Abstract
Human cytomegalovirus (CMV) is a ubiquitous pathogen which sets up a lifelong persistent infection and which can lead to significant disease in the immunosuppressed. The immunological mechanisms controlling CMV in the long term are not defined completely, but CD8+ T lymphocytes are thought to play an important role. Antiviral CD8+ T lymphocytes may exist in very large pools in healthy individuals. Although the detailed composition of these pools is not completely understood, there is known to be heterogeneity, in particular of CD45 isoform expression. We have therefore investigated the CD8+ T-lymphocyte response against CMV directly ex vivo using Class I tetramers combined with stains for a range of phenotypic markers followed by four-colour flow cytometric analysis. In particular, we examined expression of these phenotypic markers in relation to the expression of CD45 isoforms. We found that a spectrum of phenotypes exists stably, from CD45R0high/RAlow through CD45RAhigh/R0low, and that expression of other surface markers such as CD28 and CD62L, and also TCR usage, may vary in parallel with CD45 isoform expression. In some individuals, expansions of antigen-specific CD8+ T lymphocytes bearing specific TCR Vβ chains were restricted to cells of particular CD45 isoforms. Immunity against CMV comprises a large population of CD8+ T lymphocytes with heterogeneous potential, a spectrum in which CD45 isoform expression may play a central role.
Keywords: tetramer, CD8+ T lymphocyte memory, CD45 isoforms, tetramer, cytomegalovirus
INTRODUCTION
Human cytomegalovirus (HCMV) is a widespread pathogen affecting over 60% of the world's adult population. It is usually acquired in early life and persists asymptomatically except during immunosuppression [1]. The strong CTL responses it elicits in healthy seropositive individuals have been repeatedly reported, both using limiting dilution assays and recently by tetramer [2,3]. However, little is known about the composition and function of this very large memory pool.
In human T lymphocytes the expression of isoforms of CD45 has been until recently used as the major indicator of the exposure history of the lymphocyte, although in the case of CD8+ T lymphocytes, including CMV-specific cells, this is clearly not absolute [4–8]. CD45 is a glycosylated transmembrane tyrosine-specific phosphatase present in all haemopoietic cells, expressed in each cell type as a different isoform. Its isoforms are the product of differential splicing of the original CD45 transcript, which contains three exons, A, B and C, coding for the extracellular domain. When all three exons, or at least A and B, are transcribed, as occurs in naïve T cells, the marker is referred to as CD45RA. The shorter isoform lacking the three exons is known as CD45R0 and is up-regulated after activation [4,6]. However, some CD45R0+ CD8+ T cells have been shown to revert to the CD45RA+ phenotype some time after exposure to the antigen [8,9], making the memory population phenotypically heterogeneous.
Previous studies have addressed the frequency and surface marker expression of CMV-specific CD8+ T cells, as well as the functionality of different subsets within this population [2,9–13], although commonly they have used cells or clones in culture.
By using tetramer staining together with four-colour flow cytometric analysis, we were able to focus directly on CD8+ antigen-specific cells, using two fluorescence channels, and to examine both CD45 isoform expression and a further marker using the other two channels. We took advantage of this technique to study, ex vivo, the relationship between the expression of CD45 isoforms and either surface phenotype or TCR Vβ usage within populations of CMV-specific CD8+ T lymphocytes.
MATERIALS AND METHODS
HLA-A2 CMV tetramer production
An HLA-A2/CMV tetrameric complex was produced following previously described protocols [14,15], using the peptide NLVPMVATV (a reported immunodominant A2-CMV peptide derived from the matrix protein pp65 of HCMV) [10]. Tetramer validation was first performed by staining a series of negative and positive control peripheral blood mononuclear cells (PBMCs). We observed very low levels (< 0·01%) of non-specific staining in seronegative as well as seropositive HLA-mismatched individuals.
PBMC preparation
Fresh PBMCs from healthy HLA-A2+ donors were obtained from heparinized blood centrifugation over Lymphoprep (Nicomed, Norway), and washed three times (5 min, 1500 rev min, 25°C) in RPMI (Sigma Co Ltd, Poole, UK) supplemented with 50 U/ml penicillin, 2 mm/l l-glutamin and 50 μg/ml streptomycin. They were resuspended in RF10 (RPMI + 10% fetal calf serum).
PBMC staining for FACS analysis
Approximately 2–5 × 105 PBMCs in RF10 were added to 5 ml Falcon tubes, washed with PBS/0·1% Na azide and pelleted (5 min, 1500 rev min, 25°C). Approximately 0·3 μg of the HLA-A2/CMV tetramer diluted in PBS was added to each tube and the tubes were immediately incubated for 20 min at 37°C. Staining for cell surface markers was performed using antibodies against CD38-FITC, CD45R0-FITC and HLA-DR-FITC (Dako, Cambridge, UK), CD45RA-FITC, CD28-FITC, CD62L-FITC and Ki67-FITC (Immunotech, Marseilles, France), CD45R0-APC, CD27-FITC, CD57-FITC and anti-CD8 PerCP (Becton Dickinson, San José, CA, USA), and CD69-FITC, CCR5-FITC and CXCR3 (PharMingen, San José, CA, USA).
FITC-conjugated Vβ antibodies directed against Vβ 1, 2, 3, 5·1, 5·3, 8, 12, 13·1, 14, 16, 17, 20 and 22 were obtained from Immunotech. In addition, unconjugated antibodies directed against Vβ 9, 5·2 and 23 were used, followed by washing and addition of goat anti-mouse IgG Fab–FITC (Caltag, Burlingame, USA), and then addition of anti-CD8 PerCP, anti CD45R0-APC and tetramer.
Cell culture
PBMCs were plated in 24-well plates (2 × 106 cells/well) and A2-CMV peptide was added to each well to a final concentration of 10 μm. The plates were incubated at 37°C. On day 3, 10% Lymphocult T (Biotest, Germany) was added to the cultures and these were incubated for another 7 days before harvesting and staining.
PCR for CMV pp65
In order to detect CMV replication in the donors' blood, nested PCR was performed, as described previously [16]. In brief, DNA was extracted from buffy coat cells, and nested primers specific for the pp65 gene used. This assay is able to detect as few as a single infected cell in 50 000 PBMCs, and is invariably negative in uninfected donors. All donors were tested at two time-points and found to be repeatedly negative using this assay
RESULTS
Comparison of phenotypic heterogeneity amongst CMV-specific memory CTL ex vivo and in vitro
Initially, in order to examine the heterogeneity of lymphocyte phenotype amongst CMV-specific CD8+ T lymphocytes, we examined fresh PBMCs from HLA-A2+ CMV seropositive donors. These were stained with the A2-CMV tetramer, an anti CD8+/Tricolor antibody and FITC-labelled antibodies against four different surface markers (CD38+, CD45RA+, CD45R0+ and HLA-DR+). This staining is illustrated for a single donor in Fig. 1(a) (left hand column), where a mixture of CD45 isoform expression amongst the tetramer positive population can be observed (67% CD45R0 expression in the tetramer positive population). In vitro culture in the presence of antigen led to a marked distortion of expression of these surface markers (Fig. 1a middle and right hand columns), with a consistent switch from a mixed to a CD45R0high/RAlow phenotype. Accompanying this, there was a marked up-regulation of CD38 and HLA-DR (which also occurred, although to a lesser extent, in the absence of peptide, without substantial change in the CD45RA/R0 subsets).
Fig. 1.
(a) Phenotype of fresh and cultured PBMC. PBMC from a CMV seropositive HLA-A2 positive individual were examined fresh (panel A), after 10 days of culture with Lymphocult T alone (panel B) or after 10 days of culture with Lymphocult T and CMV-A2 peptide (see Methods for details; panel C). They were stained for CMV-A2 tetramer and CD8, and the other cell surface markers, as shown. The CD8high lymphocyte population is shown. The percentages shown in the right upper quadrants refer to the proportion of CD8 high/tetramer positive cells that are positive for the marker. (b) Spectrum of expression of CD45 isoforms in the CMV and specific-memory CD8+ T lymphocyte population. Four-colour staining of PBMCs from two healthy HLA-A2 HCMV seropositive individuals (donors A and B). Cells were stained with antibodies against CD8 (PerCP), CD45RA (FITC) and CD45RO (APC) and an A2-HCMV-Tetramer (PE). Left hand panels: gating on lymphocytes; right hand panels: gating only on CD8high CMV-specific T cells (Tet+ T cells).
CD45RO expression cosegregates with other cell surface markers in tetramer positive populations
Next, we took advantage of the very large size of the CMV-tetramer positive populations, coupled with four-colour staining and flow cytometry, to analyse the co-expression of CD45R0 with other cell surface markers on CMV-specific CD8+ T lymphocytes. Firstly, we observed a reciprocal expression of CD45 isoforms across a continuum within this tetramer positive population, a feature that has been reproduced recently by other workers [2]. In four healthy individuals (donors 1–4), although this continuum was seen, the dominant isoform expressed was different, and consistently so over time. We show this for two donors in Fig. 1(b). All donors were consistently CMV PCR negative, and the result cannot be explained by recent exposure to antigen.
Figure 2 illustrates the staining obtained in three donors using antibodies directed against CD28, CD27, CD57 and CD62L, after gating on CD8high, A2-CMV tetramer positive lymphocytes. We found that expression of CD45R0 was associated consistently (i.e. across patients and over time) with increased expression of CD28, compared with cells low in CD45R0. Not all cells in the CD45R0high subset were CD28high, but the CD28high set of tetramer positive cells fell largely into the CD45R0 group and similarly, CD45R0low cells were generally CD28low. For the other surface markers, a similar segregation was only observed for CD62L, which was low in most of the tetramer positive cell population; those cells which were, unconventionally, CD62Lhigh amongst the tetramer positive subset appeared to fall into the CD45R0 positive group (second row across, Fig. 2). This unusual phenotype has been associated with expansions after EBV infection [17].
Fig. 2.
Co-segregation of CD45RO and other cell surface marker expression in tetramer positive populations. PBMCs from three healthy CMV-seropositive HLA-A2 donors were stained with anti-CD8(PerCP), anti-CD45RO (APC), HLA-A2-CMV tetramer (PE) and four different FITC-labelled antibodies against CD28, CD62L, CD27 and CD57. We show plots gated on CD8+ tetramer positive T cells. Columns A, B and C correspond to each of the three donors, respectively. Rows from top to bottom correspond to the four FITC-labelled antibodies as depicted. Note the co-segregation present for CD28 and CD62L (but not for CD27 or CD57) with the CD45ROhigh population.
For the other markers studied, some did show segregation of expression according to CD45 isotype but this was not consistent between donors or over time [e.g. CD27 [11] (Fig. 2 third row across), also CD38 and perforin (data not shown)]. Others showed no segregation according to CD45 isoform expression [(e.g. CD57 [9] (Fig. 2 bottom row), also CXCR3, CCR5, HLA-DR and Ki67 (data not shown)].
Analysis of the relationship between CD45 isoform expression and TCR Vβ usage in tetramer positive populations
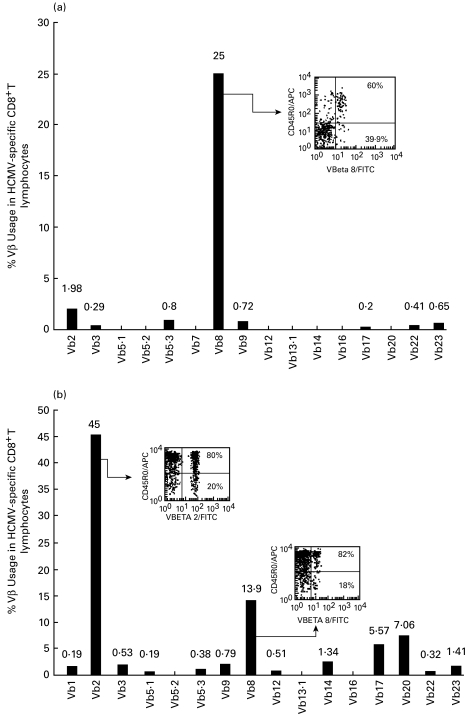
It has been shown previously [18,19] that CMV-specific CD8+ T-lymphocyte populations may contain large oligoclonal expansions. Therefore, next, the TCR Vβ usage of the tetramer positive populations and its association with CD45R0 expression was analysed using a panel of TCR Vβ-specific antibodies. PBMCs from six HLA-A2 CMV seropositive donors and a single A2-CMV CD8+ T-cell line derived from donor 4, were stained with anti-CD8+, A2-CMV tetramer, anti-CD45R0 and a panel of anti-TCR Vβ antibodies (See Materials and methods). In Fig. 3a and b, respectively, we show large Vβ expansions within the tetramer positive population of two individuals (donors 1 and 2). Interestingly, in donor 1, usage of Vβ was clearly seen to segregate according to CD45R0 expression, in that the predominant Vβ expansion (Vβ 8) was highly CD45R0 positive (60%, Fig. 3a) compared with only 18·5% CD45R0 expression in the tetramer positive population as a whole (see also Table 1). For donor 2, the CD45 isoform expression of the expanded Vβ 2 and Vβ 8 TCRs (45% and 14% of tetramer positive cells) was also high, with 80 and 82% expression, respectively (Fig. 3b and Table 1).
Fig. 3.
Vβ usage and CD45RO expression in tetramer positive populations. PBMCs from two healthy CMV seropositive HLA-A2 donors (a and b respectively) were stained as for Fig. 2, using a panel of 16 different FITC-labelled anti-TCR Vβ antibodies. Bar plots show the percentage expression of each TCR Vβ within the tetramer positive population. Insets show the CD45RO distribution within the major expansions found in each individual. Percentages shown in the insets represent the proportion of CD45RO high or low cells amongst those that express that particular TCR Vβ.
Table 1.
TCR Vβ expansions and their CD45RO expression in HCMV seropositive donor CD8+ Tet+ T lymphocytes
| Gated on CD8+ | Gated on CD8+ Tet+ | |||
|---|---|---|---|---|
| Donor | %Tet+ | %Tet+ CD45RO | Vβ (%) | (%) Vβ CD45RO |
| 1 | 0·74 | 18·5 | 8 (25) | 60 |
| 2 | 1·78 | 66 | 2 (45) | 80 |
| 8 (14) | 82 | |||
| 3 | 1·4 | 43·6 | 14 (40) | 38 |
| 22 (10·5) | 89 | |||
| 4 | 0·2 | 38 | 20 (45·5) | 25 |
| 22 (11·1) | 72 | |||
| 5 | 1·3 | 99 | 8 (54) | 100 |
| 6 | 1·44 | 58 | 1 (15·2) | 6 |
| CMV Line | 12 | 100 | 22 (96) | 99 |
%Tet+: Percentage of HCMV-specific memory T lymphocytes within the CD8+ T-cell population of the respective donor. %Tet+ CD45RO+: Percentage of CD45RO expression in Tet+ cells in the respective donor.Vβ (%): Major clonal Vβ TCR expansions for a respective donor and its percentage within the total Tet+ population. (%) Vβ CD45RO: Percentage of CD45RO expression in the respective TCR Vβ clonal expansion. In bold percentages significantly different from those in the tetramer positive population as a whole.
In four other Vβ stains, each individual showed a distinct pattern of staining (Table 1). For donor 3, for example, the distribution of CD45R0 high and low cells was approximately equivalent in the major Vβ expansion detected to that in the tetramer positive population as a whole. However, the Vβ 22 expansion of this donor showed a marked expression of CD45R0 (89%). Donor 5 expressed CD45R0 almost uniformly in all his CD8+ tetramer positive cells and accordingly, also in his largest Vβ expansion (Vβ 8). In donor 6, the largest expansion detected, Vβ1 (15% of tetramer positive cells), was predominantly CD45R0 negative (6%), in contrast to 58% of the total tetramer positive population. In this individual, since the Vβ1 expansion was relatively small, we cannot discard the possibility that there was a larger expansion in other TCR Vβ for which an antibody was not available within the panel used.
In donor 4, we observed higher expression of CD45R0 in the minor expansion Vβ22 (72%) than in the major TCR Vβ20 expansion (25%). Interestingly, the CD8+ line derived from this individual showed an almost monoclonal expansion of Vβ22 (Table 1).
DISCUSSION
Upon analysis of the surface expression of phenotypic markers in the ex vivo CMV-specific CD8+ T-lymphocyte population, the memory pool showed heterogeneous expression of CD45 isoforms, becoming significantly more homogeneous amongst CD8+ T lymphocytes stimulated with antigen in vitro. Experiments which, in the past, have relied on culture to examine the memory subset, therefore may distort the surface phenotype of the population of cells examined. While this is undoubtedly recognized for ‘activation’ markers such as HLA-DR, this may have been less obvious for ‘stable’ memory markers such as CD45 isoforms.
Our results indicate in addition a clear heterogeneity of cell surface marker expression amongst the population of CMV-specific CD8+ T lymphocyte, consistent with recent published work [2]. Here, we have taken this one step further and shown that even the CD8high/Tet+/CD45R0high subset of cells shows variability in expression of important co-stimulatory molecules, although the extent of this varies between individuals. Nevertheless, CD28 expression and CD45 isoform expression were consistently linked, which is of importance given the functional differences in activation and function that have been suggested [2,13]. CD57, whose expression has been associated with CMV specificity [12], was not consistently associated with either CD45 isoform expression, or indeed with tetramer positivity (data not shown). CD62L expression, the absence of which is generally regarded as a rigorous marker of ‘memory’ lymphocytes, may also be re-expressed in these memory pools. This may have significance as regards homing; no consistent differences were however, observed amongst chemokine receptors, at least those which we had the opportunity to test (CCR5 and CXCR3). It is possible, given the proposed role of CCR7 in ‘central memory’, that differences may be observed, although the prediction from the other markers seen is that the picture is likely to be complex in these large heterogeneous pools [20].
In the tested donors, we found very large oligoclonal expansions (up to 54% in ex vivo PBMCs) of diverse TCR Vβ within the tetramer positive populations. Vβ 2, 8, 20 and 22 were more frequently represented than other types of Vβ TCR, but not one Vβ dominated throughout. Examination of the TCR Vβ usage in these CMV-specific CD8+ CTL populations showed a variable distribution of CD45R0 isoforms within the major Vβ expansions, suggesting an independent natural history for each clonal expansion within a given tetramer positive population.
These results firstly indicate that the CD45R0low population shares, in some cases, at least the same TCR Vβ chains and probably arises from the same clonal expansions as the CD45R0high cells. Secondly, certain CMV-specific oligoclonal expansions may be more likely to retain/attain the CD45R0high phenotype, but this appears to be highly variable between individuals since in some cases, the major expansion was predominantly R0high and in others R0low.
CMV-specific oligoclonal expansions have been identified and measured by other groups using slightly different techniques. In one study [19], Vβ 8 and Vβ 17 were the two major expansions found in CTL lines derived from an HLA-A2 donor. This coincides with our own findings (expansions in Vβ 8 in three of our donors), although this should be interpreted with caution, given the distortions caused by culture and the broad individual variation we observed in healthy donors. In a recent study using tetramers, two donors were found to have expansions of Vβ 14 in their CMV-specific cells [2].
The clear ex vivo phenotypic heterogeneity we have shown here is probably associated with a functional heterogeneity of various lymphocytic subpopulations exerting different effector functions. Thus, discrepancies between frequencies of virus-specific T lymphocytes as measured by tetramer staining and by functional assays (ELISpot) have been shown for the HIV model [21] as well as for hepatitis C, EBV and, in our own hands, for CMV infections [22–24]. A recent paper by Goulder and colleagues [25], however, showed good correlation between tetramer staining and intracellular cytokine staining for IFN-γ. Further work will be necessary to clarify this issue using a variety of functional assays in parallel with tetramer staining.
The nature of long-term antiviral T-cell pools continues to tax immunologists — what is their function and capacity, how are they maintained and how are the different subsets of such cells best identified in mouse and man? This issue has become more interesting but also more complex with the advent of tetramers, which allow detection of cells independent of function [14,22,26]. The expression of isoforms of CD45 has for some time been considered important in differentiating naive and memory cells, although the distinction is becoming increasingly blurred [8,11].
Whether this marker alone is the most significant in identifying phenotypic and functional diversity, or whether other co-segregating molecules may also play a similar role, is an area beyond the scope of this paper. Nevertheless, a direct ex vivo approach using tetramers, particularly coupled with intracellular cytokine staining and four-colour flow cytometry, will allow further dissection of this complex area.
Acknowledgments
This work was sponsored by The Wellcome Trust and FUNDACYT (Ecuador). We are very grateful for help in the laboratory from Gillian Harcourt, David Price, Sarah Dawson, and Rod Dunbar, and from the Royal Free Hospital Virology Department, especially Vince Emery and Aycan Hassan-Walker. We thank V. Cerundolo and M. Salio for kindly providing us with the CMV-line and its parental PBMCs.
REFERENCES
- 1.Roullet E. Opportunistic infections of the central nervous system during HIV-1 infection (emphasis on cytomegalovirus disease) J Neurol. 1999;246:237–43. doi: 10.1007/s004150050341. [DOI] [PubMed] [Google Scholar]
- 2.Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8 (+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74:8140–50. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin X, Demoitie MA, Donahoe SM, et al. High frequency of cytomegalovirus-specific cytotoxic T-effector cells in HLA-A*0201-positive subjects during multiple viral coinfections. J Infect Dis. 2000;181:165–75. doi: 10.1086/315201. [DOI] [PubMed] [Google Scholar]
- 4.Akbar A, Terry L, Timms A, et al. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–8. [PubMed] [Google Scholar]
- 5.Miyawaki T, Kasahara Y, Kanegane H, et al. Expression of CD45R0 (UCHL1) by CD4+ and CD8+ T cells as a sign of in vivo activation in infectious mononucleosis. Clin Exp Immunol. 1991;83:447–51. doi: 10.1111/j.1365-2249.1991.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merkenschlager M, Beverley PC. Evidence for differential expression of CD45 isoforms by precursors for memory-dependent and independent cytotoxic responses: human CD8 memory CTLp selectively express CD45RO (UCHL1) Int Immunol. 1989;1:450–9. doi: 10.1093/intimm/1.4.450. [DOI] [PubMed] [Google Scholar]
- 7.Okumura M, Fujii Y, Inada K, et al. Both CD45RA+ and CD45RA- subpopulations of CD8+ T cells contain cells with high levels of lymphocyte function-associated antigen-1 expression, a phenotype of primed T cells. J Immunol. 1993;150:429–37. [PubMed] [Google Scholar]
- 8.Wills MR, Carmichael AJ, Weekes MP, et al. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J Immunol. 1999;162:7080–7. [PubMed] [Google Scholar]
- 9.Hazzan M, Labalette M, Noel C, et al. Recall response to cytomegalovirus in allograft recipients: mobilization of CD57+, CD28+ cells before expansion of CD57+, CD28- cells within the CD8+ T lymphocyte compartment. Transplantation. 1997;63:693–8. doi: 10.1097/00007890-199703150-00014. [DOI] [PubMed] [Google Scholar]
- 10.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamann D, Kostense S, Wolthers KC, et al. Evidence that human CD8+CD45RA+CD27- cells are induced by antigen and evolve through extensive rounds of division. Int Immunol. 1999;11:1027–33. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 12.Kern F, Khatamzas E, Surel I, et al. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur J Immunol. 1999;29:2908–15. doi: 10.1002/(SICI)1521-4141(199909)29:09<2908::AID-IMMU2908>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Weekes MP, Carmichael AJ, Wills MR, et al. Human CD28–CD8+ T cells contain greatly expanded functional virus-specific memory CTL clones. J Immunol. 1999;162:7569–77. [PubMed] [Google Scholar]
- 14.Altman J, Moss PAH, Goulder P, et al. Direct visualization and phenotypic analysis of virus-specific T lymphocytes in HIV-infected individuals. Science. 1996;274:94–6. [Google Scholar]
- 15.Ogg GS, Kostense S, Klein MR, et al. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J Virol. 1999;73:9153–60. doi: 10.1128/jvi.73.11.9153-9160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caliendo AM, St George K, Kao SY, et al. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients [In Process Citation] J Clin Microbiol. 2000;38:2122–7. doi: 10.1128/jcm.38.6.2122-2127.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynne JE, Schmid I, Matud JL, et al. Major expansions of select CD8+ subsets in acute Epstein-Barr virus infection: comparison with chronic human immunodeficiency virus disease. J Infect Dis. 1998;177:1083–7. doi: 10.1086/517400. [DOI] [PubMed] [Google Scholar]
- 18.Weekes MP, Wills MR, Mynard K, et al. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28- CD8+ T-cell population. Immunology. 1999;98:443–9. doi: 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weekes MP, Wills MR, Mynard K, et al. The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J Virol. 1999;73:2099–108. doi: 10.1128/jvi.73.3.2099-2108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions [see comments] Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 21.Goepfert PA, Bansal A, Edwards BH, et al. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol. 2000;74:10249–55. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callan MF, Fazou C, Yang H, et al. CD8 (+) T-cell selection, function, and death in the primary immune response in vivo. J Clin Invest. 2000;106:1251–61. doi: 10.1172/JCI10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan-Walker AF, Vargas Cuero AL, Mattes FM, et al. CD8+ cytotoxic lymphocyte responses against cytomegalovirus after liver transplantation: correlation with time from transplant to receipt of tacrolimus. J Infect Dis. 2001;183:835–43. doi: 10.1086/319260. [DOI] [PubMed] [Google Scholar]
- 25.Goulder PJ, Tang Y, Brander C, et al. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J Exp Med. 2000;192:1819–32. doi: 10.1084/jem.192.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogg G, Jin X, Bonhoeffer S, et al. Quantitation of HIV-1 specific CTL and plasma load of HIV-1. Science. 1998;279:2103–6. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]