Abstract
Peripheral administration of interleukin-1 (IL-1) is known to activate the hypothalamo–pituitary–adrenal axis (HPA axis) and brain noradrenergic systems. We studied the relationship between these responses using in vivo microdialysis to assess the release of hypothalamic norepinephrine (NE), while simultaneously sampling blood for ACTH and corticosterone, and monitoring body temperature and behavior in freely moving rats. Rats were implanted with microdialysis probes in the medial hypothalamus, with intravenous catheters, and with telethermometers in the abdomen. Each rat was injected with saline and IL-1β (1 μg ip) in random order, monitoring microdialysate NE, body temperature and plasma ACTH and corticosterone for 2–4 h after injection. Saline injections were followed by transient increases in microdialysate NE and in plasma ACTH and corticosterone. IL-1β injections resulted in prolonged elevations of microdialysate NE, as well as plasma ACTH and corticosterone, and body temperature. IL-1β also induced shivering and a prolonged depression of locomotor activity. Pretreatment with indomethacin (10 mg/kg sc) prevented the IL-1β-induced increases in body temperature and the apparent increase in hypothalamic NE release, but only attenuated the IL-1β-induced shivering and the increase in plasma ACTH. The results indicate a close temporal relationship between the release of NE and HPA axis activation. Such a relationship is also supported by the similar effects of indomethacin pretreatment on NE and ACTH. The shivering is likely involved in the increase in body temperature, but indomethacin only attenuated the shivering while it blocked the fever. However, the effects of indomethacin clearly indicate that neither the increase in body temperature nor the increase in hypothalamic NE release was essential for HPA axis activation. These results suggest that hypothalamic NE is involved in the IL-1-induced HPA axis activation, but that this is not the only mechanism by which the HPA axis is activated by intraperitoneally injected IL-1.
Keywords: Interleukin-1, HPA axis, Norepinephrine, Behavior, ACTH, Temperature, Corticosterone, Microdialysis
1. Introduction
Peripheral administration of interleukin-1 (IL-1) to rodents has been shown to induce fever (Dinarello, 1984), to activate the hypothalamo–pituitary–adrenal (HPA) axis (Besedovsky et al., 1986), to induce certain behavioral changes (Dantzer et al., 2001; Larson and Dunn, 2001; McCarthy et al., 1985), and to affect noradrenergic and serotonergic neurotransmission in the brain (Dunn, 1988; Kabiersch et al., 1988). The mechanisms involved in these various responses have been studied extensively, nevertheless, the relationships among them remain unclear. For example, it is possible that the changes in body temperature underlie the changes in noradrenergic neuronal activity, the activation of the HPA axis, and/or the behavior. Alternatively, it is possible that the changes in the activity of noradrenergic neurons underlie the fever, the HPA axis activation, and/or the behavioral changes.
We sought to determine the relationships among and between these various responses to IL-1 by studying them simultaneously. We reasoned that a critical factor was the temporal relationships among the various responses, and that by using interventions known to impair the responses we could obtain important data on their interrelationships. Therefore, we studied six of these responses simultaneously in awake rats following intraperitoneal (ip) administration of rat IL-1β. We monitored changes in body temperature using telethermometers implanted in the abdomen, used in vivo microdialysis to assess the release of norepinephrine (NE) from the medial hypothalamus, while sampling blood for ACTH and corticosterone, and scoring behavior in awake unrestrained rats.
Because cyclo-oxygenase (COX) inhibitors have been shown to prevent the IL-1-induced fever (Blatteis and Sehic, 1997, 1998), and to impair the HPA axis responses (Dunn and Chuluyan, 1992; Katsuura et al., 1988; Krymskaya et al., 1987; Rivier and Vale, 1991), we assessed the involvement of COX in these various responses by pretreating the rats with the non-selective COX inhibitor, indomethacin.
2. Materials and methods
2.1. Experimental animals
The experiments were performed using male Sprague–Dawley rats, weighing 250–300 g obtained from Harlan Sprague–Dawley, Houston, TX. The animals were housed individually, under controlled environmental conditions of temperature (22±2 °C), humidity (55±5%) and on a 12:12 light cycle (lights on at 07:00 AM). Water and Purina rat chow were available ad libitum.
2.2. Materials
Rat interleukin-1β was purchased from R&D Systems (Minneapolis, MN). All other chemicals were of analytical grade from Sigma Chemical (St. Louis, MO).
2.3. Microdialysis probes
Concentric microdialysis probes were used. The inner fused silica tube (outer diameter 150 μm: Polymicrotechnologies, Phoenix, AZ) was inserted through a polyethylene tube (Clay Adams PE-50) and then inserted into a stainless steel injector (G-312 I: Plastics One, Roanoke, VA), the upper end of which was slipped into the PE-50 tubing in such way, that the fused silica passed through it. The dialysis membrane 3 mm long and 250 μm diameter (Cuprophane Pore Fiber, molecular weight cutoff 5–6000) was attached to the end of the stainless steel tube and sealed at its tip with epoxy cement (Locktite Quickset gel). The net active length of the dialysis membrane was approximately 2 mm.
2.4. Surgical procedure
Animals were anesthetized using Innovar Plus, (3 mg fentanyl, 210 mg droperidol, and 150 mg midazolam dissolved in 174 ml of water) at a dose of 6 μl/g body weight ip. The rat was placed on its back, a short incision (1.5 cm long) was made in the abdomen, and a telethermometer was placed into the abdominal cavity. The abdomen muscles and skin were then sutured. Next, a jugular vein catheter was implanted. A short (1.5 cm) incision was made in the neck, the jugular vein was carefully exposed and ligated with surgical silk as distally as possible to prevent bleeding. A small incision was made in the vein and a catheter (4 cm long) filled with heparinized saline (80 IU in 1 ml) was inserted into the vein. A small amount of blood was then withdrawn to check the position of catheter and the catheter was held in place with surgical silk. The other end of the catheter (10 cm long, equipped with a pedestal; Plastic One, Roanoke, VA) was passed beneath the skin and Fixed to the skull with dental cement. The skin was sutured and topical antibiotic (Neosporin) applied.
The rats were then placed in a Kopf stereotaxic apparatus, and guide cannulae for microdialysis probes (C312G, 5.8 mm long: Plastics One) were implanted bilaterally and fixed to the skull using Cranioplastic cement (Plastics One). The coordinates for the microdialysis guide cannulae in the medial hypothalamus were: A–P: −2.0mm; L: ±2.2mm; V: −4.8mm, tilted medially at an angle 15.8° to prevent damage to the superior sagittal sinus. To enable the mounting of the microdialysis probes, stainless steel miniature selftapping bone screws (Fine Scientific Tools, Foster City, CA) were also inserted in the skull and Fixed with Cranioplastic cement. These procedures were approved by the Louisiana State University Health Sciences Center Animal Care and Use Committee and conform to National Institutes of Health guidelines.
2.5. Microdialysis system
The inflow to the microdialysis probe was driven by a CMA/100 Microinjection Pump. The perfusion fluid was artificial cerebrospinal fluid (aCSF) made according to Sharp et al. (1989): 1.2mM CaCl2, 1.2mM Na2HPO4, 0.3mM NaH2PO4, 3.4mM KCl, 140mM NaCl, pH 7.2. Dialysate samples were collected in 20-min periods at a flow rate of 1.2 μl/min directly into 0.5ml polypropylene vials containing 50 pg of N-methyldopamine (NMDA) as an internal standard in 5 μl of 0.15M HClO4–0.15mM ethylenediaminetetraacetic acid (EDTA). The samples were frozen immediately after collection and stored on dry ice in a −70 °C freezer until analyzed.
2.6. HPLC
HPLC with coulometric detection was used to determine the content of NE and NMDA with reference to freshly diluted standards (Sigma Chemical). The system consisted of a chromatographic syringe pump (ISCO Model 100DM), an ESA Coulochem III detector (ESA, Chelmsford, MA), and a manual injector (Rheodyne 9126). Separation was performed on a Keystone microbore analytical 125×1mm column C-18, particle size 5 μm (Thermo Hypersil). The mobile phase contained 50mM sodium acetate, 0.5mM EDTA, and 2.05mM 1-decanesulfonic acid sodium salt and 12% v/v acetonitrile. The mobile phase was adjusted to pH 6.0 with acetic acid, filtered under vacuum, through a 0.22μm Nylon membrane filter 47 mm diameter filter (Sigma) and degassed with helium. The column was pumped at a flow rate of 0.1ml/min at ambient temperature (22–24 °C).
Detection conditions were as follows: guard cell (ESA 5020)=+350 mV, microdialysis cell (ESA 5014B) E1=− 150 mV, E2=+250 mV, and a recorder range of 50 nA. The detector output was captured and analyzed using Waters Millenium32, version 3.20. The concentrations of NE in each sample were calculated from the integrated chromatographic peak height and expressed as percentage of baseline release, the mean of the first four microdialysate samples.
In vitro recovery of the microdialysis probes was determined as described previously (Lavicky and Dunn, 1995). The recoveries were between 12 and 16% for NE depending on the probe. Because the diffusion of materials from within brain tissue is likely to be different from that in a saline solution (Benveniste, 1989), we did not correct the data for these in vitro recoveries.
2.7. Experimental procedure
Data are presented from a total of 36 runs that yielded a full set of results (i.e., temperature, behavior, NE, ACTH, and corticosterone). Most of the rats were used in two separate runs, with the saline/IL-1 treatment order reversed. Each animal was familiarized with the microdialysis chamber (15 in. animal enclosure, Instech Laboratories, Plymouth Meeting, PA) and conditions, during 4 consecutive days for 1 h each day. The evening before the experiment (7:00–7:30PM) the microdialysis probes were connected to the microdialysis pump and inserted into the rats’ brains using the guide cannulae with the flow rate set at 0.5 μl/min. On the next day (ca. 8:30 AM) the flow rate was increased to 1.2 μl/min and left to stabilize for 1 h, after which microdialysate and blood samples were collected every 20min. Body temperature was measured at the same time as the microdialysate collection tube was changed and blood was drawn. The first four samples were collected to determine the baseline. Then the rats were injected subcutaneously (sc) with either saline or a suspension of indomethacin in saline at a dose of 10mg/kg. Such administrations of indomethacin have been shown to result in plasma concentrations adequate to substantially inhibit COX for up to 16 h. Twenty minutes later, the rat was injected ip with either saline (100 μl) or IL-1β (1 μg). Microdialysate and blood samples (0.6ml) were collected and body temperature recorded every 20min for the next 2 h (saline), or 4 h (IL-1β). Then, each rat was given a second ip injection; saline if IL-1β had been given the first time, or IL-1β if the first injection had been saline, so that each rat received both saline and IL-1β in the same session. Blood samples were immediately centrifuged for 10min at 4000 rpm at 4 °C. The blood cells were then resuspended in 4% bovine serum albumin dissolved in saline and returned to the same donor rat every hour through the jugular vein catheter. In most cases, the rat was tested again one week later with the order of the saline and IL-1β injections reversed. Whether the rat received saline or indomethacin was decided on a random basis. Because the insertion of microdialysis probes causes tissue damage and consequent gliosis that can impair the collection of solutes from the extracellular fluid, rats received a new microdialysis probe inserted in the contra-lateral medial hypothalamus for the second run.
The behavior of the animal was observed throughout each period following the injections. When the animal moved all four paws from one place to another in the microdialysis chamber, this was counted as one locomotor episode. Each individual shiver was recorded as an episode.
2.8. Statistical analysis
The statistical analysis was approached in two ways: 1. A repeated measures ANOVA was performed on all the data, using indomethacin, IL-1β, and time as main factors; 2. the data obtained in the 2 h following IL-1β and saline treatments were pooled separately for both treatment orders, and a repeated measures ANOVA performed, again using indomethacin, IL-1β, and time as main factors. In most cases (the exceptions being locomotor activity and corticosterone), both analyses indicated significant interactions between indomethacin and time, IL-1β and time, and indomethacin and IL-1β. Thus, the data were analyzed using Fisher’s LSD-test to determine whether or not there were significant effects of IL-1β (vs saline) in saline-pretreated rats, and indomethacin (vs saline) in IL-1β-treated rats for each 20-min time period after IL-1β or saline injections. Pearson’s correlational coeffcients were calculated to examine relationships among the various measured responses to IL-1β.
3. Results
3.1. Effect of IL-1β on locomotor activity and shivering
Locomotor activity increased transiently in the first 20 min time period after saline or IL-1β (1 μg ip) administration (Fig. 1). Subsequently, IL-1β administration decreased locomotor activity for about 4 h after injection. There was a tendency for indomethacin pretreatment to prevent the IL-1-induced reduction in locomotor activity. Repeated measures ANOVA showed that there were statistically significant effects of IL-1β (F(1,68)=15.9, p<.001) and a marginally significant effect of indomethacin pretreatment (F(1,68)=3.09, p<.08), but not for the indomethacin×IL-1β interaction (F(1,68)=0.053). However, there were statistically significant differences for time (F(11,748)=23.9, p<0.001), the interaction between time and indomethacin (F(11,748)=2.47, p<.004), and time and IL-1β (F(11, 748)=7.03, p<.001).
Fig. 1.

Locomotor activity of rats after saline and IL-1β treatments, with and without indomethacin pretreatment. Rats were placed in the microdialysis chamber overnight with microdialysis probes and intravenous catheters inserted. The next morning, locomotor activity was scored manually as the number of episodes observed in each 20 min period. After 60 min, rats were injected (arrows) with either saline (open bars) or indomethacin (10 mg/kg sc: solid bars), and 20 min later with rIL-1β (1 μg/rat) followed 4 h later by saline (upper graph), or saline followed 2 h later by rIL-1β (lower graph). IL-1β significantly depressed locomotor activity and there were no statistically significant effects of the indomethacin pretreatment (see text). N = 9 rats per group.
When the data obtained in the 2h following saline or IL-1β were analyzed together regardless of the order of administration, ANOVA indicated significant differences for IL-1β (F(1,68)=5.55, p<.02), and marginally significant differences of the indomethacin pretreatment (F(1,68)=2.92, p<.09), but not for the indomethacin×IL-1β interaction (F(1,68)=0.02). However, there were significant differences for time (F(5,340)=39.0, p<.001), the interaction between indomethacin and time (F(5,340)=3.84, p<.002), and IL-1β and time (F(5,340)=3.74, p<002). Post hoc Fisher’s tests indicated statistically significant differences from saline in each time period after IL-1β, starting 40 min after injection until the end of the experiment (p<.001). There was also a significant difference between saline- and indomethacin-pretreated rats at 80 min after IL-1β injection (p<.05).
IL-1β administration also induced shivering, with a maximal response in the first hour, lasting almost to the end of experiment (Fig. 2). Shivering also occurred in the groups pretreated with indomethacin, but less frequently than in the saline-treated groups. Repeated measures ANOVA indicated statistically significant effects of indomethacin (F(1,68)=54.9, p<.001), IL-1β (F(1,68)=101, p<.001), time (F(11,748)=40.5, p<.001), and the interactions, indomethacin×IL-1β (F(1,68)=42.1, p<.001), indomethacin×time (F(11,748)=10.9, p<.001), and IL-1β×time (F(11,748)=40.3, p<.001).
Fig. 2.

Shivering episodes of rats after saline and IL-1β treatments, with and without indomethacin pretreatment. Results are from the same rats from which the data in Fig. 1 were obtained. Shivering bouts were scored manually as the number of episodes observed in each 20 min period. Shivering was observed only after IL-1β treatment, and was significantly attenuated by indomethacin pretreatment (Filled symbols, see text). N =9 rats per group.
When the data obtained in the 2 h after saline or IL-1β were analyzed, ANOVA indicated statistically significant differences for the indomethacin treatment (F(1,68)=66.1, p<.001), IL-1β (F(1,68)=149, p<.001), time (F(5,340)=19.3, p<.01), and the interactions between indomethacin and IL-1β (F(1,68)=56.2, p<.001), indomethacin and time (F(5,340)=2,89, p<.01), and IL-1β and time (F(5,340)=19.09, p<.001). Post hoc Fisher’s tests indicated significant effects of IL-1β compared to saline in saline-pretreated animals starting from 60 min after IL-1β until the end of the experiment (p<.001). They also indicated differences between indomethacin and saline pretreatments from 20 to 60 min and from 80 to 100 min after IL-1β (p<.01).
3.2. Effect of IL-1β on body temperature
IL-1β increased body temperature compared to saline lasting for about 4 h after injection (Fig. 3). Maximum temperature differences (ca. 0.6–0.7 °C) appeared around 1.5–2 h after IL-1β administration. Pretreatment with indomethacin completely blocked the increase in body temperature. Repeated measures ANOVA indicated statistically significant effects of indomethacin (F(1,68)=42.2, p<.001), time (F(11,748)=31.1, p<.001) and the interactions: indomethacin ×time (F(11,748)=10.7, p<.001), IL-1β × time (F(11,748)=13.4, p<.001), and there was a marginally significant interaction between indomethacin and IL-1β (F(1,68)=3.10, p<.08), but not for IL-1β (F(1,68)=0.10).
Fig. 3.

Core body temperature of rats after saline and IL-1β treatments, with and without indomethacin pretreatment. Results were obtained from the same rats from which the data in Figs. 1 and 2 were obtained. Rats were injected with either saline (open symbols) or indomethacin (10 mg/kg sc: Filled symbols), and 20 min later with rIL-1β (1 μg/rat) followed 4 h later by saline (upper graph), or saline followed 2 h later by rIL-1β (lower graph). The core temperature was determined at the end of each 20 min period using Minimitters implanted in the peritoneum of each rat. IL-1β treatment induced small increases in body temperature lasting around 4 h, whereas saline injections induced only a short-lived increase in body temperature. Indomethacin pretreatment precipitated a decline in core temperature obscuring the IL-1β-induced increases. N= 9 rats per group.
When the data obtained in the 2 h after saline or IL-1β were analyzed, ANOVA indicated statistically significant differences for indomethacin (F(1,68)=28.2, p<.001), time (F(5,340)=18.4, p<.001), but only a marginally significant difference for IL-1β (F(1,68)=3.3, p<.07). However, we observed significant interactions between indomethacin and time (F(5,340)=8.20, p<.001) and IL-1β×time (F(5,340)=4.10, p<.001), but not between indomethacin and IL-1β (F(1,68)=0.30).
Subsequent post hoc Fisher’s tests indicated differences between saline- and IL-1β-treated animals in each 20 min time period from 60 min after IL-1β until the end of the experiment (p<.05). Significant differences also occurred for indomethacin pretreatment in the time periods from 60 min after IL-1β until the end of the experiment (p<.05).
3.3. Effect of IL-1β on microdialysate NE from the medial hypothalamus
Saline injections induced a brief small increase in dialysate NE from the medial hypothalamus (maximum 36%, Fig. 4). However, ip IL-1β injection induced a substantial and prolonged increase, lasting for more than 4 h. The peak (maximum 189%) appeared 1.5–2 h after IL-1β administration. Pretreatment with indomethacin completely prevented this increase in dialysate NE, whereas indomethacin pretreatment had only a small effect on the response to saline injection. Repeated measure ANOVA indicated significant effects of indomethacin (F(1,66)=46.7, p<.001), IL-1β (F(1,66)=11.9, p<.001), time (F(11,726)=6.68, p<.001), as well as the interactions between indomethacin and IL-1β (F(1,66)=7.55, p<.01), between IL-1β and time (F(11,726)=3.18, p<.001), and between indomethacin and time (F(11,726)=2.22, p<.01).
Fig. 4.

Microdialysate NE from the medial hypothalamus of rats after saline or IL-1β treatment, with and without indomethacin pretreatment. Rats were implanted with microdialysis probes in the medial hypothalamus. Results were obtained from the same rats from which the data in Figs. 1–3 were obtained. Rats were injected with either saline (open bars) or indomethacin (10 mg/kg sc: solid bars), and 20 min later with rIL-1β (1 μg/rat) followed 4 h later by saline (upper graph), or saline followed 2 h later by rIL-1β (lower graph). The NE was determined on the dialysates obtained in each 20 min period. IL-1β treatment markedly increased microdialysate NE for around 4 h, whereas saline injections induced only transient increases. Indomethacin pretreatment more or less prevented the IL-1β-induced increases in dialysate NE regardless of the order of the saline and IL-1β treatments (see text). N =9 rats per group.
When the data obtained in the 2h following saline or IL-1β were analyzed, ANOVA indicated that there were significant differences for IL-1β (F(1,66)=19.8, p<.001), indomethacin (F(1,66)=50.9, p<.001), time (F(5,330)=3.53, p<.004), and the interactions between indomethacin and IL-1β (F(1,66)=10.9, p<.002), IL-1β and time (F(5,330)=2.53, p<.03), but not for indomethacin and time (F(5,330)=1.46).
Post hoc Fisher’s tests indicated significant effects of IL-1β compared to saline in saline-pretreated animals in all samples after IL-1β injection until the end of the experiment (p<.001). The tests also indicated differences between indomethacin and saline pretreatments from 20 to 60 min and from 80 to 100 min after IL-1β (p<.01).
3.4. Effect of IL-1β on plasma ACTH and corticosterone
Injection of IL-1β increased plasma concentrations of ACTH and corticosterone, whereas saline injections had very little effect. The increase in plasma ACTH concentrations lasted about 3 h, with a peak around 20 min (Fig. 5). The increase in plasma corticosterone also lasted about 3 h with a maximal response around 1h after IL-1β (Fig. 6). Pretreatment with indomethacin attenuated the increase in plasma ACTH, but only slightly decreased that in corticosterone.
Fig. 5.
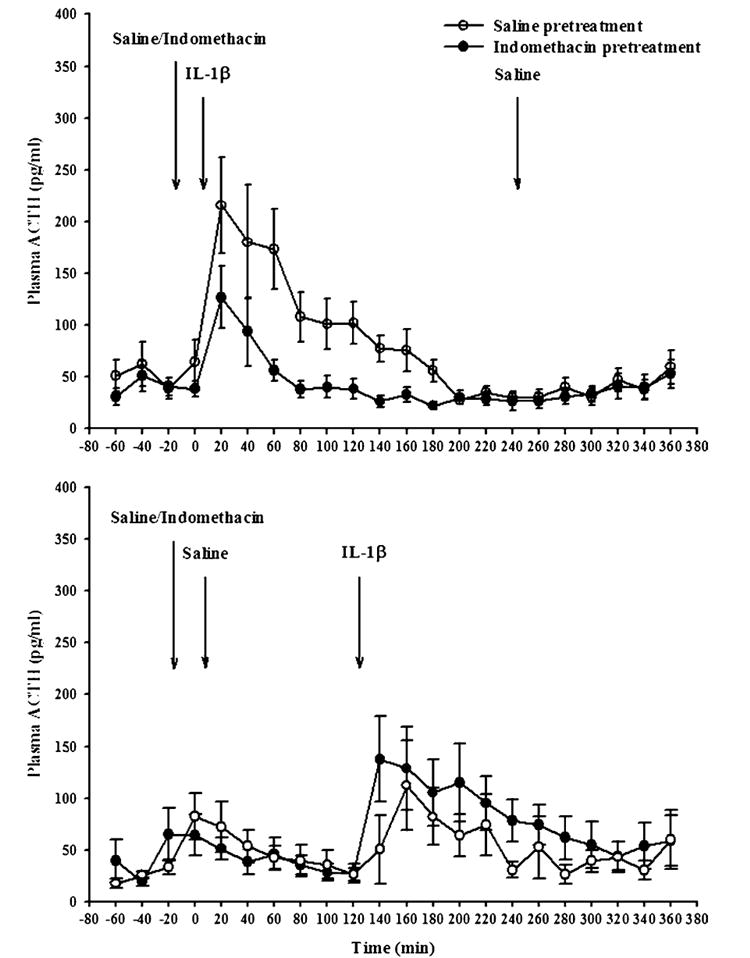
Plasma ACTH concentrations in rats after saline or IL-1β treatment, with and without indomethacin pretreatment. Results were obtained from the same rats from which the data in Figs. 1–4 were obtained. Rats were injected with either saline (open symbols) or indomethacin (10 mg/kg sc: filled symbols), and 20 min later with rIL-1β (1 μg/rat) followed 4 h later by saline (upper graph), or saline followed 2 h later by rIL-1β (lower graph). Plasma was drawn at the end of each 20 min period and ACTH was determined by radioimmunoassay. IL-1β treatment induced small increases in plasma ACTH lasting around 3 h, whereas saline injections induced only short-lived increases. Indomethacin pretreatment substantially attenuated the IL-1β-induced increases in plasma ACTH, but had little effect on the small saline-induced increases (see text). N = 9 rats per group.
Fig. 6.
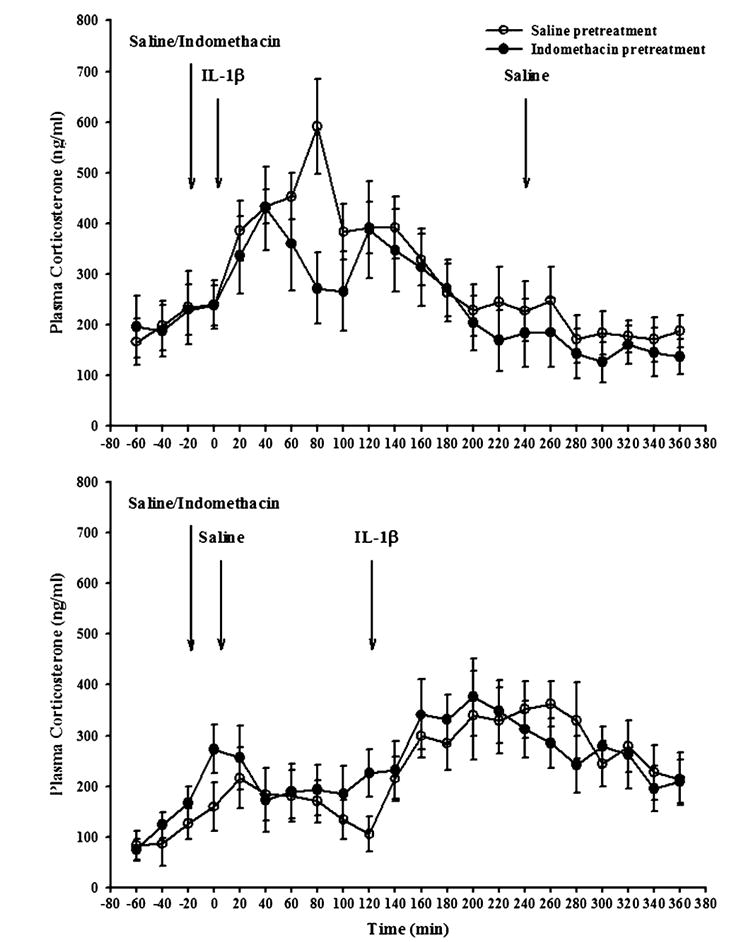
Plasma corticosterone concentrations in rats after saline or IL-1β treatment, with and without indomethacin pretreatment. Results were obtained from the same rats from which the data in Figs. 1–5 were obtained. Rats were injected with either saline (open symbols) or indomethacin (10 mg/kg sc: Filled symbols), and 20 min later with rIL-1β (1 μg/rat) followed 4 h later by saline (upper graph), or saline followed 2 h later by rIL-1β (lower graph). Plasma was drawn at the end of each 20 min period and corticosterone determined by radioimmunoassay. IL-1β treatment induced small increases in plasma corticosterone lasting around 4 h, whereas saline injections induced only short-lived increases. Indomethacin pretreatment somewhat attenuated the IL-1β-induced increases in plasma corticosterone, but had little effect on the smaller saline-induced increases. N= 9 rats per group.
Repeated measures ANOVA indicated significant effects on plasma ACTH concentrations for IL-1β (F(1,68)=13.7, p<.001), indomethacin (F(1,68)=34.7, p<.02), time (F(11,748)=10.9, p<.001), and the interactions between indomethacin and IL-1β (F(1,68)=6.71, p<.01), IL-1β and time (F(11,748)=10.02, p<.001), and a marginally significant effect for indomethacin×time (F(11,748)=1.64, p<.08).
When the data obtained in the 2 h after saline or IL-1β were analyzed, ANOVA indicated that there were significant differences in plasma ACTH for IL-1β (F(1,68)=20.2, p<.001), indomethacin (F(1,68)=5.95, p<.02), time (F(5,340)=7.92, p<.001), and the interactions between indomethacin and IL-1β (F(1,68)=6.51, p<.01), IL-1β and time (F(5,340)=6.67, p<.001), but no significant indomethacin×time interaction (F(5,340)=.53). Subsequent post hoc Fisher’s tests indicated differences between saline- and IL-1β-injected animals from 20 min after IL-1 until the end of the experiment (p<.01). They also indicated statistically significant differences of indomethacin in IL-1β-treated rats starting from 20 min after IL-1β until the end of the experiment (p<.01).
ANOVA indicated a significant effect on plasma corticosterone concentrations of IL-1β (F(1,68)=18.4, p<.001), time (F(11,748)=7.16, p<.001), and the interaction between IL-1β×time (F(11,748)=5.94, p<.001), but no significant effect of indomethacin (F(1,68)=0.36) and no significant interactions between indomethacin and IL-1β (F(1,68)=0.32) or indomethacin and time (F(11,748)=0.65).
When the plasma corticosterone data obtained in the 2 h after saline or IL-1β were analyzed, ANOVA indicated significant differences for IL-1β (F(1,68)=27.08, p<.001) and the interaction between IL-1β and time (F(5,340)=4.14, p<.001). However, the effects of indomethacin (F(1,68)=0.35), time (F(5,340)=0.32), and the interactions between indomethacin and IL-1β (F(1,68)=0.32), and indomethacin and time (F(11,340)=0.95) were not statistically significant. Post hoc Fisher’s tests indicated statistically significant effects of IL-1β injection from 40 min until the end of the experiment (p<.05). There were no statistically significant effects of indomethacin, consistent with the lack of main effects, and the interactions.
3.5. Relationships among the various responses to IL-1β
To determine the relationships among the various responses to ip IL-1β injections, we used Pearson’s correlation coefficient (Table 1). When animals were pretreated with saline, we observed significant correlations between locomotor activity, plasma ACTH and shivering episodes. Core temperature also correlated with dialysate NE and plasma corticosterone. In addition, dialysate NE correlated with shivering episodes, plasma ACTH and plasma corticosterone, and there was also a correlation between plasma ACTH, locomotor activity, shivering episodes, and plasma corticosterone. Very similar correlations were noted after indomethacin pretreatment (Table 1).
Table 1.
Pearson’s correlation coefficients for the observed measures after ip injection of IL-1β
| Variable | Locomotor activity | Shivering | Core body temperature | Hypothalamic NE | Plasma ACTH |
|---|---|---|---|---|---|
| A. Saline pretreatment | |||||
| Locomotor activity | |||||
| Shivering | 0.607, p < .002 | ||||
| Core body temperature | |||||
| Hypothalamic NE | 0.439, p < .03 | 0.635, p < .001 | |||
| Plasma ACTH | 0.5239, p < .01 | 0.89, p < .001 | 0.495, p < .01 | ||
| Corticosterone | 0.478, p < .02 | 0.477, p < .02 | 0.507, p < .01 | ||
| B. Indomethacin pretreatment | |||||
| Locomotor activity | |||||
| Shivering | 0.507, p < .01 | ||||
| Core body temperature | 0.563, p < .004 | 0.709, p < .001 | |||
| Hypothalamic NE | 0.427, p < .04 | 0.617, p < .001 | 0.491, p < .01 | ||
| Plasma ACTH | 0.698, p < .001 | 0.479, p < .02 | 0.741, p < .001 | 0.631, p < .001 | |
| Corticosterone | 0.447, p < .03 | 0.625, p < .001 |
4. Discussion
Intraperitoneal injection of IL-1β induced clear increases in body temperature relative to saline-treated rats (ca 0.6–0.7 °C) for approximately 4 h, as well as acute increases in shivering, and a more prolonged decrease in locomotor activity. These changes were accompanied by increases in the apparent release of NE from the medial hypothalamus lasting for around 4h, with a peak around 1.5–2 h. The injection of IL-1β also increased plasma concentrations of ACTH, reaching a peak within the first hour, and a more prolonged increase in plasma corticosterone reaching a peak around 1.5–2 h. Pretreatment with the cyclo-oxygenase inhibitor, indomethacin prevented the IL-1β-induced increases in body temperature and the apparent hypothalamic NE release. However, it only attenuated the increases in plasma ACTH and the shivering, and had little effect on the plasma corticosterone.
The results indicate relationships between NE release, HPA axis activation (plasma ACTH and corticosterone) and body temperature. However, the indomethacin treatment clearly differentiated the responses in body temperature and hypothalamic NE secretion, which were essentially nullified, from those on shivering and the HPA axis activation, which were only attenuated. These results suggest that there are redundant mechanisms by which the HPA axis is activated following IL-1β administration.
An association between the noradrenergic activation in the hypothalamus, and the HPA axis activation has been observed in previous studies involving measurement of NE catabolites in both mice (Dunn, 1988), and rats (Kabiersch et al., 1988). Similar relationships have been observed in microdialysis studies (Kaur et al., 1998; Shintani et al., 1993; Smagin et al., 1996, see also Dunn, 2001). This is consistent with the ability of local administration of adrenergic antagonists in the median eminence (Matta et al., 1991), or noradrenergic lesions of the ventral ascending bundle (Chuluyan et al., 1992) to decrease the IL-1β-induced increase in plasma corticosterone in rats. However, only a small effect of brain NE depletion was observed on the IL-1-induced increase in plasma corticosterone in mice (Swiergiel et al., 1996). The association between NE and HPA activation in rats was confirmed in the present study because the IL-1β-induced increases in dialysate NE parallelled to some extent the increases in plasma ACTH and corticosterone. Moreover, indomethacin pretreatment more or less completely prevented the NE response (Fig. 4) and substantially inhibited the response in plasma ACTH. However, the later responses were not completely inhibited (Figs. 5 and 6), suggesting that another mechanism is also involved in the activation of the HPA axis when IL-1β is injected ip.
Many studies have shown the ability of COX inhibitors to inhibit HPA responses to IL-1 (Dunn and Chuluyan, 1992; Katsuura et al., 1988; Krymskaya et al., 1987; Morimoto et al., 1989; Murakami and Watanabe, 1989; Rivier and Vale, 1991). However, we noted that whereas the increases in plasma corticosterone following intravenous (iv) injection of IL-1β to mice were largely prevented by COX inhibitors (as in the studies cited above), those to ip injection of IL-1 were not (Dunn and Chuluyan, 1992). This was the First clear indication that there were probably multiple mechanisms by which IL-1 could activate the HPA axis. The increase in plasma corticosterone to iv IL-1β is relatively short lived, whereas that to ip IL-1β lasts around 2 h. Interestingly, we also noted that the early response to ip IL-1 was sensitive to indomethacin, whereas the later phase was not. Because IL-1β administered ip is likely to enter the systemic circulation, this suggests that the early phase of the response to ip IL-1β may parallel that of iv IL-1β, but that later on, another mechanism operates. The present results also suggest that indomethacin inhibits the plasma ACTH response, but only slightly affected that in corticosterone in the later phases of the response. This suggests that neither preventing the activation of hypothalamic NE nor the decrease in body temperature were sufficient to prevent the HPA activation.
Consistent with the suggestions of Turnbull and Rivier (1999), we propose that iv IL-1β may act directly on the median eminence or the organum vasculosum laminae terminalis (OVLT) to elicit HPA axis activation by a mechanism involving COX. It is relevant that indomethacin prevented the HPA activation induced by local injection of IL-1 into the OVLT (Katsuura et al., 1990). We know that IL-1-receptors exist on the endothelia in the OVLT, and that they can be coupled to COX to induce the synthesis of prostaglandins (Blatteis and Sehic, 1998). However, a major mechanism by which ip IL-1β stimulates the HPA axis appears to involve hypothalamic NE and CRF (Dunn, 2005; Turnbull and Rivier, 1999). This route most probably involves stimulation of vagal afferents in the abdomen (Wieczorek et al., 2005). This would be consistent with our recent observations that in rats subdiaphragmatic vagotomy impairs both the hypothalamic noradrenergic response and the HPA responses to ip injection of IL-1β (Wieczorek and Dunn, under review).
Thus the present results indicate that the various responses to ip IL-1β are not all closely related. The changes in body temperature appear to be relatively independent of those in behavior, hypothalamic NE, and plasma ACTH and corticosterone. On the other hand, there appears to be a close relationship between the hypothalamic NE and the plasma ACTH and corticosterone responses. Nevertheless, the NE response does not appear to be essential for the HPA response, although in the absence of a noradrenergic response, the HPA activation is diminished. These results clear indicate that there are multiple redundant mechanisms by which peripherally administered IL-1 affects the various nervous system responses.
Acknowledgments
We thank Glenn Farrar for technical assistance with the microdialysis procedure, and Charles Dempsey for technical assistance with the neurochemical assays. We also thank Dr. Artur Swiergiel for his useful contributions to the manuscript. This work was supported by a grant from the National Institutes of Health (NS35370).
References
- 1.Benveniste H. Brain microdialysis. J Neurochem. 1989;52:1667–1679. doi: 10.1111/j.1471-4159.1989.tb07243.x. [DOI] [PubMed] [Google Scholar]
- 2.Besedovsky HO, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- 3.Blatteis CM, Sehic E. Fever: how may circulating pyrogens signal the brain. News Physiol Sci. 1997;12:1–9. [Google Scholar]
- 4.Blatteis CM, Sehic E. Cytokines and Fever. Ann NY Acad Sci. 1998;840:608–618. doi: 10.1111/j.1749-6632.1998.tb09600.x. [DOI] [PubMed] [Google Scholar]
- 5.Chuluyan H, Saphier D, Rohn WM, Dunn AJ. Noradrenergic innervation of the hypothalamus participates in the adrenocortical responses to interleukin-1. Neuroendocrinol. 1992;56:106–111. doi: 10.1159/000126215. [DOI] [PubMed] [Google Scholar]
- 6.Dantzer R, Bluthé R-M, Castanon N, Chauvet N, Capuron L, Goodall G, Kelley KW, Konsman J-P, Layé S, Parnet P, Pousset F. Cytokine effects on behavior. In: Ader R, Felten D, Cohen N, editors. Psychoneuroimmunology. Academic Press; San Diego, CA: 2001. pp. 703–727. [Google Scholar]
- 7.Dinarello CA. Interleukin-1. Rev Infect Dis. 1984;6:51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Dunn AJ. Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 1988;43:429–435. doi: 10.1016/0024-3205(88)90522-x. [DOI] [PubMed] [Google Scholar]
- 9.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. Academic Press; New York: pp. 649–666. [Google Scholar]
- 10.Dunn A. Cytokine activation of the hypothalamo–pituitary–adrenal axis. In: Steckler T, Kalin N, Reul JMHM, editors. Handbook of Stress and the Brain Part 2: Stress: Integrative and Clinical Aspects. Elsevier; Amsterdam: 2005. pp. 157–174. [Google Scholar]
- 11.Dunn AJ, Chuluyan H. The role of cyclo-oxygenase and lipoxygenase in the interleukin-1-induced activation of the HPA axis: dependence on the route of injection. Life Sci. 1992;51:219–225. doi: 10.1016/0024-3205(92)90078-4. [DOI] [PubMed] [Google Scholar]
- 12.Kabiersch A, del Rey A, Honegger CG, Besedovsky HO. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain Behav Immun. 1988;2:267–274. doi: 10.1016/0889-1591(88)90028-1. [DOI] [PubMed] [Google Scholar]
- 13.Katsuura G, Gottschall PE, Dahl RR, Arimura A. Adrenocorticotropin release induced by intracerebroventricular injection of recombinant human interleukin-1 in rats: possible involvement of prostaglandin. Endocrinol. 1988;122:1773–1779. doi: 10.1210/endo-122-5-1773. [DOI] [PubMed] [Google Scholar]
- 14.Katsuura G, Arimura A, Koves K, Gottschall PE. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1β-induced ACTH release. Amer J Physiol. 1990;258:E163–E171. doi: 10.1152/ajpendo.1990.258.1.E163. [DOI] [PubMed] [Google Scholar]
- 15.Kaur D, Cruess DF, Potter WZ. Effect of IL-1α on the release of norepinephrine in rat hypothalamus. J Neuroimmunol. 1998;90:122–127. doi: 10.1016/s0165-5728(98)00062-9. [DOI] [PubMed] [Google Scholar]
- 16.Krymskaya LG, Gromykhina NY, Kozlov VA. Interleukin 1 effect on adrenal gland function in mice. Immunol Lett. 1987;15:307–309. doi: 10.1016/0165-2478(87)90133-7. [DOI] [PubMed] [Google Scholar]
- 17.Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain Behav Immun. 2001;15:371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- 18.Lavicky J, Dunn AJ. Endotoxin administration stimulates cerebral catecholamine release in freely moving rats as assessed by microdialysis. J Neurosci Res. 1995;40:407–413. doi: 10.1002/jnr.490400316. [DOI] [PubMed] [Google Scholar]
- 19.Matta SG, Linner KM, Sharp BM. Interleukin-1 receptor antagonist protein equally inhibits IL1α- and IL1β-induced ACTH secretion and thymocyte proliferation. Soc Neurosci Abstr. 1991;17:1196. [Google Scholar]
- 20.McCarthy DO, Kluger MJ, Vander AJ. Suppression of food intake during infections: is interleukin-1 involved? Amer J Clin Nutr. 1985;42:1179–1182. doi: 10.1093/ajcn/42.6.1179. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto A, Murakami N, Nakamori T, Sakata Y, Watanabe T. Possible involvement of prostaglandin E in development of ACTH response in rats induced by human recombinant interleukin-1. J Physiol. 1989;411:245–256. doi: 10.1113/jphysiol.1989.sp017571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami N, Watanabe T. Activation of ACTH release is mediated by the same molecule as the Wnal mediator, PGE2, of febrile response in rats. Brain Res. 1989;478:171–174. doi: 10.1016/0006-8993(89)91492-3. [DOI] [PubMed] [Google Scholar]
- 23.Rivier C, Vale W. Stimulatory effect of interleukin-1 on adrenocorticotropin secretion in the rat: is it modulated by prostaglandins? Endocrinol. 1991;129:384–388. doi: 10.1210/endo-129-1-384. [DOI] [PubMed] [Google Scholar]
- 24.Shintani F, Kanba S, Nakaki T, Nibuya M, Kinoshita N, Suzuki E, Yagi G, Kato R, Asai M. Interleukin-1β augments release of norepinephrine, dopamine, and serotonin in the rat anterior hypothalamus. J Neurosci. 1993;13:3574–3581. doi: 10.1523/JNEUROSCI.13-08-03574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smagin GN, Swiergiel AH, Dunn AJ. Peripheral administration of interleukin-1 increases extracellular concentrations of norepinephrine in rat hypothalamus: comparison with plasma corticosterone. Psychoneuroendocrinol. 1996;21:83–93. doi: 10.1016/0306-4530(95)00019-4. [DOI] [PubMed] [Google Scholar]
- 26.Swiergiel AH, Dunn AJ, Stone EA. The role of cerebral noradrenergic systems in the Fos response to interleukin-1. Brain Res Bull. 1996;41:61–64. doi: 10.1016/0361-9230(96)00173-6. [DOI] [PubMed] [Google Scholar]
- 27.Turnbull AV, Rivier C. Regulation of the hypothalamic–pituitary–adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Wieczorek M, PournajaW-Nazarloo H, Swiergiel AH, Dunn A. Physiological and behavioral responses to interleukin-1β and LPS in vagotomized mice. Physiol Behav. 2005;85:500–510. doi: 10.1016/j.physbeh.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wieczorek, M., Dunn, A.J., The role of the vagus nerve in the febrile, behavioral, noradrenergic, and endocrine responses to interleukin-1β in the rat (under review).


