and data analysis of both Eurogentec DNA ChIP on CHIP and Affymetrix Tiling array were carried out according to Sinha et al (2006), using Gene Spring v 7.2. (Silicon Genetics). Data were quality control filtered, transformed to set measurements below 0.01–0.01, followed by normalization to the 50th percentile.
Histone H3 densities were calculated by dividing normalized histone density measurements in respective mutant, with corresponding wt measurements. Cut-off values were set to two-fold enrichment or reduction in histone H3 density in at least three of four, or four of six data points. For the binding maps, median percentile ranking was applied to statistically extract enriched binding (Hrp1 IGR 90th percentile, Hrp1 ORF 92nd percentile, Hrp3, Hrp3 IGR 94th percentile, Hrp3 ORF 92nd percentile, Nap1 IGR 90th percentile and Nap1 ORF 90th percentile) (Buck and Lieb, 2004). Nomenclature used for the GeneChip® S. pombe Tiling Array (Affymetrix) in this study was obtained from Sinha et al (2006). Similar gene lists were identified using hypergeometric distribution tests. The hypergeometric distribution test calculates the probability of overlap corresponding to k or more IGR or ORF fragments between an IGR or ORF list of n fragments compared against another gene list of m fragments when randomly sampled from a universe of u genes:
 |
In the figures, hypergeometric distribution comparisons are illustrated using Venn diagrams.
Supplementary Material
Supplementary Figures
Acknowledgments
KE is a Royal Swedish Academy of Sciences Research Fellow supported by grants from Knut and Alice Wallenberg Foundation, Swedish Cancer Society, Swedish Research Council (VR) M Bergvalls stiftelse and the EU ‘The Epigenome' NoE network. We thank I Sinha for help with data analysis, the Affymetrix core facility at KI Novum, T Schwendt for guidance with mass spectrometry, T Linder and E Choi for kindly providing pInv1 plasmids and A Wright for kindly sharing mst2 and gcn5 strains. The microarray data have been deposited in the GEO database (accession number GSE6557).
References
- Akey CW, Luger K (2003) Histone chaperones and nucleosome assembly. Curr Opin Struct Biol 13: 6–14 [DOI] [PubMed] [Google Scholar]
- Alen C, Kent NA, Jones HS, O'Sullivan J, Aranda A, Proudfoot NJ (2002) A role for chromatin remodeling in transcriptional termination by RNA polymerase II. Mol Cell 10: 1441–1452 [DOI] [PubMed] [Google Scholar]
- Allis CD, Chicoine LG, Richman R, Schulman IG (1985) Deposition-related histone acetylation in micronuclei of conjugating Tetrahymena. Proc Natl Acad Sci USA 82: 8048–8052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara H, Tartare-Deckert S, Nakagawa T, Ikehara T, Hirose F, Hunter T, Ito T, Montminy M (2002) Dual roles of p300 in chromatin assembly and transcriptional activation in cooperation with nucleosome assembly protein 1 in vitro. Mol Cell Biol 22: 2974–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A III, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951 [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Liu CL, Humphrey EL, Perlstein EO, Schreiber SL (2004) Global nucleosome occupancy in yeast. Genome Biol 5: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeger H, Griesenbeck J, Strattan JS, Kornberg RD (2004) Removal of promoter nucleosomes by disassembly rather than sliding in vivo. Mol Cell 14: 667–673 [DOI] [PubMed] [Google Scholar]
- Bouazoune K, Brehm A (2005) dMi-2 chromatin binding and remodeling activities are regulated by dCK2 phosphorylation. J Biol Chem 280: 41912–41920 [DOI] [PubMed] [Google Scholar]
- Buck MJ, Lieb JD (2004) ChIP–chip: considerations for the design, analysis, and application of genome-wide chromatin immunoprecipitation experiments. Genomics 83: 349–360 [DOI] [PubMed] [Google Scholar]
- Carey M, Li B, Workman JL (2006) RSC exploits histone acetylation to abrogate the nucleosomal block to RNA polymerase II elongation. Mol Cell 24: 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Dundr M, Wang C, Leung A, Lamond A, Misteli T, Huang S (2005) Condensed mitotic chromatin is accessible to transcription factors and chromatin structural proteins. J Cell Biol 168: 41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi ES, Shin JA, Kim HS, Jang YK (2005) Dynamic regulation of replication independent deposition of histone H3 in fission yeast. Nucleic Acids Res 33: 7102–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PJ, Arndt KM (2000) Synthetic lethal interactions suggest a role for the Saccharomyces cerevisiae Rtf1 protein in transcription elongation. Genetics 156: 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A, Liu Z, Zhu Y, Yu F, Li Z, Cao K, Shen WH (2005) Interacting proteins and differences in nuclear transport reveal specific functions for the NAP1 family proteins in plants. Plant Physiol 138: 1446–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenhofer-Murray AE (2004) Chromatin dynamics at DNA replication, transcription and repair. Eur J Biochem 271: 2335–2349 [DOI] [PubMed] [Google Scholar]
- Han M, Grunstein M (1988) Nucleosome loss activates yeast downstream promoters in vivo. Cell 55: 1137–1145 [DOI] [PubMed] [Google Scholar]
- Han M, Kim UJ, Kayne P, Grunstein M (1988) Depletion of histone H4 and nucleosomes activates the PHO5 gene in Saccharomyces cerevisiae. EMBO J 7: 2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haushalter KA, Kadonaga JT (2003) Chromatin assembly by DNA-translocating motors. Nat Rev Mol Cell Biol 4: 613–620 [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Hirosumi J, Sato W, Sugasawa K, Yokota S, Hanaoka F, Yamada M (1984) Purification and initial characterization of a protein which facilitates assembly of nucleosome-like structure from mammalian cells. Eur J Biochem 142: 431–439 [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Kojima M, Yamada M, Hanaoka F (1987) Binding mode of nucleosome-assembly protein (AP-I) and histones. Eur J Biochem 162: 19–24 [DOI] [PubMed] [Google Scholar]
- Ito T, Bulger M, Kobayashi R, Kadonaga JT (1996) Drosophila NAP-1 is a core histone chaperone that functions in ATP-facilitated assembly of regularly spaced nucleosomal arrays. Mol Cell Biol 16: 3112–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Ikehara T, Nakagawa T, Kraus WL, Muramatsu M (2000) p300-mediated acetylation facilitates the transfer of histone H2A–H2B dimers from nucleosomes to a histone chaperone. Genes Dev 14: 1899–1907 [PMC free article] [PubMed] [Google Scholar]
- Jackson V, Shires A, Tanphaichitr N, Chalkley R (1976) Modifications to histones immediately after synthesis. J Mol Biol 104: 471–483 [DOI] [PubMed] [Google Scholar]
- Jae Yoo E, Kyu Jang Y, Ae Lee M, Bjerling P, Bum Kim J, Ekwall K, Hyun Seong R, Dai Park S (2002) Hrp3, a chromodomain helicase/ATPase DNA binding protein, is required for heterochromatin silencing in fission yeast. Biochem Biophys Res Commun 295: 970–974 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Stokes DG, Perry RP (1999) CHD1 interacts with SSRP1 and depends on both its chromodomain and its ATPase/helicase-like domain for proper association with chromatin. Chromosoma 108: 10–25 [DOI] [PubMed] [Google Scholar]
- Kimura H, Cook PR (2001) Kinetics of core histones in living human cells: little exchange of H3 and H4 and some rapid exchange of H2B. J Cell Biol 153: 1341–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobor MS, Venkatasubrahmanyam S, Meneghini MD, Gin JW, Jennings JL, Link AJ, Madhani HD, Rine J (2004) A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2AZ into euchromatin. PLoS Biol 2: E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Kim M, Ahn SH, Zhong G, Kobor MS, Cagney G, Emili A, Shilatifard A, Buratowski S, Greenblatt JF (2002) RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol Cell Biol 22: 6979–6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Shibata Y, Rao B, Strahl BD, Lieb JD (2004) Evidence for nucleosome depletion at active regulatory regions genome wide. Nat Genet 36: 900–905 [DOI] [PubMed] [Google Scholar]
- Lengronne A, Katou Y, Mori S, Yokobayashi S, Kelly GP, Itoh T, Watanabe Y, Shirahige K, Uhlmann F (2004) Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature 430: 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko V, Jackson V (2004) Histone release during transcription: NAP1 forms a complex with H2A and H2B and facilitates a topologically dependent release of H3 and H4 from the nucleosome. Biochemistry 43: 2359–2372 [DOI] [PubMed] [Google Scholar]
- Linder T, Gustafsson CM (2004) The Soh1/MED31 protein is an ancient component of Schizosaccharomyces pombe and Saccharomyces cerevisiae Mediator. J Biol Chem 279: 49455–49459 [DOI] [PubMed] [Google Scholar]
- Lorch Y, Maier-Davis B, Kornberg RD (2006) Chromatin remodeling by nucleosome disassembly in vitro. Proc Natl Acad Sci USA 103: 3090–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch Y, Zhang M, Kornberg RD (1999) Histone octamer transfer by a chromatin-remodeling complex. Cell 96: 389–392 [DOI] [PubMed] [Google Scholar]
- Loyola A, Almouzni G (2004) Histone chaperones, a supporting role in the limelight. Biochim Biophys Acta 1677: 3–11 [DOI] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389: 251–260 [DOI] [PubMed] [Google Scholar]
- Lusser A, Urwin DL, Kadonaga JT (2005) Distinct activities of CHD1 and ACF in ATP-dependent chromatin assembly. Nat Struct Mol Biol 12: 160–166 [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Mosammaparast N, Ewart CS, Pemberton LF (2002) A role for nucleosome assembly protein 1 in the nuclear transport of histones H2A and H2B. EMBO J 21: 6527–6538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen PR, Nietlispach D, Buscaino A, Warner RJ, Akhtar A, Murzin AG, Murzina NV, Laue ED (2005) Structure of the chromo barrel domain from the MOF acetyltransferase. J Biol Chem 280: 32326–32331 [DOI] [PubMed] [Google Scholar]
- Nielsen PR, Nietlispach D, Mott HR, Callaghan J, Bannister A, Kouzarides T, Murzin AG, Murzina NV, Laue ED (2002) Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature 6876: 103–107 [DOI] [PubMed] [Google Scholar]
- Ohkuni K, Shirahige K, Kikuchi A (2003) Genome-wide expression analysis of NAP1 in Saccharomyces cerevisiae. Biochem Biophys Res Commun 306: 5–9 [DOI] [PubMed] [Google Scholar]
- Okuwaki M, Kato K, Shimahara H, Tate S, Nagata K (2005) Assembly and disassembly of nucleosome core particles containing histone variants by human nucleosome assembly protein I. Mol Cell Biol 25: 10639–10651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel M, Lando DBonilla C, Trewick S, Boubaka A, Walfridsson J, Cauwood J, Werler P, Carr A, Kouzarides T, Murzina N, Allsire R, Ekwall K, Laue E (2007) Genome-wide studies of histone demethylation catalysed by the fission yeast homologues of mammalian LSD1. PLoS ONE 18: e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D (1998) FACT, a factor that facilitates transcript elongation through nucleosomes. Cell 92: 105–116 [DOI] [PubMed] [Google Scholar]
- Owen-Hughes T, Workman JL (1996) Remodeling the chromatin structure of a nucleosome array by transcription factor-targeted trans-displacement of histones. EMBO J 15: 4702–4712 [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Chodaparambil JV, Bao Y, McBryant SJ, Luger K (2005) Nucleosome assembly protein 1 exchanges histone H2A–H2B dimers and assists nucleosome sliding. J Biol Chem 280: 1817–1825 [DOI] [PubMed] [Google Scholar]
- Phelan ML, Schnitzler GR, Kingston RE (2000) Octamer transfer and creation of stably remodeled nucleosomes by human SWI-SNF and its isolated ATPases. Mol Cell Biol 20: 6380–6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Allshire RC (2004) Kinetochore and heterochromatin domains of the fission yeast centromere. Chromosome Res 12: 521–534 [DOI] [PubMed] [Google Scholar]
- Pray-Grant MG, Daniel JA, Schieltz D, Yates JR III, Grant PA (2005) Chd1 chromodomain links histone H3 methylation with SAGA- and SLIK-dependent acetylation. Nature 433: 434–438 [DOI] [PubMed] [Google Scholar]
- Reinke H, Horz W (2003) Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol Cell 11: 1599–1607 [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Pelletier J, Price GB, Zannis-Hadjopoulos M (2000) NAP-2: histone chaperone function and phosphorylation state through the cell cycle. J Mol Biol 298: 225–238 [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A, Wangh LJ, Allfrey VG (1975) Processing of newly synthesized histone molecules. Science 190: 117–128 [DOI] [PubMed] [Google Scholar]
- Simic R, Lindstrom DL, Tran HG, Roinick KL, Costa PJ, Johnson AD, Hartzog GA, Arndt KM (2003) Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J 22: 1846–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha I, Wiren M, Ekwall K (2006) Genome-wide patterns of histone modifications in fission yeast. Chromosome Res 14: 95–105 [DOI] [PubMed] [Google Scholar]
- Spahr H, Khorosjutina O, Baraznenok V, Linder T, Samuelsen CO, Hermand D, Makela TP, Holmberg S, Gustafsson CM (2003) Mediator influences Schizosaccharomyces pombe RNA polymerase II-dependent transcription in vitro. J Biol Chem 278: 51301–51306 [DOI] [PubMed] [Google Scholar]
- Spahr H, Samuelsen CO, Baraznenok V, Ernest I, Huylebroeck D, Remacle JE, Samuelsson T, Kieselbach T, Holmberg S, Gustafsson CM (2001) Analysis of Schizosaccharomyces pombe mediator reveals a set of essential subunits conserved between yeast and metazoan cells. Proc Natl Acad Sci USA 98: 11985–11990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes DG, Tartof KD, Perry RP (1996) CHD1 is concentrated in interbands and puffed regions of Drosophila polytene chromosomes. Proc Natl Acad Sci USA 93: 7137–7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong JK, Hassig CA, Schnitzler GR, Kingston RE, Schreiber SL (1998) Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature 395: 917–921 [DOI] [PubMed] [Google Scholar]
- Tran HG, Steger DJ, Iyer VR, Johnson AD (2000) The chromo domain protein chd1p from budding yeast is an ATP-dependent chromatin-modifying factor. EMBO J 19: 2323–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler JK (2002) Chromatin assembly. Cooperation between histone chaperones and ATP-dependent nucleosome remodeling machines. Eur J Biochem 269: 2268–2274 [DOI] [PubMed] [Google Scholar]
- Walfridsson J, Bjerling P, Thalen M, Yoo EJ, Park SD, Ekwall K (2005) The CHD remodeling factor Hrp1 stimulates CENP-A loading to centromeres. Nucleic Acids Res 33: 2868–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren M, Silverstein RA, Sinha I, Walfridsson J, Lee HM, Laurenson P, Pillus L, Robyr D, Grunstein M, Ekwall K (2005) Genomewide analysis of nucleosome density histone acetylation and HDAC function in fission yeast. EMBO J 24: 2906–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodage T, Basrai MA, Baxevanis AD, Hieter P, Collins FS (1997) Characterization of the CHD family of proteins. Proc Natl Acad Sci USA 94: 11472–11477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick JJ, Holstege FC, Jennings EG, Causton HC, Shore D, Grunstein M, Lander ES, Young RA (1999) Chromosomal landscape of nucleosome-dependent gene expression and silencing in yeast. Nature 402: 418–421 [DOI] [PubMed] [Google Scholar]
- Xue Y, Haas SA, Brino L, Gusnanto A, Reimers M, Talibi D, Vingron M, Ekwall K, Wright AP (2004) A DNA microarray for fission yeast: minimal changes in global gene expression after temperature shift. Yeast 21: 25–39 [DOI] [PubMed] [Google Scholar]
- Zhang Y, LeRoy G, Seelig HP, Lane WS, Reinberg D (1998) The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell 95: 279–289 [DOI] [PubMed] [Google Scholar]
- Zhao J, Herrera-Diaz J, Gross DS (2005) Domain-wide displacement of histones by activated heat shock factor occurs independently of Swi/Snf and is not correlated with RNA polymerase II density. Mol Cell Biol 25: 8985–8999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures


