Abstract
Retinochoroiditis caused by Toxoplasma gondii infection results in inflammation and necrosis of the retina. We have used human retinal pigment epithelial cultures (HRPE) as an in vitro model to investigate the role of TGF-β in T. gondii-induced retinochoroiditis. RT-PCR analyses showed enhanced steady state levels of TGF-β1 and TGF-β2 mRNA in T. gondii-infected HRPE. Uninfected HRPE secrete TGF-β1 in a latent form while 10–30% of the secreted TGF-β2 was in the active form. T. gondii infection induced a significant increase (P < 0·01) in total TGF-β1 and TGF-β2 secretion by HRPE. In addition, soluble extracts of T. gondii (ST) stimulated secretion of both TGF-β1 and TGF-β2 significantly (P < 0·01). Interestingly, T. gondii infection as well as ST of the parasites completely inhibited secretion of the active form of TGF-β2. Studies evaluating the effect of TGF-β on T. gondii replication in HRPE revealed that TGF-β enhanced parasite replication. The interactions between host retinal cells and T. gondii may play an active role in the pathogenesis of retinochoroiditis.
Keywords: immunopathogenesis, retinochoroiditis, TGF-β, Toxoplasma gondii
INTRODUCTION
Ocular toxoplasmosis results in inflammation and disorganization of the retina and is one of the common causes of uveitis, an intraocular inflammatory disease [1–5]. The intensity of the damage to the retina and choroid depends on the severity of infection and the associated inflammatory reaction [3–5]. Infiltrating inflammatory cells, predominantly macrophages and lymphocytes, play an active role in the pathogenesis associated with T. gondii- induced retinochoroiditis [4,5]. However, the roles of native pro- and anti-inflammatory cytokines and retinal resident cells in the regulation of primary and recurrent T. gondii infections have not been elucidated clearly.
Transforming growth factor-β (TGF-β) is a multi-functional cytokine with anti-inflammatory activities, such as inhibition of proliferation, maturation and/or activation of macrophages, lymphocytes and natural killer (NK) cells [6–8]. TGF-β is secreted in a latent form from which the mature form is released from the latency-associated peptide by proteolytic cleavage and other unknown mechanisms [9,10]. The roles of TGF-β in ocular pathophysiology and in immunoregulation are well documented [11–13]. Elevated expression of TGF-β in vitreous, retina and retinal pigment epithelium (RPE) has been correlated closely with retinal fibrosis and choroidal neovascularization [11]. On the other hand, TGF-β levels in ocular fluids have been shown to be lower in uveitis patients [13].
Retinal pigment epithelium (RPE), a single layer of epithelial cells that form a tight junction, acts as a barrier between the choroid and the neuroretina in the eye. RPE plays a vital role in the normal physiology and pathophysiology of the retina and choroid [14,15]. RPE respond to inflammatory stimuli and secrete molecules such as IL-6, IL-8, GMCSF, nitric oxide and ICAM-1 [16–19]. Histopathological examination of the eyes of patients with toxoplasma-induced retinochoroiditis revealed the presence of free tachyzoites and tissue cysts in the RPE and the neuroretina [20–22]. In our previous studies, we have characterized the replication of T. gondii in human RPE cultures [23] and the secretion of inflammatory molecules upon T. gondii infection [24]. In this report, we have identified retinal host cell–parasite interactions in which T. gondii infection stimulates and alters the active form of TGF-β, while administration of TGF-β augments parasite replication.
MATERIALS AND METHODS
Reagents
Human TGF-β1, TGF-β2 ELISA kits and human rTGF-β1 and rTGF-β2 were bought from R&D Systems, Minneapolis, MN, USA. Monoclonal mouse anti-T. gondii (RH strain) antibodies were purchased from Biogenex Laboratories, San Ramos, CA, USA. Fetal bovine serum and cell culture media were obtained from Life Technologies, Grand Island, NY, USA. PCR amplimer sets for human GAPDH, TGF-β1 and TGF-β2 were purchased from Continental Laboratory Products, San Diego, CA, USA. GeneAmp RNA PCR reagent kit with AmpliTaq DNA polymerase was purchased from Perkin Elmer, Foster City, CA, USA.
HRPE cultures and infection of cells with T. gondii
Primary cell lines of human retinal pigment epithelial cells (HRPE) were prepared from human donor eyes and characterized as described previously [16,17]. HRPE cultures at passages 6–12 were used for the experiments reported in this study. HRPE cultures were grown to confluence in 24-well tissue culture plates in MEM containing 10% FBS and other components as described [16,24]. Tachyzoites of T. gondii (RH strain) were grown in HRPE cultures and prepared for inoculation as reported previously [23,24]. The cultures were washed with serum free medium (SFM) and incubated in SFM overnight before inoculating with tachyzoites of T. gondii, suspended in SFM (0·5 ml/well), at a multiplicity of infection (moi) of 5. The cultures were incubated for 2 h at 37°C in a tissue culture incubator, with mild agitation every 15 min. Then, the cultures were washed twice with SFM to remove non-adherent parasites and incubated in 1 ml/well of fresh SFM. Supernatant fluids from duplicate wells of control (uninfected) and T. gondii infected cultures were collected at various times (days 1, 2, 3 and 4) post-inoculation and frozen until used for analysis.
Preparation of T. gondii soluble extracts
Tachyzoites of T. gondii (RH strain) were harvested from infected HRPE cells as described earlier [23,24]. Parasites were suspended in PBS at 2 × 108/ml, and lysed by freeze–thaw three times on dry-ice followed by sonication for three cycles of 30 s duration. Parasite extracts were centrifuged for 15 min at 14 000 r.p.m. in an Eppendorf microfuge and the supernatant was used as T. gondii soluble extract. Parasite extracts were heat inactivated by incubating at 60°C for 30 min. For cytokine secretion studies, 50 μl of soluble extract, equivalent to 10 million tachyzoites of T. gondii, was added to each well of a 24-well plate containing 950 μl of SFM.
Detection of intracellular T. gondii by immunostaining
HRPE cultures grown to confluence in 8-well chamber slides were inoculated with tachyzoites of T. gondii at an moi of 1–10. At various post-inoculation times, cultures were fixed for 10 min in an acetone–methanol mixture [11], precooled to – 20°C. The slides were air-dried and incubated with mouse MoAb raised against T. gondii (RH strain), for 1h at room temperature. After washing, cultures were incubated further with FITC conjugated horse antimouse IgG for 1h. The slides were washed again with PBS and mounted with aqua-mount and observed under a fluorescence microscope.
Analysis of TGF-β1 and TGF-β2 mRNA expression by RT- PCR
The PCR primers for TGF-β1, TGF-β2 and GAPDH are as follows: TGF-β1, 5′-CAG AAA TAC AGC AAC AAT TCC TGG-3′ and 5′-TTG CAG TGT GTT ATC CGT GCT GTC-3′; TGF-β2, 5′-TCC AAA GAT TTA ACA TCT CCA ACC-3′ and 5′-CAT GCT CCA GCA CAG AAG TTG G-3′; GAPDH, 5′- CCA CCC ATG GCA AAT TCC ATG GCA-3′ and 5′-TCT AGA CGG CAG GTC AGG TCC ACC-3′. Total cellular RNA from the control and T. gondii-infected cultures was prepared by using RNASTAT-60 solution [24]. A RNA PCR kit (Perkin Elmer, Applied Biosystems Division, Foster City, CA, USA) was used according to the manufacturer’s instructions. PCR products obtained after 30 cycles were separated on ethidium bromide containing agarose gel, photographed under UV light and integrated in Eagle Eye system (Stratagene, San Diego, CA, USA).
Analysis of TGF-β1 and TGF-β2 levels in culture supernatant fluids by ELISA
Culture supernatant fluids from uninfected and T. gondii-infected HRPE cells were clarified by centrifugation for 5 min at 14 000 r.p.m. in an Eppendorf microfuge. Levels of TGF-β1 and TGF-β2 in the culture supernatant fluids were determined by enzyme immunoassays following the manufacturer’s instructions. TGF-β1 and TGF-β2 present in untreated samples were considered as mature (active) forms whereas those detected following acid activation were taken as the total (mature and latent) TGF-β. Results obtained from the same batch of the cultures grown under similar conditions were used for the statistical evaluation of the data for any given experiment.
Effects of TGF-β1 and TGF-β2 on T. gondii replication in HRPE
HRPE cultures were grown to confluence in 8-well glass chamber slides and were infected with tachyzoites of T. gondii (RH strain) as described previously [23]. The cultures were washed with SFM and incubated in SFM overnight before inoculation with 105 tachyzoites suspended in (400 μl/well) SFM. The cultures were incubated for 2h at 37°C in a tissue culture incubator, with mild agitation every 15 min. Then, the cultures were washed twice with SFM to remove non-adherent parasites and incubated in 0·5 ml/well of fresh SFM or SFM containing indicated concentrations of human recombinant TGF-β or other growth factors. After 5 days of inoculation, cultures were gently rinsed twice, supernatant fluids collected and the parasite count determined by using a haemocytometer [23].
RESULTS
Detection of T. gondii replication in HRPE cells by immunofluorescence
Experiments were conducted initially to determine the ideal conditions for T. gondii infection of HRPE cells for TGF-β studies. HRPE cells grown to confluence on glass chamber slides were inoculated with tachyzoites of T. gondii (RH strain) at an moi of 1, 5 and 10. At 1, 2, 3 and 4 days post-inoculation, cultures were fixed and the presence of T. gondii within the HRPE cells was detected by immunofluorescence using MoAb raised against the 30 kDa membrane antigen (SAG-1) of T. gondii (RH strain). Cultures inoculated at an moi of 1 were not uniformly infected whereas cultures inoculated at an moi of 5 were uniformly infected [24]. At an moi of 10, HRPE cultures were rapidly destroyed. For all the studies reported in this paper, we have selected an moi of 5 for T. gondii inoculation, since it results in uniform infection of HRPE cell layers. Representative immunofluorescent images of T. gondii within HRPE cells are shown in Fig. 1. At one day post-inoculation (pi), parasites are seen as free tachyzoites intracellularly (Fig. 1a), which divide and increase in numbers by 2 days pi (Fig. 1b). At day 3 pi (Fig. 1c), T. gondii continue to divide to form multicellular conglomeration (rosettes) of tachyzoites. By day 4 pi, parasites occupy most of the cytoplasm, sparing the nucleus (Fig. 1d).
Fig. 1.
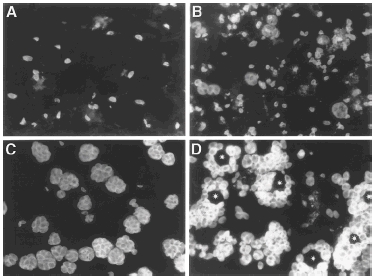
Immunofluorescence detection of T. gondii in HRPE cultures. Cultures were grown on 8-well glass chamber slides and inoculated with T. gondii (RH strain) at an m.o.i of 5. Cultures were fixed and stained with MoAb made against T. gondii antigen. Representative images of T. gondii- infected HRPE cells at various post-inoculation days; post- inoculation day 1(a), day 2 (b), day 3 (c), day 4 (d). All pictures are at the same magnification (400×). Asterisks in (d) indicate the position of nucleus in parasite-filled cells.
Analyses of mRNA expression of TGF-β1 and TGF-β2
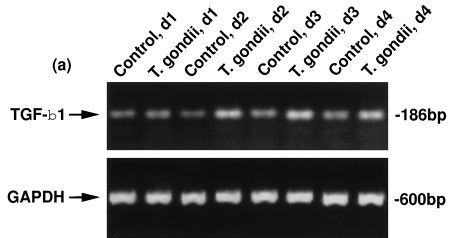
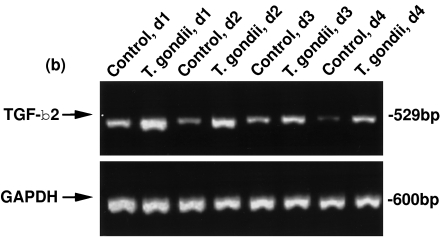
We evaluated the steady state levels of TGF-β1 and TGF-β2 mRNA in T. gondii-infected HRPE cultures to determine if T. gondii infection influenced their mRNA levels. Total RNA prepared from the untreated cells and T. gondii-infected cultures were used for RT-PCR analyses. Uninfected and T. gondii-infected HRPE expressed both TGF-β1 and TGF-β2 mRNA at days 1–4 post-inoculation (Fig. 2). The intensity of the bands of the PCR products representing TGF-β1 and TGF-β2 are higher in T. gondii-infected HRPE when compared to uninfected (control) cultures. Lower panels show the bands of PCR products amplified with GAPDH primers with the same total RNA preparations as indicated in the upper panel.
Fig. 2.
RT-PCR analyses of TGF-β1 (a) and TGF-β2 (b) mRNA expression in T. gondii-infected HRPE. Total RNA was prepared from control (uninfected) and T. gondii-infected HRPE and 1 u g of RNA was used for reverse transcription and amplification (30 cycles) in a single tube using an RNA PCR kit as described in the Methods section. Control, d1; Control, d2; Control, d3 and Control, d4=uninfected cultures incubated for 1, 2, 3 or 4 days in serum free medium (SFM). T. gondii, d1; T. gondii, d2; T. gondii, d3 and T. gondii, d4=cultures infected with T. gondii for 1, 2, 3 or 4 days post-inoculation in SFM. The position of TGF-β1, TGF-β2 and GAPDH PCR products, 186, 529 and 600 bp, respectively, are indicated by arrows.
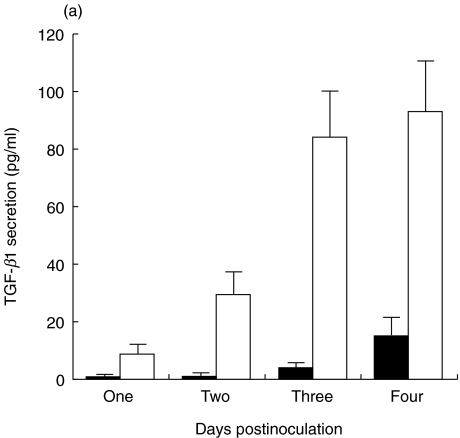
Time-course of total and active TGF-β1 and TGF-β2 secretion by HRPE cells
For all these studies, cultures were washed in SFM and incubated in SFM to avoid the effects of various growth factors and cytokines, including high levels of TGF-β present in the serum, on the secretion of TGF-β. Levels of TGF-β1 and β2 in the culture supernatants were determined without or with acid activation to identify mature and total (latent and mature) forms, respectively. The time-course of TGF-β secretion was followed up to 4 days. Levels of total TGF-β2 secreted were higher than total TGF-β1 secreted by HRPE cells (Fig. 3). Almost all of the secreted TGF-β1 was in a latent form while 10–40% of the secreted TGF-β2 was in a mature form.
Fig. 3.
Time-course of total and activeTGF-β1, and TGF-β2 secretion by HRPE. Cultures were serum starved for 24 h and incubated in SFM for 1, 2, 3 or 4 days and culture supernatants were collected and the levels of active (mature) and total TGF-β1 and TGF-β2 were determined by ELISA. Results are the means ± s.e. for four experiments each with duplicate samples. ▪, TGF-β1(total); □, TGF-β1 (active); •,TGF-β2 (total); ○, TGF-β2 (active).
TGF-β1 and TGF-β2 secretion by T. gondii-infected HRPE
Next, we examined the secretion of TGF-β protein by HRPE cells infected with T. gondii to verify whether enhanced levels of TGF-β mRNA resulted in translation. HRPE cultures were inoculated with T. gondii at an moi of 5. In uninfected cultures, there was a small increase in TGF-β1 secretion from days 1–4. Secretion of TGF-β1 increased significantly (P < 0·01) in T. gondii-infected cultures at all post-inoculation times (Fig. 4a). Progressive increase in TGF-β2 secretion was observed in uninfected HRPE cultures (Fig. 4b). Significant differences (P < 0·01) between control and infected cultures were observed in TGF-β2 secretion at days 1–4 post-inoculation times.
Fig. 4.
Secretion of TGF-β1(a) and TGF-β2 (b) by T. gondii-infected HRPE. Culture supernatant fluids were harvested at various post- inoculation times and the levels of total TGF-β determined by ELISA. Results are the means ± s.e. for four separate experiments each with duplicate samples. ▪, Control; □, T. gondii.
Effect of T. gondii soluble extracts on TGF-β1 and TGF-β2 secretion by HRPE
We have prepared soluble extracts of T. gondii tachyzoites to examine their possible effect on TGF-β secretion. Results of the effects of the parasite extracts on TGF-β1 and TGF-β2 secretion are shown in Tables 1 and 2. For comparison, effects of T. gondii infection, soluble extract and heat-inactivated soluble extract of one set of HRPE cultures are presented as one experiment. As there is a variation in the basal levels of TGF-β secretion by HRPE cultures, comparisons are always made with the same batch of cultures. Soluble extracts of T. gondii are at least as effective as parasite infection, in inducing both TGF-β1 and TGF-β2 secretion. Heat inactivation of the soluble extracts did not reduce significantly the TGF-β-inducing activity, suggesting that active component(s) are not heat labile.
Table 1.
TGF-β1(total) secretion by HRPE incubated with soluble extracts of T. gondii
| TGF-β1 secretion (pg/ml medium) | |||
|---|---|---|---|
| Treatment | Experiment 1 | Experiment 2 | Experiment 3 |
| Uninfected HRPE (control) | 3·8 ± 1·7 | 29·6 ± 6·6 | 103·8 ± 42·5 |
| T. gondii-infected HRPE | 84·1 ± 15·9* | 101·3 ± 27·0* | 526·8 ± 42·8* |
| Soluble extract-1 | 198·6 ± 15·7* | n.d. | 434·6 ± 79·8* |
| HI soluble extract-1 | 135·6 ± 14·2* | n.d. | 362·5 ± 97·2* |
| Soluble extract-2 | n.d. | 127·1 ± 18·1* | n.d. |
| HI soluble extract-2 | n.d. | 100·9 ± 20·4* | n.d. |
Cultures were incubated with native or heat inactivated soluble extracts of T. gondii (RH strain) tachyzoites for 3 or 4 days and the levels of total TGF-β1were determined by ELISA. Soluble extract-1 and soluble extract-2 are the extracts prepared from two different T. gondii tachyzoite preparations. Heat-inactivated (HI) soluble extracts were prepared by heating the extracts at 60°C for 30 min. For comparison, total TGF-β1 secreted by the same batch of HRPE infected with T. gondii are given. Results are the means ± s.e. for four separate cultures each with duplicate samples.
Significantly different from uninfected cultures P < 0·01; n.d.: not determined.
Table 2.
TGF-β2 (total) secretion by HRPE incubated with soluble extracts of T. gondii
| TGF-β2 secretion (pg/ml medium) | |||
|---|---|---|---|
| Treatment | Experiment 1 | Experiment 2 | Experiment 3 |
| Uninfected HRPE (control) | 203·8 ± 39·2 | 231·0 ± 52·2 | 237·1 ± 66·2 |
| T. gondii-infected HRPE | 378·3 ± 53·8* | 393·4 ± 91·4* | 452·5 ± 110·8* |
| Soluble extract-1 | 606·2 ± 83·3* | n.d. | 533·2 ± 151·6* |
| HI soluble extract-1 | 604·1 ± 89·0* | n.d. | 548·7 ± 194·6* |
| Soluble extract-2 | n.d. | 407·5 ± 69·1* | n.d. |
| HI soluble extract-2 | n.d. | 362·6 ± 62·0* | n.d. |
Culture supernatants prepared for TGF-β1 analyses (Table 1) were used for total TGF-β2 assay. For comparison, total TGF-β2 secreted by the same batch of HRPE infected with T. gondii are given. Results are the means ± s.e. for four separate cultures each with duplicate samples.
Significantly different from uninfected cultures P < 0·01; n.d.: not determined.
T. gondii infection or T. gondii extracts inhibit formation of mature TGF-β2
Uninfected HRPE cultures were shown to secrete TGF-β2, but not TGF-β1, in both mature and latent forms (Fig. 3). T. gondii infection as well as soluble extracts enhanced the secretion of both TGF-β1 and TGF-β2 (Fig. 4 and Tables 1 and 2). Therefore, we examined whether there are any changes in the ratio of secreted latent and mature TGF-β forms. TGF-β1 was always secreted in latent form in uninfected as well as in HRPE infected with T. gondii or incubated with T. gondii extracts (data not shown), in spite of significant increases in total TGF-β1 secretion. In two experiments, about 30–50% of the secreted TGF-β2 by uninfected HRPE cells was in the mature form (Table 3). T. gondii infection or T. gondii extracts induced significant increase in total TGF-β2 secretion. Interestingly, there is a tremendous drop in mature TGF-β2 levels, to less than 5% of the total TGF-β2, in T. gondii-infected HRPE as well as in HRPE incubated with T. gondii soluble extracts.
Table 3.
T. gondii infection and T. gondii soluble extracts inhibit secretion (formation) of mature TGF-β2 by HRPE
| TGF−β2 secretion (pg/ml medium) | ||||
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | |||
| Treatment | Total | Mature | Total | Mature |
| Uninfected HRPE | 150·1 ± 46·0 | 54·5 ± 17·3 | 203·8 ± 39·2 | 101·4 ± 18·6 |
| T. gondii-infected HRPE | 351·5 ± 71·8 | 8·0 ± 3·3* | 378·2 ± 53·8 | 0·3 ± 0·3* |
| Soluble extract | 407·5 ± 69·1 | 15·0 ± 7·8* | 607·4 ± 89·6 | 0·0 |
| HI soluble extract | 362·6 ± 62·0 | 24·1 ± 12·1* | 606·2 ± 83·4 | 0·0 |
Cultures were incubated with native or heat inactivated soluble extracts of T. gondii (RH strain) tachyzoites for 3 or 4 days, and the levels of mature (active) and total TGF-β2 were determined by ELISA. Soluble and heat-inactivated soluble extracts were prepared as described in Methods. For comparison, mature and total TGF-β2 secreted by the same batch of HRPE infected with T. gondii are given. Results are the means ± s.e. for four separate cultures each with duplicate samples.
Significantly different from uninfected cultures P < 0·01.
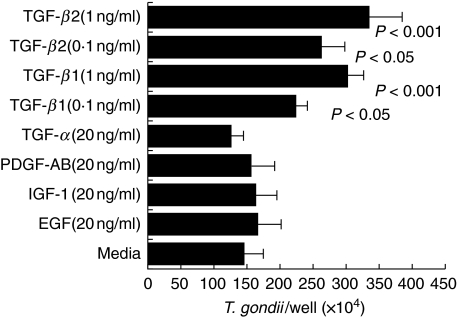
Effects of human recombinant TGF-β1 and TGF-β2 on T. gondii replication in HRPE
Because enhanced secretion of TGF-β1 and TGF-β2 was observed in HRPE infected with T. gondii, it is of interest to see whether addition of TGF-β have any effect on T. gondii replication in HRPE. HRPE were treated with biologically active recombinant TGF-β1,TGF-β2,TGF-α, PDGF-AB, IGF-1 or EGF and parasite replication was evaluated. There were significant increases (P-value < 0·05 and < 0·001) in the T. gondii replication in HRPE treated with 0·1 and 1·0 ng/ml of TGF-β1 or TGF-β2 (Fig. 5). TGF-α, PDGF-AB, IGF-1 and EGF had no significant effects on T. gondii replication in HRPE (Fig. 5).
Fig. 5.
Effect of TGF-β on T. gondii replication in HRPE. HRPE were infected with T. gondii and treated with human recombinant TGF-β1,TGF-β2 or other growth factors as described in Material and methods section. After 5 days, T. gondii replication in HRPE was quantified by counting the parasites using a haemocytometer. Results are the means ± s.e. for four experiments each with duplicate samples.
DISCUSSION
Retinochoroiditis caused by T. gondii infection is a frequent cause of uveitis, an intraocular inflammatory disease of the eye in humans [1–5]. The hallmark of toxoplasma-induced retinochoroiditis is the recurrent episodes of reactivation and localized inflammatory responses to these episodes. Immunopathological changes observed in the retina are directly due to invasion, replication and destruction of retinal cells followed by the secondary effects due to immune reactivity to parasite antigens as well as retinal antigens released from the necrotizing retina [4,5,25,26]. However, it is difficult to analyse the initial responses of retinal cells to parasite invasion and injury to the target tissue. Therefore, we have used cultured human RPE (HRPE) cells to investigate the primary cellular responses of retinal resident cells to intracellular T. gondii replication. The present study demonstrated that T. gondii infection up-regulate mRNA levels and enhance secretion of TGF-β1 and TGF-β2 by HRPE cells. The study also showed that soluble T. gondii antigens induce the secretion of TGF-β1 and β2 by HRPE cells. Finally, our results indicate that TGF-β can act directly on HRPE cells to augment replication of T. gondii.
A combination of parasite trigger and host cell status may be important factors in eliciting TGF-β secretion by HRPE in response to T. gondii infection. It should be emphasized here that cells were incubated in SFM during T. gondii infection studies and therefore no stimulating agents or serum factors were responsible for induction and/or interference with the TGF-β secretion. We suggest that the enhanced secretion of TGF-β by T. gondii-infected HRPE may be due to the interaction of the parasite with host cells by (a) the initiation of a cascade of signal transduction pathways at the membrane level during parasite infiltration; (b) interfering with host cell metabolic pathways during intracellular parasite replication; and/or (c) the release of parasite secretion and degradation products [23,24,27,28]. The secretion of TGF-β by HRPE may not be due to non-specific effect of cellular damage caused by T. gondii, as soluble as well as heat-inactivated soluble extracts of T. gondii could induce TGF-β secretion without causing any microscopically noticeable cellular injury. Because heat inactivation of the extracts had no significant reduction in stimulating TGF-β secreting activity, the causative factors in the extract are non-protein components, possibly glycosylphosphatidyl moieties of the parasite. Additional support that TGF-β secretion by T. gondii-infected HRPE is not non-specific comes from the studies in which incubation of HRPE with IL-6 or GM-CSF, cytokines known to be produced by HRPE upon T. gondii infection [24], failed to enhance TGF-β1 or TGF-β2 secretion (unpublished observations).
The interactions between TGF-β and parasite were studied in in vitro cell culture systems. Elevated levels of active TGF-β were produced by spleen cells from mice infected with Trypanosoma cruzi[29] and by normal mouse peritonial macrophages infected with Leishmania braziliensis[30]. However, there are no studies on TGF-β secretion or effects of TGF-β on T. gondii replication in either retinal cells or cells of the immune system. In our studies, we observed elevated levels of both latent TGF-β1 and TGF-β2 in T. gondii-infected HRPE. Interestingly, secretion of active (mature) TGF-β2, observed in uninfected HRPE, was abolished in T. gondii-infected HRPE suggesting possible interactions between host cells and parasite in the regulation of TGF-β2 production and activation. Activation of latent TGF-β is a complex process involving extracellular matrix and proteolytic enzymes, such as urokinase type plasminogen activator, matrix metalloproteinases and tissue inhibitors of metalloproteinases [9,10]. Because T. gondii infection inhibits the formation of active form of TGF-β2 in spite of elevated latent TGF-β2, we suspect that some of these activation mechanisms are affected in T. gondii-infected HRPE. Further studies are needed to investigate the detailed mechanisms of TGF-β activation in uninfected and T. gondii-infected HRPE
Latent TGF-β produced by HRPE cells, if converted to the mature form, may promote parasite replication in vivo by inhibiting the actions of lymphocytes, macrophages and other immunoregulatory cells that control parasite replication. It is also possible that TGF-β acts directly on HRPE and other retinal cells altering their potential to promote T. gondii replication as observed in the present study (Fig. 5). Similar observations were made in which addition of recombinant TGF-β1 in vitro to murine peritonial macrophages or human macrophages increased the intracellular replication of L. braziliensis and T. cruzi, respectively [29,31].
The role of cell-mediated immunity has been shown to play a critical role in the defence against T. gondii infections [5,25]. A number of recent studies indicate that balanced interactions among the various cytokines may be critical in effectively controlling the parasite growth and minimizing the host tissue damage [25,26,32]. Cytokines such as IFN-γ and TNF-α[32] and TGF-β and IL-6 [26] are shown to be associated with the immunopathogenesis in animal models of ocular toxoplasmosis. Cytokines produced by macrophages, lymphocytes and NK cells are involved in mediating cellular immune responses to the parasite or its antigens. In addition, resident non-lymphoid cells may also take an active part in secreting some of the inflammatory mediators that could affect the recruitment, proliferation and activation of lymphoid cells. Finally, we suggest that HRPE and parasite interactions generate TGF-β, a natural immunosuppressive agent, which may regulate T. gondii replication and immunopathogenesis in toxoplasma-induced retinochoroiditis.
REFERENCES
- 1.Wilder HP. Toxoplasma chorioretinitis in adults. Arch Ophthalmol. 1952;48:127–36. doi: 10.1001/archopht.1952.00920010132001. [DOI] [PubMed] [Google Scholar]
- 2.Perkins ES. Ocular toxoplasmosis. Br J Ophthalmol. 1973;57:1–17. doi: 10.1136/bjo.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland GN, Engstrom RE, Jr, Glasgow BJ. Ocular toxoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1988;106:653–67. doi: 10.1016/0002-9394(88)90697-6. [DOI] [PubMed] [Google Scholar]
- 4.Jabs DA. Ocular toxoplasmosis. Int Ophthalmol Clin. 1990;30:264–70. doi: 10.1097/00004397-199030040-00009. [DOI] [PubMed] [Google Scholar]
- 5.Nussenblatt RB, Whitcup SM, Palestine AG. Ocular toxoplasmosis. In: Nussenblatt RB, Whitcup SM, Palestine AG, editors. Uveitis Fundamentals and clinical practice. St Louis: Mosby Yearbook; 1996. pp. 211–28. [Google Scholar]
- 6.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Ann Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 7.Wahl SM. Transforming growth factor beta (TGF-β) in inflammation. A cause and a cure. J Clin Immunol. 1992;12:61–74. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz DA, Gray JD, Ohtsuka K, Hirokawa M, Takahashi T. The immunoregulatory effects of NK cells: the role of TGF-β and implications for autoimmunity. Immunol Today. 1997;18:538–42. doi: 10.1016/s0167-5699(97)01149-3. [DOI] [PubMed] [Google Scholar]
- 9.Flaumenhaft R, Kojima S, Abe M, Rifkin DB. Activation of latent transforming growth factor β. Adv Pharmacol. 1993;24:51–76. doi: 10.1016/s1054-3589(08)60933-3. [DOI] [PubMed] [Google Scholar]
- 10.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloprotease-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–76. [PMC free article] [PubMed] [Google Scholar]
- 11.Connor TB, Roberts AB, Sporn MB, et al. Correlation of fibrosis and transforming growth factor-β type 2 levels in the eye. J Clin Imvest. 1989;83:1661–6. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forrester JV, Lumsden L, Liversidge J, Kuppener M, Mesri M. Immunoregulation of uveoretinal inflammation. Prog Reti Eye Res. 1995;14:393–412. [Google Scholar]
- 13.de Boar JH, Limpens J, Orengo-Nania S, de Jong PVTM, Heij EL, Kojlstra A. Low mature TGF-β2 levels in aqueous humor during uveitis. Invest Opthalmol Vis Sci. 1994;35:3702–10. [PubMed] [Google Scholar]
- 14.Liversidge J, Forrester JV. Regulation of immune responses by the retinal pigment epithelium. In: Marmor MF, Wolfensburger TJ, editors. The retinal pigment epithelium: function and disease. New York: Oxford University Press; 1998. pp. 511–27. [Google Scholar]
- 15.Rao NA. Inflammations and infections of the retinal pigment epithelium. In: Marmor MF, Wolfensburger TJ, editors. The retinal pigment epithelium: function and disease. New York: Oxford University Press; 1998. pp. 528–41. [Google Scholar]
- 16.Nagineni CN, Detrick B, Hooks JJ. Synergistic effects of gamma interferon on inflammatory mediators that induce interleukin-6 gene expression and secretion by human retinal pigment epithelial cells. Clin Diagn Laboratory Immunol. 1994;1:569–77. doi: 10.1128/cdli.1.5.569-577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagineni CN, Kutty RK, Detrick B, Hooks JJ. Inflammatory cytokines induce intercellular adhesion molecule-1 (ICAM-1) mRNA synthesis and protein secretion by human retinal pigment epithelial cell cultures. Cytokine. 1996;8:622–30. doi: 10.1006/cyto.1996.0083. 10.1006/cyto.1996.0083. [DOI] [PubMed] [Google Scholar]
- 18.Elner VM, Strieter RM, Elner SG, Baggiolini M, Lindle I, Kunkel SL. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990;136:745–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Kutty RK, Kutty G, Hooks JJ, Wiggert B, Nagineni CN. Transforming growth factor-β inhibits the cytokine-mediated expression of the inducible nitric oxide synthase mRNA in human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1995;215:386–93. doi: 10.1006/bbrc.1995.2477. [DOI] [PubMed] [Google Scholar]
- 20.Friedman AH. Uveitis affecting the retina and posterior segment. In: Freeman WR, editor. Practical atlas of the retinal diseases and therapy. New York: Raven Press; pp. 37–70. [Google Scholar]
- 21.Rao NA, Font RL. Toxoplasmic retinochoroiditis. Electron- microscopic and immunofluorescence studies of formalin-fixed tissue. Arch Ophtalmol. 1977;95:273–7. doi: 10.1001/archopht.1977.04450020074012. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson DH, Wolchok EB. Ocular toxoplasmosis in an adult receiving long-term corticosteroid therapy. Arch Ophthalmol. 1976;94:248–54. doi: 10.1001/archopht.1976.03910030120009. [DOI] [PubMed] [Google Scholar]
- 23.Nagineni CN, Pardhasaradhi K, Martins MC, Detrick B, Hooks JJ. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect Immun. 1996;64:4188–96. doi: 10.1128/iai.64.10.4188-4196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagineni CN, Detrick B, Hooks JJ. Toxoplasma gondii infection induces gene expression and secretion of interleukin 1 (IL-1), IL-6, granulocyte-macrophage colony stimulating factor, and intracellular adhesion molecule 1 by human retinal pigment epithelial cells. Infect Immun. 2000;68:407–10. doi: 10.1128/iai.68.1.407-410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander J, Hunter CA. Immunoregulation during toxoplasmosis. Chem Immunol. 1998;70:81–102. doi: 10.1159/000058701. [DOI] [PubMed] [Google Scholar]
- 26.Lyons RE, Anthony JP, Ferguson DJP, Byrne N, Alexander J, Roberts F, Roberts CW. Immunological studies of chronic ocular toxoplasmosis: up-regulation of major histocompatibility complx class I and transforming growth factor B and a protective role for interleukin-6. Infect Immun. 2001;69:2589–95. doi: 10.1128/IAI.69.4.2589-2595.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesbron-Delaw MF. Dense-granule organelles of Toxoplasma gondii: their role in the host–parasite relationship. Parasitol Today. 1994;10:293–6. doi: 10.1016/0169-4758(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 28.Nichols BA, Chiappino ML, O’Connor GR. Secretion from the rhoptries of Toxoplasma gondii during cell division. J Ultrastruct Res. 1983;83:85–98. doi: 10.1016/s0022-5320(83)90067-9. [DOI] [PubMed] [Google Scholar]
- 29.Silva JS, Twardzik DR, Reed SG. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor (TGF-β) J Exp Med. 1991;174:539–45. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barral A, Barrol-Netto M, Yong EC, Brownell CE, Twardzik DR, Reed SG. Transforming growth factor as a virulance mechanism for Leishmania braziliensis. Proc Natl Acad Sci USA. 1993;90:3442–6. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barral-Netto M, Barral A, Brownwell CE et al. Transforming growth factor-β in Leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–8. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 32.Gazzinelli RT, Brezin A, Li Q, Nussenblatt RB, Chan CC. Toxoplasma gondi. Aquired ocular toxoplasmosis in the murine model, protective role of TNF-α and IFN-γ. Exp Parasitol. 1994;78:217–29. doi: 10.1006/expr.1994.1022. 10.1006/expr.1994.1022. [DOI] [PubMed] [Google Scholar]