Abstract
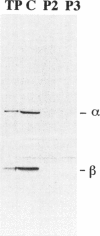
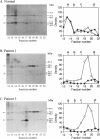
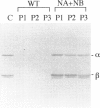
Mitochondrial trifunctional protein (TP) is an enzyme complex with three activities: enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase. Studies on defects in this enzyme in patients with TP deficiency suggest that there are two types of defect. Patients in group 1 have normal amount of cross-reacting material by immunoblot and lack only long-chain 3-hydroxyacyl-CoA dehydrogenase activity. Patients in group 2 have a trace amount of cross-reacting material, with all three activities being low. We identified three patients in group 2, and analysis was made at the cDNA level. In patient 2, there was a heterozygous 71-bp deletion at position 110-180 in the alpha-subunit. In patients 1 and 3, there was an abnormal beta-subunit; patient 1 had an A-788-to-G substitution, and patient 3 had G-182-to-A and G-740-to-A substitutions in each of separate alleles. This is the first demonstration of disease-causing mutations in the beta-subunit. cDNA-expression experiments in patients' fibroblasts, using a vaccinia virus system, and gel filtration analysis, using patients' fibroblasts, revealed that the existence of both normal alpha- and beta-subunits, and possibly their association, are important for stabilizing TP and that A-788-to-G substitution on the beta-subunit in patient 1 seems to interfere with the association, the result being a rapid decomposition of TP.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama T., Nagata K., Yamazoe Y., Kato R., Matsunaga E., Gelboin H. V., Gonzalez F. J. Cytochrome b5 potentiation of cytochrome P-450 catalytic activity demonstrated by a vaccinia virus-mediated in situ reconstitution system. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5425–5429. doi: 10.1073/pnas.87.14.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T., Nakajima-Nasu T., Souri M., Hashimoto T. A quantitative method for cDNA-directed expression in cultured mammalian cells. Anal Biochem. 1995 May 1;227(1):85–89. doi: 10.1006/abio.1995.1256. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Souri M., Kamijo T., Ushikubo S., Hashimoto T. Peroxisomal acyl-coenzyme A oxidase is a rate-limiting enzyme in a very-long-chain fatty acid beta-oxidation system. Biochem Biophys Res Commun. 1994 Jun 30;201(3):1541–1547. doi: 10.1006/bbrc.1994.1879. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Souri M., Ueno I., Kamijo T., Yamaguchi S., Rhead W. J., Tanaka K., Hashimoto T. Cloning of human very-long-chain acyl-coenzyme A dehydrogenase and molecular characterization of its deficiency in two patients. Am J Hum Genet. 1995 Aug;57(2):273–283. [PMC free article] [PubMed] [Google Scholar]
- Aoyama T., Souri M., Ushikubo S., Kamijo T., Yamaguchi S., Kelley R. I., Rhead W. J., Uetake K., Tanaka K., Hashimoto T. Purification of human very-long-chain acyl-coenzyme A dehydrogenase and characterization of its deficiency in seven patients. J Clin Invest. 1995 Jun;95(6):2465–2473. doi: 10.1172/JCI117947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T., Tsushima K., Souri M., Kamijo T., Suzuki Y., Shimozawa N., Orii T., Hashimoto T. Molecular cloning and functional expression of a human peroxisomal acyl-coenzyme A oxidase. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1113–1118. doi: 10.1006/bbrc.1994.1158. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Ueno I., Kamijo T., Hashimoto T. Rat very-long-chain acyl-CoA dehydrogenase, a novel mitochondrial acyl-CoA dehydrogenase gene product, is a rate-limiting enzyme in long-chain fatty acid beta-oxidation system. cDNA and deduced amino acid sequence and distinct specificities of the cDNA-expressed protein. J Biol Chem. 1994 Jul 22;269(29):19088–19094. [PubMed] [Google Scholar]
- Aoyama T., Yamano S., Waxman D. J., Lapenson D. P., Meyer U. A., Fischer V., Tyndale R., Inaba T., Kalow W., Gelboin H. V. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem. 1989 Jun 25;264(18):10388–10395. [PubMed] [Google Scholar]
- Brackett J. C., Sims H. F., Rinaldo P., Shapiro S., Powell C. K., Bennett M. J., Strauss A. W. Two alpha subunit donor splice site mutations cause human trifunctional protein deficiency. J Clin Invest. 1995 May;95(5):2076–2082. doi: 10.1172/JCI117894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter K., Middleton B., Pollitt R. J. The long-chain 3-hydroxyacyl-CoA dehydrogenase of human liver mitochondria. J Inherit Metab Dis. 1991;14(3):321–324. doi: 10.1007/BF01811693. [DOI] [PubMed] [Google Scholar]
- Carpenter K., Pollitt R. J., Middleton B. Human liver long-chain 3-hydroxyacyl-coenzyme A dehydrogenase is a multifunctional membrane-bound beta-oxidation enzyme of mitochondria. Biochem Biophys Res Commun. 1992 Mar 16;183(2):443–448. doi: 10.1016/0006-291x(92)90501-b. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Furuta S., Miyazawa S., Osumi T., Hashimoto T., Ui N. Properties of mitochondria and peroxisomal enoyl-CoA hydratases from rat liver. J Biochem. 1980 Oct;88(4):1059–1070. doi: 10.1093/oxfordjournals.jbchem.a133057. [DOI] [PubMed] [Google Scholar]
- Harger C., Skupski M., Allen E., Clark C., Crowley D., Dickinson E., Easley D., Espinosa-Lujan A., Farmer A., Fields C. The Genome Sequence DataBase version 1.0 (GSDB): from low pass sequences to complete genomes. Nucleic Acids Res. 1997 Jan 1;25(1):18–23. doi: 10.1093/nar/25.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJlst L., Wanders R. J., Ushikubo S., Kamijo T., Hashimoto T. Molecular basis of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: identification of the major disease-causing mutation in the alpha-subunit of the mitochondrial trifunctional protein. Biochim Biophys Acta. 1994 Dec 8;1215(3):347–350. doi: 10.1016/0005-2760(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Ijlst L., Uskikubo S., Kamijo T., Hashimoto T., Ruiter J. P., de Klerk J. B., Wanders R. J. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: high frequency of the G1528C mutation with no apparent correlation with the clinical phenotype. J Inherit Metab Dis. 1995;18(2):241–244. doi: 10.1007/BF00711778. [DOI] [PubMed] [Google Scholar]
- Imamura S., Ueda S., Mizugaki M., Kawaguchi A. Purification of the multienzyme complex for fatty acid oxidation from Pseudomonas fragi and reconstitution of the fatty acid oxidation system. J Biochem. 1990 Feb;107(2):184–189. doi: 10.1093/oxfordjournals.jbchem.a123023. [DOI] [PubMed] [Google Scholar]
- Izai K., Uchida Y., Orii T., Yamamoto S., Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. I. Purification and properties of very-long-chain acyl-coenzyme A dehydrogenase. J Biol Chem. 1992 Jan 15;267(2):1027–1033. [PubMed] [Google Scholar]
- Jackson S., Bartlett K., Land J., Moxon E. R., Pollitt R. J., Leonard J. V., Turnbull D. M. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Pediatr Res. 1991 Apr;29(4 Pt 1):406–411. doi: 10.1203/00006450-199104000-00016. [DOI] [PubMed] [Google Scholar]
- Jackson S., Kler R. S., Bartlett K., Briggs H., Bindoff L. A., Pourfarzam M., Gardner-Medwin D., Turnbull D. M. Combined enzyme defect of mitochondrial fatty acid oxidation. J Clin Invest. 1992 Oct;90(4):1219–1225. doi: 10.1172/JCI115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S., Schaefer J., Middleton B., Turnbull D. M. Characterisation of a novel enzyme of human fatty acid beta-oxidation: a matrix-associated, mitochondrial 2-enoyl-CoA hydratase. Biochem Biophys Res Commun. 1995 Sep 5;214(1):247–253. doi: 10.1006/bbrc.1995.2281. [DOI] [PubMed] [Google Scholar]
- Kamijo T., Aoyama T., Komiyama A., Hashimoto T. Structural analysis of cDNAs for subunits of human mitochondrial fatty acid beta-oxidation trifunctional protein. Biochem Biophys Res Commun. 1994 Mar 15;199(2):818–825. doi: 10.1006/bbrc.1994.1302. [DOI] [PubMed] [Google Scholar]
- Kamijo T., Aoyama T., Miyazaki J., Hashimoto T. Molecular cloning of the cDNAs for the subunits of rat mitochondrial fatty acid beta-oxidation multienzyme complex. Structural and functional relationships to other mitochondrial and peroxisomal beta-oxidation enzymes. J Biol Chem. 1993 Dec 15;268(35):26452–26460. [PubMed] [Google Scholar]
- Kamijo T., Wanders R. J., Saudubray J. M., Aoyama T., Komiyama A., Hashimoto T. Mitochondrial trifunctional protein deficiency. Catalytic heterogeneity of the mutant enzyme in two patients. J Clin Invest. 1994 Apr;93(4):1740–1747. doi: 10.1172/JCI117158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYNEN F., HENNING U., BUBLITZ C., SORBO B., KROPLIN-RUEFF L. Der chemische Mechanismus der Acetessigsäurebildung in der Leber. Biochem Z. 1958;330(4):269–295. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mantamadiotis T., Sobieszczuk P., Weinstock J., Baldwin G. S. Nucleotide sequence encoding a novel member of the hydratase/dehydrogenase family. Biochim Biophys Acta. 1993 Oct 13;1170(2):211–215. doi: 10.1016/0005-2760(93)90073-i. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T. The presence of a new 3-oxoacyl-CoA thiolase in rat liver peroxisomes. Eur J Biochem. 1980 Feb;103(3):589–596. doi: 10.1111/j.1432-1033.1980.tb05984.x. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Occurrence of two 3-hydroxyacyl-CoA dehydrogenases in rat liver. Biochim Biophys Acta. 1979 Aug 30;574(2):258–267. [PubMed] [Google Scholar]
- Saijo T., Welch W. J., Tanaka K. Intramitochondrial folding and assembly of medium-chain acyl-CoA dehydrogenase (MCAD). Demonstration of impaired transfer of K304E-variant MCAD from its complex with hsp60 to the native tetramer. J Biol Chem. 1994 Feb 11;269(6):4401–4408. [PubMed] [Google Scholar]
- Sims H. F., Brackett J. C., Powell C. K., Treem W. R., Hale D. E., Bennett M. J., Gibson B., Shapiro S., Strauss A. W. The molecular basis of pediatric long chain 3-hydroxyacyl-CoA dehydrogenase deficiency associated with maternal acute fatty liver of pregnancy. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):841–845. doi: 10.1073/pnas.92.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman H. M., Hill R. L. Bovine liver crotonase (enoyl coenzyme A hydratase). EC 4.2.1.17 L-3-hydroxyacyl-CoA hydrolyase. Methods Enzymol. 1975;35:136–151. doi: 10.1016/0076-6879(75)35149-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y., Izai K., Orii T., Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. J Biol Chem. 1992 Jan 15;267(2):1034–1041. [PubMed] [Google Scholar]
- Wanders R. J., Duran M., Ijlst L., de Jager J. P., van Gennip A. H., Jakobs C., Dorland L., van Sprang F. J. Sudden infant death and long-chain 3-hydroxyacyl-CoA dehydrogenase. Lancet. 1989 Jul 1;2(8653):52–53. doi: 10.1016/s0140-6736(89)90300-0. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., IJlst L., Poggi F., Bonnefont J. P., Munnich A., Brivet M., Rabier D., Saudubray J. M. Human trifunctional protein deficiency: a new disorder of mitochondrial fatty acid beta-oxidation. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1139–1145. doi: 10.1016/0006-291x(92)91350-y. [DOI] [PubMed] [Google Scholar]
- Zhang Q. X., Baldwin G. S. Structures of the human cDNA and gene encoding the 78 kDa gastrin-binding protein and of a related pseudogene. Biochim Biophys Acta. 1994 Oct 18;1219(2):567–575. doi: 10.1016/0167-4781(94)90091-4. [DOI] [PubMed] [Google Scholar]