Abstract
Airway epithelial cells are simultaneously exposed to and produce cytokines and reactive oxygen species (ROS) in inflammatory settings. The signaling events and the physiologic outcomes of exposure to these inflammatory mediators remain to be elucidated. Previously we demonstrated that in cultured mouse lung epithelial cells exposed to bolus administration of H2O2, TNF-α–induced NF-κB activity was inhibited, whereas c-Jun-N-terminal kinase (JNK) activation was enhanced via a mechanism involving TNF receptor-1 (TNF-RI). In this study we used the nonphagocytic NADPH oxidase (Nox1) to study the effects of endogenously produced ROS on a line of mouse alveolar type II epithelial cells. Nox1 expression and activation inhibited TNF-α–induced inhibitor of κB kinase (IKK), and NF-κB while promoting JNK activation and cell death. Nox1-induced JNK activation and cell death were attenuated through expression of a dominant-negative TNF-RI construct, implicating a role for TNF-RI in Nox1 signaling. Furthermore, Nox1 used the TNF-RI adaptor protein TNF-receptor–associated factor-2 (TRAF2), and the redox-regulated JNK MAP3K, apoptosis signal kinase-1 (ASK1), to activate JNK. In addition, ASK1 siRNA attenuated both Nox1-induced JNK activity and cell death. Collectively, these studies suggest a mechanism by which ROS produced in lung epithelial cells activate JNK and cause cell death using TNF-RI and the TRAF2–ASK1 signaling axis.
Keywords: Nox1, c-Jun-N-terminal kinase, hydrogen peroxide, cell death, TNF-RI
CLINICAL RELEVANCE
JNK and NF-κB pathways are plausible candidates for therapeutic intervention to control inflammation and epithelial cell death in patients with acute lung injury associated with enhanced oxidant production.
Under inflammatory conditions airway epithelial cells are simultaneously exposed to and produce cytokines and reactive oxygen species (ROS). Two signaling pathways known to be activated and regulated by both cytokines and ROS are the NF-κB and c-Jun-N-terminal kinase (JNK) signaling pathways, which converge at TNF receptor-1 (TNF-RI). These two pathways can determine cellular fate as NF-κB activation ensures cell survival (1), while its inhibition promotes prolonged JNK activity and cell death (2, 3), demonstrating reciprocal regulation of these two pathways (4). ROS production and subsequent protracted JNK activation in the absence of NF-κB activity has been implicated as a mechanism of TNF-α–induced cell death (5). The functional interplay between NF-κB and JNK signaling through TNF-RI therefore is likely to influence phenotypic outcomes of airway epithelial cells exposed to inflammatory mediators such as cytokines and ROS. We have previously demonstrated that exposure of mouse lung epithelial cells to bolus H2O2 inhibited TNF-α–induced inhibitor of κB kinase (IKK) and NF-κB activity and enhanced JNK activation. H2O2-induced JNK activation was attenuated in TNF-RI−/− lung fibroblasts, and in mouse type II airway epithelial cell cultures expressing a TNF-RI construct lacking the intracellular death domain, termed dominant-negative (dn)TNF-RI (6). The mechanism whereby H2O2-induced activation of JNK occurs through this receptor remains to be fully elucidated.
One pathway of TNF-α and ROS-induced JNK activity that has been garnering recent attention is apoptosis signal kinase-1 (ASK1). ASK1 is a 170-kD ubiquitously expressed MAP3K, and its over-expression promotes cell death in various cell types (7–9). ASK1 can activate JNK in response to TNF-α, which involves the TNF-RI adaptor protein, TNF receptor–associated factor-2 (TRAF2). ASK1 directly associates with TRAF2 in response to TNF-α through the amino terminal zinc and ring finger and TRAF domains of TRAF2 (10). Interestingly, binding of TRAF2 to ASK1 in response to TNF-α requires the prior dissociation of the ASK1 inhibitory protein thioredoxin, demonstrating the redox dependence of TRAF2–ASK1 signaling to JNK (11).
The Nox and Duox family of NADPH oxidases are expressed in nonphagocytic cells, such as lung epithelium (12–14), and produce ROS in response to cytokines, calcium signals, and growth factors. These proteins are structurally related to the phagocytic NADPH oxidases and are a source of deliberate production of low levels of superoxide and H2O2 (15, 16). Cells can use ROS as second messengers to transduce signals (17), and ROS have the potential to alter cytokine responses (18).
The goal of the present study was to determine whether ROS produced by the nonphagocytic oxidase, Nox1, in lung epithelial cells alter TNF-RI signaling in favor of JNK, and to determine the mechanism and physiologic outcome of Nox1-induced JNK activity. In response to Nox1-induced ROS, we demonstrate inhibition of NF-κB, and activation of JNK, which requires TNF-RI. ROS-induced JNK signaling through TNF-RI involved the death domain of TNF-RI and its signaling components, as dominant-negative TRAF2 (dnTRAF2) decreased Nox1-induced JNK activation. We also provide evidence in support of activation of ASK1 by Nox1, and that knock-down of ASK1 by siRNA attenuated Nox1-induced JNK activation and cell death. These data implicate a role for the TNF-RI–TRAF2–ASK1 signaling axis in eliciting the response to ROS in lung epithelial cells.
MATERIALS AND METHODS
Cell Culture and Reagents
A line of spontaneously transformed mouse alveolar type II epithelial cells (C10) was propagated in CRML-1066 medium containing 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM L-glutamine, and 10% fetal bovine serum, all from Invitrogen (Carlsbad, CA). Murine recombinant TNF-α was purchased from Calbiochem (San Diego, CA). JNK, IKK, and ASK1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and TNF-R1 antibody was obtained from R&D Systems (Minneapolis, MN). Antibody to phospho-ASK1 was purchased from Cell Signaling Technology (Danvers, MA) and the FLAG antibody was from Sigma-Aldrich (St. Louis, MO). TNF-α–neutralizing antibody was purchased from eBioscience (San Diego, CA). The dnTNF-R1 (p60ΔCD) plasmid lacking the intracellular death domain was provided by Dr. Michael Lenardo (National Institutes of Health, Bethesda, MD); Flag-dnTRAF2 was provided by Dr. David Riches (National Jewish, Denver, CO); and the Nox1, NoxO1, and NoxA1 plasmids were the kind gift of Dr. David Lambeth (Emory University, Atlanta, GA). NF-κB–luciferase was provided by Dr. Patrick Baeuerle (Micromet, Martinsreid, Germany). Glutathione S-transferase (GST)-IκB was provided by Dr. Rosa Ten (Mayo Clinic, Rochester, MN).
Kinase Assays
Cells were exposed to test agents and, at the indicated times, transferred to ice, washed once with cold PBS, and lysed as described elsewhere (6). Lysates were cleared by centrifugation at 14,000 rpm, 4°C for 10 min. Protein concentrations were determined, and a fraction of each sample was frozen for Western Blot Analyses to confirm that protein expression of JNK or IKK was equal between samples. In addition, IKK or JNK was immunoprecipitated from 200 μg of protein with antibodies recognizing IKKγ or JNK, respectively, at 4°C for 1.5 h using protein G-agarose beads (Invitrogen). For TNF-R1 immunoprecipitation and JNK kinase assay, TNF-R1 was immunoprecipitated from 700 μg of protein using an anti–TNF-R1 antibody from R&D Systems. Precipitates were washed twice with lysis buffer and once with kinase buffer. Kinase reactions were performed using 1 μg of GST-c-Jun as a substrate and 5 μCi of [γ-32P] adenosine triphosphate at 30°C for 30 min. Reactions were stopped by the addition of 2× sample buffer. Samples were boiled and stored at −20°C. Proteins were separated on a 15% polyacrylamide gel. Gels were dried and examined by autoradiography. In all kinase assays, gels were scanned by a phosphor-imager and each band was given an adjusted volume based on counts per millimeter squared, after correction from background signals. Where appropriate, duplicate values were averaged and the average volume for sham samples was set at 1. All “fold inductions” were determined by dividing the band density value for each experimental treatment by the value for the sham control sample.
Transfection
Cells were transfected using LipofectAMINE-Plus (Invitrogen) according to the manufacturer's directions. For plasmid transfections, cells were transfected and incubated in DMEM/F12 (Invitrogen) media containing 0.5% serum. Control and ASK1 siRNA were purchased from Ambion (Austin, TX) and transfected into the cells at a concentration of 50 nM using siPORTamine according to the manufacturer's directions. Forty-eight hours after transfection, test agents were added and experiments performed.
Immunoprecipitation and Western Blotting
A fraction of lysates for protein expression or co-immunoprecipitation were added to 2× Laemmli sample buffer, boiled, and loaded on a 10% polyacrylamide gel. Proteins were transferred to nitrocellulose (Schleicher and Shuell, Keene, NH), and membranes were subsequently blocked in 5% milk in tris-buffered saline (TBS). Membranes blocked overnight in TBS/milk were washed two times for 15 min in TBS containing 0.05% Tween 20, and incubated with primary antibody for 1 h at 4°C. Membranes were washed three times for 20 min in TBS/Tween 20 and incubated with a peroxidase-conjugated secondary antibody (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. After a 30-min wash with TBS/Tween 20, conjugated peroxidase was detected by ECL according to the manufacturer's instructions (Amersham Biosciences, GE Healthcare UK, Buckinghamshire, UK). For immunoprecipitations, cells were grown to confluence in 100-mm dishes, washed three times with PBS, and treated with test agents in PBS. Cells were lysed in immunoprecipitation buffer (50 mM Hepes, pH 7.4, 250 mM NaCl, 0.1% Nonidet P-40, 5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 1% aprotinin), and lysates were incubated with TNF-R1 antibody for 2 h with rocking at 4°C. After incubation with antibody, protein G-agarose beads (Invitrogen) were added for 1 h. Precipitates were washed three times with lysis buffer, and 2× Laemmli was added followed by boiling, loading onto a 10% polyacrylamide gel, and Western analysis.
Assessment of NF-κB Transcriptional Activity
C10 cells were transiently transfected with a 6 κB-tk-luc plasmid containing 6 NF-κB DNA elements, and either Nox1, NoxO1, and NoxA1 or pCDNA3. Cells were treated with 1 ng/ml TNF-α for 4 h. Cells were lysed in Luciferase Assay Lysis Buffer (Promega, Madison, WI), and a luciferase assay was performed as previously described and normalized for protein content.
Determination of Cell Death
Sixteen hours after transfection, cells were incubated with 10 μM of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl Tetrazolium bromide) reagent (Molecular Probes, Invitrogen) which was used to assess overall viability by monitoring the production of formazan at 540 nm. Results were expressed as percent survival compared with sham controls, and represent an n of three. Error bars in figures represent SEM.
Assessment of Intracellular H2O2
C10 cells were incubated for 30 min at 37°C with dichlorofluorescein (DCF-HA) diacetate (Molecular Probes). Cells were washed once with PBS, harvested by trypsinization, centrifuged, and resuspended in Hanks' balanced salt solution (Life Technologies, Inc., Gaithersburg, MD) to a density of 500,000 cells/ml. DCF oxidation was detected by flow cytometry.
Statistics
All experiments were repeated at least twice, and selected data were evaluated by the Student t test.
RESULTS
Overexpression of Nox1 Alters TNF-α Signaling
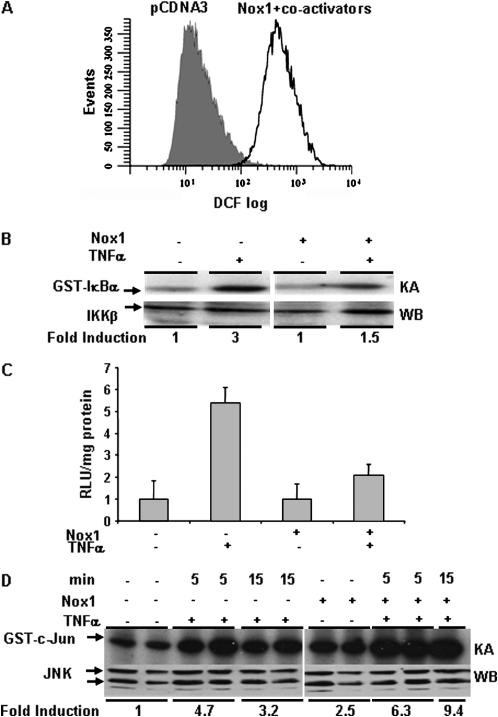
We first established that expression of Nox1 and its co-activators NoxO1 and NoxA1 in C10 cells caused increased oxidant production by evaluation of DCF oxidation via flow cytometry. Results in Figure 1A confirm that DCF oxidation was enhanced after transfection of Nox1 plus co-activators, compared with pCDNA3 transfected controls. We next addressed the impact of Nox-generated ROS on TNF-RI signaling. Results in Figures 1B and 1C demonstrate that expression of Nox1 in C10 cells inhibited TNF-α–induced IKK activation, and NF-κB transactivation, respectively. Interestingly, Nox1 expression was sufficient to induce JNK activity, and led to an increase in the ability of TNF-α to activate JNK as compared with pCDNA3 control transfected cells (Figure 1D). These data are consistent with our previous findings obtained with bolus H2O2, and suggest that ROS produced by Nox1 can also induce signaling imbalances at TNF-RI.
Figure 1.
Nox1 expression and ROS production leads to altered TNF-α signaling. (A) Twenty-four hours after transfection of C10 cells with 1.5 μg of pCDNA3 or 500 ng each of Nox1, NoxO1 and NoxA1, cells were incubated with 10 uM DCF-DA for 30 min followed by cytometry. The x-axis represents log DCF fluorescence, and the y-axis represents cell counts. A rightward shift in the signal is representative of enhanced DCF oxidation. (B) C10 cells expressing 500 ng of Nox1 plus co-activators were treated with 10 ng/ml of TNF-α for 5 min, and IKK activity was assessed using an in vitro kinase assay with GST-IκBα as the substrate. Top panel: kinase assay (KA) demonstrating GST-IκBα substrate phosphorylation; bottom panel: IKKγ Western blot (WB), performed on separate aliquots of the samples used for immunoprecipitation. Kinase assays were quantified by phosphor-image analysis and data expressed as fold induction compared with sham controls. (C) C10 cells were transfected with either 1.5 μg of pCDNA3 or 500 ng each of Nox1, NoxO1, and NoxA1 along with 250 ng of NF-κB luciferase reporter construct. Cells were treated with 10 ng/ml of TNF-α for 4 h and NF-κB reporter activity was determined using a luminometer. Data are expressed as relative luciferase units (RLU) after correction for protein. Error bars represent SEM. (D) C10 cells were transfected with 500 ng each of Nox1, NoxO1, and NoxA1 or 1.5 μg of pCDNA3 and treated with TNF-α for 5 or 15 min, and JNK activity was determined using an in vitro kinase assay using GST-c-Jun as the substrate. Top panel: kinase assay (KA) demonstrating GST-c-Jun substrate phosphorylation; bottom panel: JNK Western blot (WB) performed on separate aliquots of the samples used in immunoprecipitation. The two separate lanes per condition represent independent samples. Kinase assays were quantified by phosphor-image analysis, results averaged, and data expressed as fold induction compared with sham controls.
Nox1 Induces JNK and Cell Death in a TNF-RI–Dependent Manner
To determine whether TNF-RI is involved in Nox1-induced JNK activation, C10 cells were transfected with Nox1 in the presence or absence of dnTNF-RI, a construct in which the intracellular death domain is deleted, and JNK activity was evaluated. As seen in Figure 2A, dnTNF-RI decreased basal, TNF-α–, and Nox1-induced JNK activity. Given the involvement of TNF-RI in Nox1-induced JNK activation, it is conceivable that Nox1 expression increased levels or post-translational processing of TNF-α. To address this possibility, C10 cells expressing pCDNA3 or Nox1 were treated with a TNF-α–neutralizing antibody, or an isotype control, for 16 h before harvest. As expected, TNF-α–neutralizing antibody decreased the ability of TNF-α to induce JNK, while an isotype control antibody had a small effect (Figure 2B). In contrast, Nox1-induced JNK activity was still apparent in cells treated with TNF-α–neutralizing antibody. These results imply that Nox1-induced JNK activation required TNF-RI (Figure 2A), but occurred independently of TNF-α.
Figure 2.
Nox1-induced JNK activity and cell death are TNF-RI dependent. (A) C10 cells were transfected with either 2 μg of pCDNA3 alone; pCDNA3 plus 500 ng of dnTNF-RI; or 500 ng of Nox1, NoxO1, and NoxA1 alone or with the addition of 500 ng of dnTNF-RI. Cells were treated with 10 ng/ml of TNF-α for 15 min, and JNK activity was determined using an in vitro kinase assay. Top panel: kinase assay (KA) demonstrating GST-c-Jun phosphorylation; bottom panel: JNK Western blot (WB) run on separate aliquots of samples used for immunoprecipitation. The two separate lanes per condition represent independent samples. Note that because of the shorter electrophoresis time the two JNK isoforms appear as one band. Kinase assays were quantified by phosphor-image analysis, results averaged, and data expressed as fold induction compared with sham controls. (B) C10 cells were transfected with pCDNA3 or Nox1 and co-activators in the presence of 6.25 μg/ml of TNF-α–neutralizing antibody (anti–TNF-α) or isotype control for 16 h, at which time lysates were prepared for evaluation of JNK activity, as described in A. As a reagent control, cells were incubated with anti–TNF-α antibody or isotype control IgG for 15 min, followed by addition of 10 ng/ml TNF-α for 15 min before assessment of JNK activity. (C) C10 cells were transfected with either pCDNA3, Nox1 plus NoxO1, or NoxA1 in the presence of dnTNF-RI. Sixteen hours after transfection, digital microscopic images were taken to assess cell morphology and death (left panel, ×20 magnification). Right panel: Cells were incubated with 50 μg/ml of MTT reagent for 3 h for assessment of cell viability. Data are represented as mean ± SEM. * P < 0.05 (Student's t test).
Prolonged JNK activation induced by ROS is known to cause cell death when NF-κB is inhibited (19). In addition, ROS-induced JNK activation in C10 cells has been shown to result in cell death (20, 21). To determine whether Nox1 induced cell death, and whether this was TNF-RI dependent, cell death was assessed in Nox1-expressing cells in the presence or absence of dnTNF-RI. As is shown in Figure 2C, expression of Nox1 caused marked cell death, which was attenuated by dnTNF-RI. These data suggest a role for TNF-RI not only in Nox1-induced JNK activation, but also subsequent cell death.
TRAF2 Is Involved in Nox1-Induced JNK Activation
TRAF2 is required for TNF-α–induced JNK activity (22). However, the role of TRAF2 in transducing H2O2 triggered signals and death in lung epithelial cells is not known, although evidence points toward a role of TRAF2 in ROS-induced JNK activation and death of other cell types (9, 23). Using a FLAG-dnTRAF2 construct, the role of TRAF2 in ROS-induced JNK activation was examined. Transfection of C10 cells with dnTRAF2 led to expression of the protein (Figure 3A), and attenuated TNF-α, H2O2 or Nox-1–induced JNK activation (Figure 3B and 3C), as well as TNF-α–induced JNK activity in Nox1-expressing cells (Figure 3C). Collectively these findings support the emerging role of TRAF2 not only as a TNF-RI adaptor protein, but also as a mediator of ROS-induced JNK activation in lung epithelial cells.
Figure 3.
Nox1-induced JNK activation involves TRAF2. (A) C10 cells were transfected with either 250 ng of pCDNA3 (−) or FLAG-dnTRAF2 (+), and 16 h after transfection lysates were collected and assessed for FLAG-dnTRAF2 expression by Western analysis for the FLAG tag. (B) C10 cells were transfected with pCDNA3 (−) or 500 ng dnTRAF2 (+). Cells were treated with either 10 ng/ml of TNF-α for 15 min or 200 μM H2O2 for 20 min, and JNK activity was tested using an in vitro kinase assay. Top panel: Kinase Assay (KA) demonstrating GST-c-Jun phosphorylation; bottom panel: JNK Western blot (WB) performed on separate aliquots of samples used for immunoprecipitation. (C) C10 cells were transfected with Nox1 as in Figure 2A in the presence or absence of 500 ng of dnTRAF2 (+) or pCDNA3 (−). Cells were treated with 10 ng/ml of TNF-α for 15 min and JNK activity was assessed by an in vitro kinase assay. Top panel: kinase assay (KA) demonstrating GST-c-Jun phosphorylation; bottom panel: JNK Western blot (WB) run on separate aliquots of the samples used in immunoprecipitation. Note that Phosphor Image Analysis was performed on Nox1-transfected cells, and that the designated value of 1 represents a sample with activated JNK, not a baseline value.
Evidence for ASK1 Activation by Nox1 and its Involvement in Nox1-Induced Cell Death
As previously mentioned, ASK1 is a MAP3K known to activate JNK in response to TNF-α by phosphorylating JNK kinases, MKK7/4. ASK1 can also activate JNK in a redox sensitive manner through oxidation of the ASK1 inhibitor, Trx (11). We previously demonstrated that in response to H2O2, JNK activity was associated with immunoprecipitated TNF-RI (6). To establish a role for ASK1 in TNF-RI–dependent signaling to JNK, the ability of ASK1 to associate with TNF-RI was determined. Expression of wild-type (WT) ASK1 resulted in its co-immunoprecipitation with TNF-RI in response to H2O2 (Figure 4A, upper panel). Furthermore, immunoprecipitated TNF-RI from cells overexpressing WT-ASK1 displayed increased JNK activity, which was further enhanced by H2O2, (Figure 4A, lower panel). These data suggest that ASK1 can associate with TNF-RI, possibly facilitating JNK activation in response to ROS. Using an antibody to phosphor-serine 83, a serine residue which is phosphorylated by Akt to keep ASK1 inactive (24) in unstimulated cells, we demonstrate that Nox1 expression resulted in decreased phosphorylation at this site, indicating activation of ASK1. Co-expression of dnTNF-RI in the presence of Nox1 did not reverse the de-phosphorylation of ASK1 at Serine 83 (Figure 4C), indicating that while ASK1 could associate with TNF-RI its activation may be independent of presence at the TNF-receptor signaling complex.
Figure 4.
Nox1 activates ASK1. (A) Top panel: 1 μg of WT-ASK1 was expressed in C10 cells, and cells were treated with 200 μM H2O2 for 30 min or 1 h. TNF-RI was immunoprecipitated from cell extracts followed by immunoblotting for ASK1. WCL represents whole cell lysate. Note that irrelevant lanes were cut out of the gel. Bottom panel: cells were transfected with 1 μg of pCDNA3 (−) or WT-ASK1 (+) and treated with 200 μM H2O2 for 15 min to 1 h. TNF-RI was immunoprecipitated and JNK kinase activity associated with the receptor complex was assessed by a JNK in vitro kinase assay using GST-c-Jun as the substrate. (B) C10 cells were transfected with either pCDNA3 or Nox1 as described in previous Figures. Sixteen hours after transfection, cells were treated with 10 ng/ml of TNF α for 15 min. Cells were lysed and assessed for Serine 83 phosphorylation of ASK1 by Western blotting, using a phospo-specific antibody to Serine 83. Note that irrelevant lanes were cut out of the gel. (C) Cells were transfected as in B, with the addition of 500 ng of dnTNF-RI. Cells were treated as above and Serine 83 phosphorylation of ASK1 was determined as in B. Bottom panel: JNK Western blot run on separate aliquots of samples.
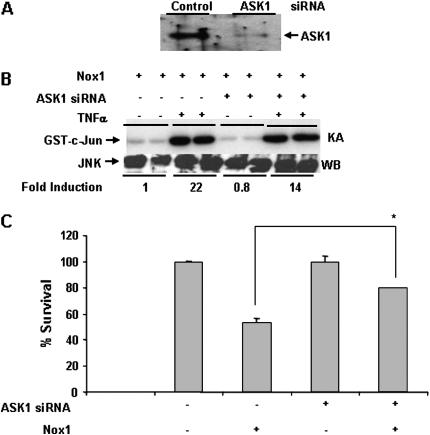
To assess the role of ASK1 in Nox1-induced JNK activation and cell death, siRNA specific to ASK1 was employed. Introduction of ASK1 siRNA into C10 cells markedly decreased ASK1 protein expression (Figure 5A). Furthermore, ASK1 siRNA decreased JNK activation in cells expressing Nox1 in presence of TNF-α by 40% (Figure 5B). Lastly, Nox1-induced cell death was also attenuated in cells expressing ASK1 siRNA (Figure 5C). These results suggest that while ASK1 activation in response to ROS produced by Nox1 seems to be independent of TNF-RI, ASK1 appears to play a role in Nox1-induced JNK activation and cell death.
Figure 5.
ASK1 plays a role in Nox1-induced JNK activation and cell death. (A) C10 cells were transfected with either 50 nM of control or ASK1 specific siRNA and ASK1 expression was determined 36 h after transfection by Western blot analysis. (B) C10 cells were transfected with Nox1 as in previous figures, in the presence of control (−) or ASK1 siRNA (+). Sixteen hours after transfection, cells were treated with 10 ng/ml of TNF-α, and JNK activity was determined as by an in vitro kinase assay, according to previous figures. Note that the phosphoimage analysis was performed on Nox1-transfected cells, and that the designated value of 1 represents a sample with activated JNK, not a baseline value. (C) siRNA and plasmid transfections were performed as in B, followed by incubation with MTT reagent for 3 h for assessment of cell viability. Data are represented as mean ± SEM. * P < 0.05 (Student's t test).
DISCUSSION
Many pulmonary diseases, such as asthma, chronic obstructive pulmonary disease, and acute respiratory distress syndrome, are characterized by chronic inflammation, production of cytokines, ROS generation, and epithelial cell damage and death (25, 26). While ROS were once considered to act nonspecifically to induce cell damage, it is now accepted that ROS, such as H2O2, can act as signaling molecules altering protein function while eliciting specific cellular responses (17). The signaling events and physiologic outcome of ROS on airway epithelial cells remain to be fully elucidated. Deliberate production of low levels of ROS by the nonphagocytic NADPH oxidases has been demonstrated to play a role in signaling cascades involved in innate host defense (27) and proinflammatory cascades. ROS generation by NADPH oxidases has been shown to be important in LPS-induced signals through TLR4 (28), in EGF receptor signaling (29), TLR5 signaling in colonic epithelial cells (30), and FasL signaling in rat hepatocytes (31). While many studies have investigated the role of bolus H2O2 on TNF-α signaling, and activation of JNK, the role of the Nox family in TNF-RI signaling in epithelial cells of the lung has yet to be determined. It should be pointed out that in endothelial cells, the phagocytic NADPH oxidase subunit, p47phox, has been implicated in TNF-α–mediated induction of JNK (32). Our current work confirms these earlier observations in endothelial cells and also elucidates the role of a TRAF2–ASK1 platform that communicates signals between TNF-R1 and JNK. Specifically we demonstrate here that Nox1-derived ROS inhibits TNF-α–induced IKK and NF-κB activation, while promoting JNK activation through TNF-RI, TRAF2, and ASK1.
Recent studies have demonstrated that ASK1 can bind to and be activated by TRAF2 to signal to JNK. Interestingly, TRAF2-induced JNK activation was redox dependent, as it required prior dissociation of Trx from ASK1. Trx binds to the N-terminal inhibitory domain of ASK1, inhibiting ASK1 activity, only when its two critical cysteines are in a reduced state (33). In response to ROS, Cys32 and Cys35 of Trx are oxidized and form an intramolecular disulfide and dissociate from ASK1 (11). This unequivocally demonstrates the redox sensitivity of ASK1 activation. Once liberated from Trx, ASK1 oligomerizes and undergoes autophosphorylation at Thr845 (34, 35).
While the aforementioned studies have demonstrated a causal role of ASK1 in TNF-α– and ROS-induced JNK activation, the precise role of TNF-RI, and its death domain in eliciting these signals, and their consequence had not been clearly addressed. We demonstrate in the present study that ASK1 co-immunoprecipitates with TNF-RI in response to H2O2, and that overexpression of ASK1 can enhance JNK association with TNF-RI. We also demonstrate that Nox1 expression induced death in lung epithelial cells, which was partially dependent on TNF-R1 and ASK1. These findings suggest that ASK1 may be a part of the TNF-RI signalsome after exposure to oxidants, and that it facilitates JNK activation and subsequent cell death.
A proposed mechanism of Nox-induced activation of JNK and subsequent cell death is depicted in Figure 6. This model is based on previous studies (11, 33), as well as on our current observations. We propose that Nox1 leads to the oxidation of Trx, liberating ASK1, which then can bind to TRAF2. TRAF2–ASK1 may then translocate to, and use the death domain of, TNF-RI to cause JNK activation. The observed JNK activation at TNF-R1 occurs in the absence of NF-κB activity. As mentioned previously, NF-κB inhibition by ROS is proposed to promote sustained JNK activation, and subsequent cell death. In support of the proposed role of TRAF2 and ASK1 in Nox1-induced JNK activation and cell death, TRAF2 has been implicated as a player in ROS-induced cell death (23), and it has recently been demonstrated that TRAF2 and ASK1 associate in response to H2O2 (9). While the molecular link between TRAF2, ASK1, and JNK activation under conditions of oxidative stress was clearly illuminated in that report, the explicit role of TNF-RI in this signaling event was not addressed. Although we are proposing Trx oxidation to be the major target for ASK1 liberation, other pathways cannot be overlooked. Both Hsp90 and Akt have recently been found to associate with and keep ASK1 inactive (24). Interestingly, these two proteins also play a role in IKK activation by TNF-α (36, 37), and therefore may play a role in redox-induced changes in the cross-talk between IKK/NF-κB and JNK. Moreover, glutaredoxin, a protein important in redox signaling, is also known to bind to ASK1 and maintain its inactivity (38).
Figure 6.
Proposed model for JNK activation by Nox1. A proposed model in which ROS produced by Nox1 dissociate ASK1 from Trx, allowing ASK1 to complex with TRAF2, possibly at TNF-RI (right), to facilitate JNK activation, and subsequent cell death. Nox1-induced inactivation of NF-κB (left) also shown in this study may further facilitate JNK activation and cell death.
The outcome of activation of nonphagocytic oxidases in airway epithelium in vivo remains to be fully unraveled and likely depends of the magnitude and duration of their activation as well as the host defense status. The data presented in this study provides a plausible mechanism by which airway epithelial cells undergo cell death in response to ROS, which involves inhibition of the pro-survival transcription factor, NF-κB, and marked activation of the pro-death JNK signaling cascade, via a mechanism involving the TNF-RI and the TRAF2-ASK1 signaling axis.
Acknowledgments
The authors thank Drs. Michael Lenardo, David Riches, David Lambeth, Patrick Baeuerle, and Rosa Ten for kindly providing various plasmid contructs, and Jennifer Díaz for secretarial assistance.
This work was supported by NIH R01 HL60014 (Principal Investigator Y.M.W.J.-H.), Public Health Service P20 RL15557 (NCRR COBRE), and P01 HL67004.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0109OC on November 1, 2006
Conflict of Interest Statement: C.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.H.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.M.W.J.-H. received $1,500 from Sepracor for a visiting professor engagement in North Carolina in 2005.
References
- 1.Mizgerd JP, Lupa MM, Kogan MS, Warren HB, Kobzik L, Topulos GP. Nuclear factor-κB p50 limits inflammation and prevents lung injury during Escherichia coli pneumonia. Am J Respir Crit Care Med 2003;168:810–817. [DOI] [PubMed] [Google Scholar]
- 2.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature 2001;414:313–317. [DOI] [PubMed] [Google Scholar]
- 3.Tang F, Tang G, Xiang J, Dai Q, Rosner MR, Lin A. The absence of NF-kappaB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol 2002;22:8571–8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen-Heininger YM, Persinger RL, Korn SH, Pantano C, McElhinney B, Reynaert NL, Langen RC, Ckless K, Shrivastava P, Poynter ME. Reactive nitrogen species and cell signaling: implications for death or survival of lung epithelium. Am J Respir Crit Care Med 2002;166:S9–S16. [DOI] [PubMed] [Google Scholar]
- 5.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 2003;22:3898–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantano C, Shrivastava P, McElhinney B, Janssen-Heininger Y. Hydrogen peroxide signaling through tumor necrosis factor receptor 1 leads to selective activation of c-Jun N-terminal kinase. J Biol Chem 2003;278:44091–44096. [DOI] [PubMed] [Google Scholar]
- 7.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997;275:90–94. [DOI] [PubMed] [Google Scholar]
- 8.Kadowaki H, Nishitoh H, Urano F, Sadamitsu C, Matsuzawa A, Takeda K, Masutani H, Yodoi J, Urano Y, Nagano T, et al. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ 2005;12:19–24. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue JI, Ichijo H. Recruitment of TRAF family proteins to the ASK1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem 2005;44:37033–37040. [DOI] [PubMed] [Google Scholar]
- 10.Hoeflich KP, Yeh WC, Yao Z, Mak TW, Woodgett JR. Mediation of TNF receptor-associated factor effector functions by apoptosis signal-regulating kinase-1 (ASK1). Oncogene 1999;18:5814–5820. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol 2000;20:2198–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem 2004;279:36454–36461. [DOI] [PubMed] [Google Scholar]
- 13.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J 2003;17:1502–1504. [DOI] [PubMed] [Google Scholar]
- 14.Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 2005;102:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambeth JD. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr Opin Hematol 2002;9:11–17. [DOI] [PubMed] [Google Scholar]
- 16.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004;4:181–189. [DOI] [PubMed] [Google Scholar]
- 17.Forman HJ, Torres M, Fukuto J. Redox signaling. Mol Cell Biochem 2002;234–235:49–62. [PubMed] [Google Scholar]
- 18.Korn SH, Wouters EF, Vos N, Janssen-Heininger YM. Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J Biol Chem 2001;276:35693–35700. [DOI] [PubMed] [Google Scholar]
- 19.Ventura JJ, Cogswell P, Flavell RA, Baldwin AS Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev 2004;18:2905–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McElhinney B, Poynter ME, Shrivastava P, Hazen SL, Janssen-Heininger YM. Eosinophil peroxidase catalyzes JNK-mediated membrane blebbing in a Rho kinase-dependent manner. J Leukoc Biol 2003;74:897–907. [DOI] [PubMed] [Google Scholar]
- 21.Shrivastava P, Pantano C, Watkin R, McElhinney B, Guala A, Poynter ML, Persinger RL, Budd R, Janssen-Heininger Y. Reactive nitrogen species-induced cell death requires Fas-dependent activation of c-Jun N-terminal kinase. Mol Cell Biol 2004;24:6763–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinhard C, Shamoon B, Shyamala V, Williams LT. Tumor necrosis factor alpha-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J 1997;16:1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen HM, Lin Y, Choksi S, Tran J, Jin T, Chang L, Karin M, Zhang J, Liu ZG. Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol Cell Biol 2004;24:5914–5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene 2005;24:3954–3963. [DOI] [PubMed] [Google Scholar]
- 25.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2003;167:1027–1035. [DOI] [PubMed] [Google Scholar]
- 26.Ward PA, Hunninghake GW. Lung inflammation and fibrosis. Am J Respir Crit Care Med 1998;157:S123–S129. [DOI] [PubMed] [Google Scholar]
- 27.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science 2005;310:847–850. [DOI] [PubMed] [Google Scholar]
- 28.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 2004;173:3589–3593. [DOI] [PubMed] [Google Scholar]
- 29.Fan C, Katsuyama M, Nishinaka T, Yabe-Nishimura C. Transactivation of the EGF receptor and a PI3 kinase-ATF-1 pathway is involved in the upregulation of NOX1, a catalytic subunit of NADPH oxidase. FEBS Lett 2005;579:1301–1305. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara T, Kuwano Y, Teshima-Kondo S, Kawai T, Nikawa T, Kishi K, Rokutan K. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. J Med Invest 2001;48:190–197. [PubMed] [Google Scholar]
- 31.Reinehr R, Becker S, Eberle A, Grether-Beck S, Haussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem 2005;280:27179–27194. [DOI] [PubMed] [Google Scholar]
- 32.Gu Y, Xu YC, Wu RF, Souza RF, Nwariaku FE, Terada LS. TNFalpha activates c-Jun amino terminal kinase through p47(phox). Exp Cell Res 2002;272:62–74. [DOI] [PubMed] [Google Scholar]
- 33.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 1998;17:2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotoh Y, Cooper JA. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem 1998;273:17477–17482. [DOI] [PubMed] [Google Scholar]
- 35.Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol 2002;191:95–104. [DOI] [PubMed] [Google Scholar]
- 36.Lewis J, Devin A, Miller A, Lin Y, Rodriguez Y, Neckers L, Liu ZG. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem 2000;275:10519–10526. [DOI] [PubMed] [Google Scholar]
- 37.Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell 2002;9:401–410. [DOI] [PubMed] [Google Scholar]
- 38.Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem 2002;277:46566–46575. [DOI] [PubMed] [Google Scholar]