Abstract
A number of cytokines and costimulatory molecules involved in immune activation have recently been identified including IL-12, a heterodimeric cytokine that supports the development of cell-mediated immunity, and B7-1, a costimulatory molecule involved in the activation of T lymphocytes. We explored the use of these immunomodulants as molecularly defined adjuvants in the function of recombinant anticancer vaccines using a murine model adenocarcinoma, CT26, transduced with a model Ag, β-galactosidase (β-gal). Although IL-12 given alone to mice bearing tumors established for 3 days did not have consistent antitumor activity, a profound therapeutic effect was observed when IL-12 administration was combined with a recombinant vaccinia virus (rVV) encoding β-gal called VJS6. On the basis of the reported synergistic effects of IL-12 and the costimulatory molecule B7-1 (CD80) in vitro, we immunized mice with a double recombinant vaccinia encoding both the model tumor Ag the costimulatory molecule B7-1, designated B7-1β-gal rVV. The adjuvant administration of IL-12 after immunization with this virus significantly enhanced survival in tumor-bearing animals. T cell subset depletions demonstrated that the in vivo activity of IL-12 was largely independent of CD4+ T lymphocytes, whereas the in vivo activity of a B7-1 rVV required both CD4+ and CD8+ T cells to elicit maximal therapeutic effect. To our knowledge, this is the first description of B7-1 and IL-12 cooperation in vivo and represents a novel strategy to enhance the efficacy of recombinant anticancer vaccines.
Effective antiviral vaccines have been developed to prevent numerous viral diseases. Vaccinia virus, e.g., was administered to millions of people in the successful eradication of smallpox. In its recombinant form, vaccinia virus has potential value in cancer therapy because it can be manipulated to deliver tumor Ags within the host cell cytoplasm where it can elicit specific immune responses targeted against engineered proteins (1-6). Studies have demonstrated that immunizing with a recombinant vaccinia virus (rVV)3 expressing a tumor Ag prevented tumor development when challenged with Ag-expressing tumor and could be used in the active treatment of low burdens of established tumor (5, 7-9). Moreover, the immunogenicity of rVV has been enhanced when molecularly defined adjuvants such as IL-2 were given along with rVV (5).
A recently characterized cytokine, IL-12, holds promise as a vaccine adjuvant because it can specifically enhance cell-mediated immunity. In murine models, both IL-12 adjuvant and Leishmania Ag were required to protect BALB/c mice from otherwise fatal infection with Leishmania major (10). IL-12 induced a shift from Th2, i.e., primarily Ab responses, which were ineffectual against the intracellular parasite, to Th1 responses, which resulted in protective cell-mediated immunity (11-13). Similar findings were obtained in other studies in which the induction of cell-mediated immunity was required, such as in murine models of toxoplasmosis, schistosomiasis, and HIV (14-18).
IL-12 has been used as an adjuvant to cancer vaccination attempts in two settings. NIH3T3 cells transfected to produce IL-12 delayed the appearance of tumor when given along with irradiated BL-6 tumor cells, an aggressive and weakly immunogenic clone of B16 murine melanoma (19). Additionally, IL-12 given exogenously along with mutant p53 peptide was able to cause the regression of an established subcutaneous Meth A sarcoma (20). The adjuvant effects of IL-12 with peptide immunization were dependent on CD8+ but not on CD4+ T cells and resulted in an increased lysis of targets expressing mutant p53 peptide. In both the Meth A and BL-6 models, IL-12 administered alone had significant antitumor activity in addition to its adjuvant effects. Systemically administered IL-12 induced the regression of established cancer in several experimental tumor models and was particularly effective against murine renal cell cancer (21, 22). The direct antitumor effects of IL-12 were dependent on IFN-γ and CD8+ T lymphocytes and independent of NK cells and TNF-α. Transducing the murine adenocarcinoma CT26 with the cDNA for IL-12 resulted in slowed growth in animals bearing either subcutaneous or visceral established tumors (23). In contrast, exogenously administered IL-12 was unable to treat effectively CT26, the tumor that we used in the present study (23).
Specialized APCs possessing costimulatory molecules, such as dendritic cells, are thought to be capable of enhancing the activation and clonal proliferation of T lymphocyte responses, including those directed against tumor. Anergy or apoptotic cell death can result when signaling occurs through the TCR in the absence of this Ag-independent “second signal” (24). Two cloned molecules capable of providing a costimulatory signal have been designated B7-1 (CD80) and B7-2 (CD86) (25, 26). These molecules interact with T lymphocyte ligands, CD28 and CTLA-4, and initiate a cascade of effects mediated, in part, by up-regulation of IL-2 production (27). B7-1 or B7-2 transfection of tumors can result in increased tumor immunogenicity, leading both to protection against subsequent challenge with the wild-type tumor and to the active treatment of established disease (28-30).
Recent reports have demonstrated an in vitro synergistic effect between IL-12 and the costimulatory molecule B7-1 in inducing proliferation and cytokine production by both human T cells (31) and mouse T helper clones normally unresponsive to B7 costimulation (32). These studies suggest that the synergistic effect of these two molecules is primarily mediated via Th cells. In particular, terminally differentiated Th1 cells were shown to require both molecules for maximal proliferation and cytokine production (32). We know of no reports to date of a demonstrated in vivo synergy between these immunomodulatory molecules.
The recent cloning of the genes encoding tumor-associated Ags (TAAs) processed and presented by human cancer cells that are targets for CTL has rekindled interest in therapeutic cancer vaccination (33-38). In this study we examined the impact of using IL-12 as an adjuvant to therapeutic cancer vaccination with a rVV engineered to express a model tumor Ag. In addition, we explored the synergistic effects of IL-12 and the costimulatory molecule B7-1 in vivo by using a rVV constructed to express both a model tumor Ag and B7-1, coupled with IL-12 adjuvant in the treatment of established cancer.
Materials and Methods
Animals and cell lines
Six- to 8-wk-old female BALB/c (H-2d) mice were obtained from the Frederick Cancer Research Center (Frederick, MD). CT26 is an N-nitroso-N- methylurethan-induced BALB/c (H-2d) undifferentiated colon carcinoma supplied by Dr. D. Pardoll (Baltimore, MD). CT26 was cloned to produce a parental wild-type (WT) cell line, CT26.WT. The gene for lacZ was stably transfected into CT26.WT to create CT26.CL25 as described (4). A clone of the mouse thymoma EL-4 (H-2b) stably transfected with β-galactosidase (β-gal), designated E22, was used as a negative control in 51Cr release assays. BSC-1 cells were used to expand and titer all viruses (American Type Culture Collection, Rockville, MD; CCL 26). Cell lines were maintained in RPMI 1640, 10% heat-inactivated FCS (Biofluids, Rockville, MD), 0.03% l-glutamine, 100 mg/ml streptomycin, 100 U/ml penicillin, and 50 mg/ml gentamicin sulfate (National Institutes of Health Media Center, Bethesda, MD). CT26.CL25 and E22 cells were maintained in media containing 400 mg/ml G418 (Life Technologies, Grand Island, NY).
Recombinant viruses
All of the single rVVs used in this study were originated by inserting the foreign genes into the VV thymidine kinase (TK) gene by homologous recombination, resulting in the generation of TK− progeny as described (6). Viral stocks were propagated in BSC-1 cells and were purified by ultracentrifugation on a 36% sucrose cushion. The BSC-1 cell line was also used to determine virus concentration by plaque titration. The rVVs used in a single experiment were titered together to maximize the accuracy of the relative titers. VJS6, the rVV expressing β-gal used in this report, has been previously characterized (5). Preparation of the rVV expressing the influenza A/PR/8/34 nucleoprotein (NP), V69NP rVV (NP-VAC), constructed from the plasmid pGS69, has been previously described (39). All recombinant viruses were selected for both TK− phenotype and their ability to express β-gal.
Construction and characterization by surface staining, an in vitro functional assay, and Western blotting of rVV containing murine B7-1 and measles hemagglutinin (MHA) will be described in greater detail elsewhere (M. Carroll et al., manuscript in preparation). Double recombinant viruses were prepared using the method described by Blasco and Moss, and B7-1 and its control molecule, MHA, were inserted into the VP37 site and were under the control of the synthetic early/late promoter (40). rVV names have been truncated for clarity. Designations for the viruses constructed are as follows in parentheses: B7-lβ-gal rVV (vMCB7-lβ-gal), MHAβ-gal rVV (vMCMHAβ-gal), and B7-1/NP rVV (vMCB7-1NP).
In vivo treatment studies
Three or six days after i.v. challenge with tumor cells (105 to 5 × 105) to establish pulmonary metastases, BALB/c mice were immunized with virus (101 to 107 PFU). All animals were randomized before receiving virus. Treatment with cytokine adjuvant was initiated at the doses and times indicated. Mice were killed 12 days after tumor injection, and lungs were harvested, stained, and counted with a blinded procedure as described previously (5). Identically treated groups of mice were followed for survival.
Primary in vivo adoptive transfer studies involved i.v. immunization of BALB/c mice with HBSS, B7-1β-gal rVV, or MHAβ-gal rVV at l07 PFU. After 21 days, mice were killed and splenectomized. Spleens were homogenized, passed through a Nytex membrane, and suspended in HBSS. Splenocytes (2 × 107) were adoptively transferred to syngeneic mice that had been injected with 5 × 105 tumor cells i.v. of either CT26.WT or CT26.CL25 3 days earlier. Nine days after adoptive transfer of primed splenocytes, the mice were randomized and killed, and lung metastases were enumerated in a blinded procedure. All animals in this study inoculated with tumor in the absence of other treatments had >250 pulmonary tumor nodules (deemed too numerous to count), unless otherwise stated.
Cytokines and Abs
The dosing and schedule of recombinant murine IL-12 were derived from published reports on the direct antitumor effects of IL-12 alone. IL-12 was administered at 0.02, 0.1, 0.5, or 2.5 mg i.p. according to the schedules given in the figure legends. IL-12 was a generous gift of Dr. M. Gately (Hoffmann-La Roche, Nutley, NJ) and was reconstituted in 1% homologous mouse serum in PBS before use. The reported bioactivity of the stocks used in these experiments was 2.3 × 108 Roche U/mg. Culture supernatants of anti-CD4 mAb GK1.5 (ATCC; TIB 207) and ascites fluid of hybridoma 2.43 (anti-CD8) (ATCC; TIB 210) were generously supplied by Dr. P. Cohen (National Cancer Institute, Bethesda, MD) and were diluted in HBSS before use in vivo. For in vivo depletion, BALB/c mice were given i.v. injections of GK1.5 at 100 mg/ml or of empirically determined levels of 2.43 mAbs (1 ml) 2 days before and 5 days after tumor.
Statistics
Data involving lung metastases does not follow a normal distribution because a11 lungs that contained >250 metastases were deemed too numerous to count. Therefore, the data from in vivo studies were analyzed with the nonparametric two-tailed Kruskal-Wallis test. Survival curves were analyzed with Kaplan-Meier survival curves (41).
Results
IL-12 was an adjuvant to virally based vaccination
We examined the effect of IL-12 administration following vaccination with a virus (VJS6) encoding a model tumor Ag, β-gal, in animals bearing 3-day-old pulmonary metastases. Six distinct experiments demonstrated the adjuvant effects of IL-12 (Table I). In these experiments, mice (5 to 10 per group) were inoculated i.v. with CT26.CL25 tumor cells. Three days later, the mice were given 106 PFU of VJS6 followed 12 h later by IL-12 adjuvant at 0.5 mg i.p. once a day for 5 consecutive days (hence referred to as the standard dose) or HBSS (reported as VJS6 alone). Administration of IL-12 alone mediated a statistically significant reduction in metastases in only one of six experiments. Treatment of tumors established for 3 days with virus alone resulted in significantly fewer metastases when enumerated 12 days after tumor inoculation compared with the no treatment group (p < 0.05 in all six experiments). When IL-12 and virus were combined, however, tumor burden assessed on day 12 was significantly diminished (Table I). The dose of IL-12 required to achieve significant antitumor effects as an adjuvant could be reduced to as low as 0.1 mg per injection, whereas IL-12 alone did not have consistent antitumor activity at a dose one log higher, suggesting that the therapeutic index for the adjuvant uses of IL-12 was much greater than its use as direct therapy (data not shown). In two other experiments, adjuvant IL-12 following immunization with a rVV expressing an irrelevant Ag, NP, generated no therapeutic response in mice bearing CT26.CL25 tumors established for 3 days (data not shown). These results demonstrated that IL-12 specifically enhanced the antitumor effects of rVV, although a residual tumor burden remained in all treatment groups.
Table I.
IL-12 is an adjuvant to therapeutic tumor vaccination with VJS6a
| Mean No. of Pulmonary Metastases | ||||||
|---|---|---|---|---|---|---|
| Treatment | Expt. 1 | Expt. 2 | Expt. 3 | Expt. 4 | Expt. 5 | Expt. 6 |
| None | 250 | 250 | 250 | 250 | 250 | 250 |
| IL-12 | 250 | 212 | 250 | 172 | 250 | 64b |
| VJS6 | 150 | 119 | 198 | 64 | 179 | 144 |
| VIS6 + IL-12 | 35* | 21** | 78* | 26* | 43* | 19*** |
In each experiment nonirradiated BALB/c mice (5 to 10 per group) were injected i.v. with 5 × 105 CT26.CL25 tumor cells, followed 3 days later by 106 PFU of VJS6. Mice were given IL-12 adjuvant or HBSS (reported as VIS6 alone) at 0.5 μg i.p. once a day for 5 days starting 12 h after viral inoculation. Mice were ear tagged, randomized, and euthanized 12 days after tumor injection. Lungs were harvested and pulmonary metastases were enumerated in a blinded, coded procedure. In one additional experiment not shown, no IL-12 adjuvant effect was observable.
p < 0.05 comparing VJS6 alone vs VJS6 + IL-12.
p < 0.01 comparing VJS6 alone vs VJS6 + IL-12.
p < 0.05 comparing VJS6 alone or IL-12 alone vs VJS6 + IL-12.
In all six experiments p < 0.05 comparing VJS6 alone vs no treatment. In Expt. 6, p < 0.05 comparing IL-12 alone vs no treatment.
IL-12 adjuvant plus vaccination specifically prolonged survival in tumor-bearing animals
To assess whether treatment with IL-12 adjuvant could prolong the survival of mice bearing tumor established for either 3 or 6 days, mice were immunized with VJS6 on day 3 or 6, followed by the adjuvant administration of IL-12. No prolongation of survival was observed in any treatment group for mice bearing CT26.WT tumors, with all mice dying within 12 to 25 days after receiving tumor inoculation (Fig. 1A). In mice bearing CT26.CL25 tumors established for 3 days then vaccinated with VJS6, the adjuvant administration of IL-12 prolonged survival when compared with mice receiving VJS6 immunization alone (Fig. 1B). In this experiment, although therapy with VJS6 alone was partially effective by extending survival when compared with the no treatment group (p < 0.05), treatment with VJS6 plus adjuvant IL-12 significantly prolonged survival compared with either therapy alone (p < 0.0024).
FIGURE 1. Effect of IL-12 adjuvant with rVV vaccination on mice bearing 3- and 6-day-old pulmonary metastases.

Nonirradiated BALB/c mice (10 per group) were injected i.v. with 105 tumor cells of CT26.WT (A) or CT26.CL25 (B to D) and then randomized. Three (A and B) or 6 (C and D) days later, mice were immunized with either 106 PFU of rVV encoding β-gal (VJS6) or nothing. Adjuvant HBSS or IL-12 (0.5 mg i.p.) once a day for 5 days was started 12 h after immunization. Mice were then followed for survival. This figure represents data pooled from two distinct, but identically performed experiments in which all experimental groups were included in each experiment. A, IL-12 plus vaccination did not prolong the survival of mice bearing TAA-negative tumors established for 3 days. B, IL-12 plus vaccination prolonged the survival of mice bearing tumors established for 3 days, which expressed the model TAA. Treatment with VJS6 + IL-12 was superior to the next best treatment, VJS6 alone (p < 0.0024). C, IL-12 plus vaccination with a rVV expressing the relevant TAA prolonged the survival of mice bearing tumor established for 6 days which expressed the model TAA. V69NP refers to a rVV that expressed NP and did not express β-gal. Treatment with MHA β-gal rVV + IL-12 was superior to the next best treatment, MHAβ-gal rVV alone (p < 0.048). This experiment rendered similar results when VJS6 was used as the model Ag-expressing virus (not shown). D, IL-12 plus vaccination prolonged survival in mice bearing 6-day-old pulmonary metastases. Treatment with VJS6 + IL-12 was superior to all other groups (p < 0.0018). This experiment also yielded similar results when IL-12 was started on day 4 or 12 h after viral administration (not shown).
In mice bearing CT26.CL25 tumors established for 6 days, treatment with MHAβ-gal rVV alone, a rVV expressing similar levels of β-gal in vivo as VJS6 (data not shown), was able to extend survival compared with mice receiving no treatment (p < 0.05), where as treatment with V69NP, a rVV encoding the irrelevant Ag NP, provided no benefit (Fig. 1C). The adjuvant administration of IL-12 following MHAβ-gal rVV immunization resulted in a significant prolongation of survival compared with therapy with MHAβ-gal rVV alone (p < 0.048). No therapeutic benefit was observed when IL-12 was given after V69NP immunization (Fig. 1C), illustrating that adjuvant therapy was effective only after immunization with an Ag expressing virus.
In a separate experiment, we observed that the administration of adjuvant IL-12 after VJS6 immunization doubled the survival time of animals bearing tumors established for 6 days (Fig. 1D). In this experiment, several animals were killed 6 days after tumor injection and their lungs were removed and stained X-gal for the presence of β-gal. These animals had grossly visible tumor nodules that stained positive for β-gal, clearly indicating that at the time of initial treatment animals suffered from well established disease (data not shown). Treatment with VJS6 alone or with IL-12 alone had no impact on survival of animals bearing such advanced tumors (Fig. 1D), whereas the combination of these two therapies significantly prolonged the survival of treated mice compared with mice receiving no treatment (p < 0.0018). Together, these findings established that vaccination with 106 PFU of rVV coupled with IL-12 at the standard dose prolonged the survival of animals bearing 3- and 6-day-old pulmonary metastases. Collectively, these experiments indicated the specificity of the immune response, because treatment was effective only when a virus expressing the tumor Ag was used.
IL-12 adjuvant enhanced antitumor responses against low levels of Ag
To evaluate whether lower doses of the therapeutic vector could be used if given together with IL-12, we immunized mice with VJS6 at doses ranging from 104 to 107 PFU/mouse. In these experiments, IL-12 administered alone had no significant antitumor effects against CT26.CL25 (Fig. 2). Treatment with VJS6 alone mediated a partial reduction in the number of pulmonary metastases when compared with unimmunized mice or mice treated with IL-12 alone (p < 0.05 vs tumor alone or IL-12 alone at 105, 106, and 107 PFU of virus; treatment with 104 PFU was not statistically superior to no treatment or IL-12 alone). The administration of IL-12 along with VJS6 resulted in an enhanced response to viral immunization and produced statistically significant reductions in metastases vs treatment with VJS6 alone (p < 0.05 comparing 105, 106, and 107 PFU VJS6 alone vs VJS6 + 1L-12 at these doses of virus; 104 PFU VJS6 + IL-12 was not statistically better than virus alone). Vaccination plus IL-12 administration induced tumor regression similar to virus alone at a 100-fold lower dose of virus (Fig. 2). In previous experiments we have established that lower doses of virus produce lower levels of Ag expression in vivo (V. Bronte et al., manuscript in preparation). By improving the function of low levels of virus, IL-12 adjuvant seemed capable of transforming weak antitumor responses, presumably limited by Ag availability, into effective treatments for established disease.
FIGURE 2. Adjuvant effect of IL-12 enhances antitumor responses against low levels of Ag.

Nonirradiated BALB/c mice (10 per group) were injected i.v. with CT26.CL25 tumor cells and then randomized. Three days later, mice were immunized i.v. with varying doses (104 to 107 PFU) of rVV encoding β-gal (VJS6) or nothing. Adjuvant HBSS or IL-12 (0.5 mg i.p.) once a day for 5 days was started 12 h after immunization. Mice were randomized and killed 12 days after tumor injection. Lungs were harvested and pulmonary metastases were enumerated in a blinded procedure. *, p < 0.05 comparing 105 PFU VJS6 + IL-12 vs 105 PFU VJS6 alone; **, p < 0.05 comparing 106 PFU VJS6 + IL-12 vs 106 PFU VJS6 alone; ***, p < 0.05 comparing 107 PFU VJS6 + IL-12 vs 107 PFU VJS6 alone. This figure represents data pooled from two distinct, but identically performed experiments.
IL-12 cooperated with B7-1 in the treatment of animals bearing 3-day-old pulmonary metastases
Based on reports of a synergism between IL-12 and B7-1 in vitro (31, 32), we explored whether a similar effect could be observed in vivo in a tumor model system. We thus used rVVs capable of expressing our model TAA with either B7-1 (CD80) or a control molecule, MHA. Mice bearing 3-day CT26.CL25 tumors were treated with B7-lβ-gal rVV, B7-1NP rVV, or MHAβ-gal rVV alone or in combination with IL-12. The therapeutic effects of MHAβ-gal rVV and B7-1β-gal rVV were statistically significant (Fig. 3A; comparing B7-1β-gal or MHAβ-gal with no virus or B7-1NP rVV, p < 0.05). However, B7-1 did not enhance the therapeutic effectiveness of the virus in this set of experiments. We have found that at higher doses of virus than the 105 PFU per mouse used here, the expression of B7-1 along with tumor Ag can enhance the antitumor effects of vaccination (R. S. Chamberlain et al., manuscript in preparation).
FIGURE 3. IL-12 plus vaccination with a virus expressing B7-1 and tumor Ag prolongs survival of mice bearing 3-day-old pulmonary metastases.

Nonirradiated BALB/c mice (10 per group) were injected i.v. with CT26.CL25 tumor cells and then randomized. Three days later, mice were immunized with 105 PFU of MHAβ-gal rVV, B7-1β-gal rVV, B7-1 NP rVV, or nothing. Adjuvant IL-12 (0.5 mg i.p.) once a day for 5 days was started 12 h after immunization. Mice were followed for survival. This figure represents data pooled from two distinct, but identically performed experiments, each of which included all experimental groups. A, Experimental groups receiving no treatment or immunized with rVV without adjuvant IL-12. B, Treatment groups receiving IL-12 alone or as an adjuvant to rVV immunization. Treatment with B7-1 β-gal rVV + IL-12 was superior to treatment with any other group (B7-1β-gal rVV + IL-12 vs B7-1 β-gal rVV alone, p < 0.0018).
In the same experiments, the extension of survival resulting from IL-12 administered alone was small but significant (Fig. 3B) when compared with mice receiving no treatment (Fig. 3A; p < 0.05). Furthermore, although IL-12 reproducibly augmented the function of VJS6, no significant augmentation of the therapeutic effectiveness of MHAβ-gal rVV was observed (Fig. 3).
Addition of exogenous IL-12 to the B7-1β-gal rVV, however, significantly prolonged the survival of mice with established tumor (Fig. 3; B7-I β-gal + IL-12 vs B7-1β-gal alone, p < 0.01). To assess the potential nonspecific effects of the combination of exogenous IL-12 and B7-1, we administered IL-12 along with a virus expressing B7-1 and an irrelevant Ag, NP. The antitumor effects of combination therapy with IL-12 adjuvant and the virus B7-1NP rVV was not superior to either treatment alone (Fig. 3). These results indicated that the antitumor effects of vaccination could be significantly enhanced when IL-12 administration was given following treatment with a virus encoding the genes for both the model tumor Ag and B7-1.
Adoptive transfer of splenocytes from mice immunized with rVV encoding B7-1 and a model tumor Ag leads to regression of established tumor in syngeneic mice
To explore the components of the antitumor immune responses observed, we first examined the role for B7-1. The previous set of experiments (Fig. 3) had indicated that the therapeutic activity of the rVV encoding B7-1 that was observed in conjunction with IL-12 was Ag-specific, because the B7-1NP rVV did not have activity. We hypothesized that the B7-1 molecule encoded by the rVV was activating the cellular immune system, specifically T lymphocytes, and that this activation was occumng in the absence of tumor cells.
Mice were immunized with B7-lβ-gal rVV, MHAβ-gal rVV, and B7-1NP rVV. Twenty-one days later, their splenocytes were harvested and immediately transferred to mice bearing 3-day-old pulmonary metastases. Therapeutic effectiveness was evaluated 9 days after adoptive transfer. No response was seen following the adoptive transfer of splenocytes to animals bearing CT26.WT in any group or when animals were immunized with B7-1NP rVV (data not shown). Mice bearing 3-day-old CT26.CL25 tumors that were treated with B7-1β-gal rVV-primed splenocytes had significantly fewer pulmonary metastases when compared with the no treatment group (HBSS) (p < 0.007; Fig. 4). No significant reduction in tumor burden was seen in mice receiving MHAβ-gal rVV-primed splenocytes compared with splenocytes from the no treatment control group (p > 0.05; Fig. 4). Furthermore, the adoptive transfer of B7-1β-gal rVV-primed splenocytes resulted in greater antitumor immunity compared with the adoptive transfer of MHAβ-gal rVV-primed splenocytes (p < 0.005; Fig. 4). This experiment demonstrated that immunization of mice with a rVV encoding a tumor Ag and B7-1 resulted in the specific activation of splenocytes, in the absence of tumor cells, that were capable of inducing the regression of 3-day-old pulmonary metastases.
FIGURE 4. Primary adoptive transfer of lymphocytes after in vivo priming with rVV.

Nonirradiated BALB/c mice (10 per group) were injected i.v. with B7-1β-gal rVV, MHAβ-gal rVV, or HBSS. Twentyone days later a splenectomy was performed on all immunized mice. Splenocytes from designated groups of immunized mice were immediately adoptively transferred to similar mice (10 per group) injected i.v. 3 days earlier with either CT26.CL25 or CT26.WT tumor cells. Mice were randomized and then killed 12 days after tumor injection. Lungs were harvested and stained, and pulmonary metastases were enumerated in blinded procedure. All mice inoculated with a CT26.WT had large tumor burdens (data not shown). The adoptive transfer of lymphocytes from animals immunized with either HBSS or MHAβ-gal rVV did not mediate a statistically significant reduction in tumor burden. The adoptive transfer of lymphocytes from mice immunized with B7-1β-gal rVV (*) resulted in a statistically significant reduction in pulmonary metastases vs both the MHA β-gal rVV group and the HBSS group (p < 0.05 and p < 0.007, respectively). This figure represents data pooled from two distinct, but identically performed experiments. The x-axis designates the rVV and the dose (PFU) used for immunization.
Both CD4+ and CD8+ T cells were required for B7-1β-gal rVV to mediate the regression of established tumor
To identify the T cell subset responsible for the observed immunomodulatory effects of B7-1β-gal rVV, mice were selectively depleted of either CD4+ or CD8+ T cells. In mice treated with the anti-CD4+ Ab, FACS analysis revealed >99.8% depletion of CD4+ cells, whereas in mice treated with the anti-CD8 Ab, >97% depletion of CD8+ cells was achieved (data not shown). We confirmed these results in three other FACS analyses completed 1 day before and 4 days after immunization with rVV.
Depletions were performed 2 days before the injection of tumor cells. Three days after tumor inoculation, mice were immunized with B7-1β-gal rVV, MHAβ-gal rVV, B7-1NP rVV or received no treatment. In mice immunized with B7-1β-gal rVV, the none depleted group showed a significant reduction in the number of pulmonary metastases when compared with the no treatment group (data not shown) or the group immunized with MHAβ-gal rVV (p < 0.005; Fig. 5). When either CD4+ or CD8+ T cell subsets were depleted, however, the therapeutic effects of immunization with B7-1β-gal rVV were lost. Mice receiving no treatment or mice immunized with B7-1NP rVV showed no reduction in the number of pulmonary metastases in any group (data not shown). These results not only confirmed that the reduction in pulmonary metastases was T cell mediated but also demonstrated that both CD8+ and CD4+ T lymphocytes were required to mediate this phenomenon.
FIGURE 5. Effect of in vivo T cell subset depletions on the therapeutic effectiveness of rVV vectors.

Two days before tumor administration, nonirradiated BALB/c mice (five per group) were injected with anti-CD4 and anti-CD8 mAbs (GK1.5 (anti-CD4) and 2.43 (anti-CD8)). Mice were then inoculated i.v. with CT26.CL25 tumor cells. Three days after tumor inoculation, mice were immunized i.v. with B7-lβ-gal rVV, MHAβ-gal rVV, B7-1 NP rVV, or HBSS. Repeat treatment with anti-CD4 and anti-CD8 mAbs was done 3 days after immunization. Mice were randomized and then killed on day 12. Lungs were harvested and pulmonary metastases were enumerated in a blinded procedure. FACS analysis was pet formed 1 day before immunization and again at day 7 to verify depletion. Only data from B7-1β-gal rVV (designated virus containing B7-1) and MHAβ-gal rVV (designated virus not containing B7-1) vaccination groups are shown. In the absence of any depleting Ab, immunization with both MHAβ-gal rVV and B7-lβ-gal rVV was able to mediate a significant reduction in the number of pulmonary metastases (*, p < 0.02 and **, p < 0.005, respectively), although the therapeutic effect elicited after immunization with B7-1β-gal rVV was superior to that with MHAβ-gal rVV immunization (p < 0.03). No significant tumor rejection was found in any of the B7-1 NP rVV or HBSS treatment groups (data not shown). This figure represents data pooled from two distinct, but identically performed experiments.
Adjuvant effect of IL-12 is not abrogated by the depletion of CD4+ T cells
To elucidate the roles of CD4+ and CD8+ T cells in the antitumor response following vaccination and IL-12 therapy, we examined the effects of our immunization strategy in mice depleted of CD4+ and CD8+ T cell subsets. FACS analysis revealed that >97% depletion of the appropriate CD4+or CD8+ T cell subset was achieved in each case (data not shown). Depletion of either CD4+ or CD8+ populations completely abrogated the antitumor effects of treatment with VJS6 alone (Fig. 6). The absence of CD8+ T cells resulted in the complete abrogation of the effectiveness of adjuvant IL-12 following VJS6 vaccination. However, an adjuvant effect of IL-12 given together with VJS6 was still significant in the absence of CD4+ T cells (p < 0.048; duplicate experiments confirmed these results). This suggested that the adjuvant effect of IL-12 may be CD4+ T cell independent and that it may partially supplant the requirement of CD4+ T cells in the generation of vaccine-elicited antitumor responses.
FIGURE 6. Effect of IL-12 on mice treated with rVV and depleted of CD4+ and CD8+ T lymphocytes.
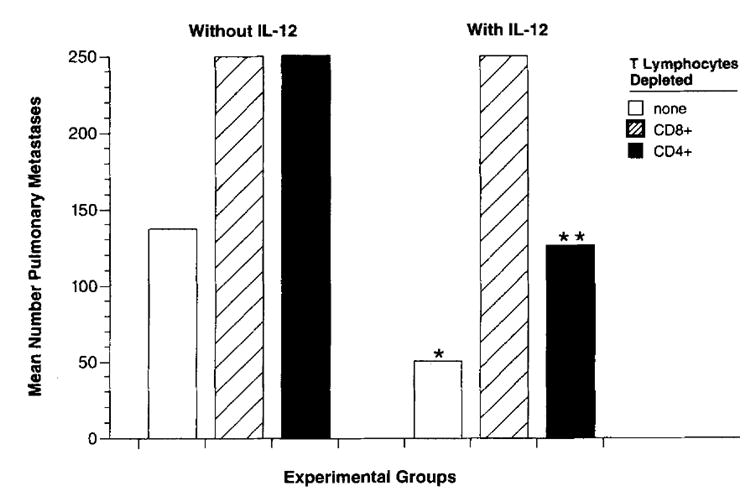
Nonirradiated BALB/c mice(10 per group) were depleted of CD8+ T cells or CD4+ T cells 2 days before and 5 days after tumor administration. Mice were injected i.v. with CT26.CL25 tumor cells and then randomized. Three days later mice were immunized with 106 PFU of a rVV encoding β-gal, VJS6, or nothing. Adjuvant HBSS or IL-12 (0.5 mg i.p.) once a day for 5 days was started 12 h after immunization. Mice were randomized and killed 12 days after tumor injection. Lungs were harvested and pulmonary metastases were enumerated in a blinded procedure. In the absence of any depleting Ab, treatment with VJS6 + IL-12 (*) was superior to treatment with VJS6 alone (p < 0.05). In the absence of CD4+ T cells, treatment with VJS6 + IL-12 (**) was superior to treatment with VJS6 alone (p < 0.05). This figure represents data pooled from two distinct but identically performed experiments.
Discussion
Cell-mediated immunity has been identified as a key mechanism of tumor rejection. Th1 immunity and IFN-γ have been identified as essential components of the cellular immune responses against vaccinia and other viruses in normal and nude mice (42, 43). IL-12 is a potent inducer of IFN-γ secretion from NK cells and macrophages. IL-12 also appears to be capable of enhancing antiviral immune responses (44, 45). Moreover, IL-12 enhanced the function of rVV even at low doses of virus (Fig. 2) and could be used at doses as low as 0.1 mg, further reducing concerns related to the toxicity of IL-12 administration. In an experimental model of respiratory syncytial virus (RSV) infection, IL-12 given at the time of vaccination along with formalin-inactivated, alum-precipitated RSV resulted in the increased immunogenicity of vaccination. Four weeks after vaccination, live RSV challenge of animals immunized with virus plus IL-12 resulted in a shift toward a Th1 cytokine expression pattern and a significant reduction of virus titer in lungs 4 days after challenge (46). These studies collectively established that IL-12 could be used to augment immune responses against a virus.
When compared with the adjuvant effects of IL-12 administration in our model, the antitumor effects of IL-12 given alone were inconsistent. In a few cases, therapy with IL-12 given alone was effective both in reducing pulmonary metastases and in extending survival of the animals (Fig. 3B; Table I), although in most experiments IL-12 alone did not have a significant antitumor effect. IL-12 administered as an adjuvant consistently improved the antitumor effects of rVV vaccination both in reducing metastatic tumor burden and in extending survival when greater than 106 PFU of rVV were used. On the basis of these findings, we concluded that IL-12 had adjuvant effects that were distinct from its direct antitumor effects. Although adjuvant IL-12 enhanced the effect of VJS6 (105 PFU) in mediating a reduction in tumor burden for mice bearing 3-day-old pulmonary metastases, it was incapable of enhancing the survival benefit obtained with VJS6 alone. Given these results, we deduced that it may be easier to see subtle antitumor effects in a pulmonary metastases model than in a survival model.
The results of subset depletion experiments obtained from mice treated with adjuvant IL-12 following VJS6 immunization vs those from mice immunized with B7-1β-gal rVV reveal interesting differences. In the B7-1β-gal rVV-treated mice, depletion of either CD4+ or CD8+ T cells abrogated the therapeutic response (Fig. 5). In this case, although both were important, CD4+ T cells exert a more significant effect. In the IL-12- and VJS6-treated mice, CD8+ T cells were critical to elicit maximal therapeutic effect, and the CD4+ T cells were less important (Fig. 6).These differences present several possible explanations for the enhanced cooperative and therapeutic effect when these two treatments are combined in vivo. Most interestingly, the effects of IL-12 may be mediated via CD4+-independent mechanisms, such as a direct effect of IL-12 on the activation or proliferation of CD8+ T cells (21). When both B7-1β-gal rVV and adjuvant IL-12 are administered together, IL-12 may be directly affecting not only the generation but also the proliferation of Ag-specific CD8+ T cells, supplanting the need for CD4+ T cells. We are continuing to investigate the underlying mechanism using rVVs encoding the genes for B7-1, IL-12, and β-gal individually, as well as in a single recombinant virus.
Further investigations into the contribution of IL-12 on the immune response following vaccination and IL-12 therapy led to the surprising finding that IL-12 appears to exert its effect in the absence of CD4+ T cells. We had initially hypothesized that IL-12 adjuvant functioned primarily through promoting CD4+ Th1 cells. Indeed, this mechanism may still be contributing to the adjuvant effect of IL-12, as treatment with IL-12 and VJS6 in the absence of CD4+ T cells was not as effective as when CD4+ T cells were present (Fig. 6; p < 0.05). Similar findings showing a requirement for CD8+ but not CD4+ T cells have been reported when IL-12 was added to vaccination with a mutant p53 peptide in the treatment of an established subcutaneous Meth A sarcoma (21). Also, when DNA encoding IL-12 was transduced into CT26, the tumor line used in our study, an enhanced antitumor response was seen after depleting CD4+ T cells (19). In these studies, the authors speculated that a suppressor CD4+ T cell population may be inhibiting antitumor immune responses. This is unlikely in our model because CD4+ T cell depletion neither enhanced treatment with VJS6 alone nor improved vaccination efficacy when VJS6 and IL-12 were co-administered. In cytotoxicity assays, we found that IL-12 adjuvant therapy did not result in an increased β-gal-specific lysis by CTL or in increased nonspecific lysis of YAC-I targets, minimizing the possibility that NK or LAK cell activation was the predominant source of the observed antitumor effects (data not shown). Instead, these observations led us to hypothesize that the adjuvant effects of IL-12 in our tumor system involved a direct effect on CD8+ T cell activation or proliferation, because the absence of CD8+ T cells completely eliminated the effects of IL-12 therapy.
Although tumor samples often express abundant levels of Ag and are infiltrated by tumor-reactive lymphocytes, patients nevertheless succumb to progressive disease. The lack of sufficient costimulation has been posited as a potential mechanism for tumor escape. It is known that optimal T cell activation requires at least two interactions, one from the MHC/peptide complex and the TCR, and a second Ag-independent signal between the APC-bound costimulatory molecules B7-1 and B7-2 and the T cell ligands, CD28 or CTLA-4 (47). Anergy or quiescence following MHC class I/peptide/TCR interaction may be induced in the absence of the second signal provided by B7-1 or B7-2 interaction with CD28 or CTLA-4 (24). Tumor cells often possess abundant MHC class I molecules but not B7-1. Transfecting B7-1 into tumors increases tumor immunogenicity and can be used effectively to treat established disease in selected tumor models (29, 30). In other studies, a tumor cell infected with a rVV expressing B7-1 in vitro before tumor implantation grew more slowly than tumor infected with a control vaccinia (48).
Primary adoptive transfer studies were conducted in an attempt to characterize the contribution of the viral expression of B7-1 in the priming of an antitumor immune response. These studies demonstrated that vaccination with B7-lβ-gal rVV primed a splenocyte effector population with enhanced in vivo tumoricidal capacity, and that the genes for both B7-1 and β-gal were necessary. These results suggest that the provision of a costimulatory signal by virally expressed B7-1 produces a splenocyte population with either a quantitative increase in Ag-specific precursor frequency, enhanced survival or trafficking ability, or a qualitative increase in cytotoxic potential. Active immunotherapy studies involving CD8+ and CD4+ subset depletions demonstrated that T lymphocytes (both CD8+ and CD4+) were critical to the mediation of tumor regression in vivo. The molecular mechanisms by which B7-1 can enhance T cell activation are now being elucidated. B7/CD28 interaction can enhance IL-2 mRNA stability (27) important for T cell proliferative responses, as well as enhance IFN-γ production important in Th1 induction and IL-4 production important in Th2 induction(49). In addition, costimulatory signals provided by the B7/CD28 interaction have also been shown to prolong T cell survival by inducing increased expression of the Bcl-xL gene which provides T cells with inherent resistance to apoptotic death induced by either TCR cross-linking, Fas cross-linking, or IL-2 withdrawal (50).
It is important to realize that the tumor model system in which these studies were completed is artificial. β-gal represents a large xenogeneic Ag introduced into a tumor cell line and tested in syngeneic animals, whereas many of the human TAAs cloned thus far, as well as the mouse Ag P1A, are nonmutated “self” proteins. Thus, the question arises as to whether data derived from the use of such a foreign Ag as a TAA will have relevance to the human situation in which most TAAs appear to be predominantly self Ags (34). It is worth noting, however, that similar systems, although using foreign proteins as model TAAs, have been instructive, e.g., transfection of the NP gene from vesicular stomatitis virus into EL4 thymoma (51) or transfection of the human carcinoembryonic Ag (CEA) into MC38, a murine adenocarcinoma (8). Interestingly, the host response to challenge with either CT26.WT or CT26.CL25, expressing β-gal, was unaltered, and we found no evidence of systemic immunity elicited to β-gal. Both CT26.WT and CT26.CL25 grow equally well and are equally lethal after i.v. injection (7). Indeed, the β-gal model system may be most relevant to human tumors possessing TAAs that originate from viruses (52), fusion proteins resulting from translocations (53), or genetic events that result in the expression of foreign proteins arising from mutations, frame-shifts, translation of introns, and the loss of stop codons (54, 55).
Although current efforts using B7-1 alone in the prevention and active treatment of tumor are promising, even more exciting is the potential to combine the potent effects of B7-1 with other treatment strategies. In vitro data suggest that B7-1 and IL-12 may specifically synergizes to induce IFN-γ secretion as well as activate T cells (31, 32). In this study, we describe the potent antitumor effects of combining a rVV expressing tumor Ag and B7-1 with IL-12 adjuvant. To our knowledge, this is the first description of B7-1 and IL-12 cooperating in vivo in the effective treatment of established cancer. Studies using B7-1 and IL-12 delivered in the same viral vector are ongoing and will help provide greater insight into the immunologic mechanisms responsible for the enhanced antitumor effects seen when both agents are combined (M. Carroll et al., manuscript in preparation).
This report establishes both that IL-12 is a potent and specific adjuvant to a virally delivered tumor vaccine and that it cooperates with B7-1 in the treatment of established tumor. This strategy represents a novel approach to enhancing the function of therapeutic recombinant poxvirus-based vaccines by exploiting rVV to apply synergies identified in vitro for the treatment of established disease in vivo.
Acknowledgments
The authors thank Martha Blalock for expert assistance with graphics; David Jones and Paul Spiess for help with animal experiments; Peter Cohen and Stan Wolf for helpful discussion; and Karen Hathcock for technical expertise and advice.
Abbreviations used in this paper
- rVV
recombinant vaccinia virus
- β-gal
β-galactosidase
- MHA
measles hemagglutinin
- NP
nucleoprotein from influenza A/PR/8/34
- TAA
tumor-associated antigen
- TK
thymidine kinase
- PFU
plaque-forming unit
- RSV
respiratory syncytial virus
References
- 1.Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991;252:1662. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- 2.Moss B. Poxvirus vectors: cytoplasmic expression of transferred genes. Curr Opin Genet Dev. 1993;3:86. doi: 10.1016/s0959-437x(05)80346-6. [DOI] [PubMed] [Google Scholar]
- 3.Restifo NP, Bacik I, Irvine KR, Yewdell JW, McCabe BJ, Anderson RW, Eisenlohr LC, Rosenberg SA, Bennink JR. Antigen processing in vivo and the elicitation of primary CTL responses. J Immunol. 1995;154:4414. [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, Restifo NP. Active immunotherapy of cancer with a nonreplicating recombinant fowl poxvirus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685. [PMC free article] [PubMed] [Google Scholar]
- 5.Bronte V, Tsung K, Rao JB, Chen PW, Wang M, Rosenberg SA, Restifo NP. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J Immunol. 1995;154:5282. [PMC free article] [PubMed] [Google Scholar]
- 6.Mackett M, Smith GL, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Biotechnology. 1992;24:495. [PubMed] [Google Scholar]
- 7.Blancou J, Kieny MP, Lathe R, Lecocq JP, Pastoret PP, Soulebot JP, Desmettre P. Oral vaccination of the fox against rabies using a live recombinant vaccinia virus. Nature. 1986;322:373. doi: 10.1038/322373a0. [DOI] [PubMed] [Google Scholar]
- 8.Kantor J, Irvine K, Abrams S, Kaufman H, DiPietro J, Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992;84:1084. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 9.Estin CD, Stevenson US, Plowman GD, Hu SL, Sridhar P, Hellstrom I, Brown JP, Hellstrom KE. Recombinant vaccinia virus vaccine against the human melanoma antigen p97 for use in immunotherapy. Proc Natl Acad Sci USA. 1988;85:1052. doi: 10.1073/pnas.85.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 11.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper I cell development in experimental leishmaniasis. J Immunol. 1995;154:5320. [PubMed] [Google Scholar]
- 12.Ghalib HW, Whittle JA, Kubin M, Hasbim FA, el-Hassan AM, Grabstein KH, Trinchieri G, Reed SG. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623. [PubMed] [Google Scholar]
- 13.Murray HW, Hariprashad J. Interleukin-12 is effective treatment for an established systemic intracellular infection: experimental visceral leishmaniasis. J Exp Med. 1995;181:387. doi: 10.1084/jem.181.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter CA, Candolfi E, Subauste C, Van Cleave V, Remington JS. Studies on the role of interleukin-12 in acute murine toxoplasmosis. Immunology. 1995;84:16. [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn TA, Jankovic D, Hieny S, Cheever AW, Sher A. IL-12 enhances vaccine-induced immunity to Schisrosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J Immunol. 1995;154:4701. [PubMed] [Google Scholar]
- 16.Sieling PA, Wang XH, Gately MK, Oliveros JL, McHugh T, Barnes PF, Wolf SF, Golkar L, Yamamura M, Yogi Y. IL-12 regulates T helper type 1 cytokine responses in human infectious disease. J Immunol. 1994;153:3639. [PubMed] [Google Scholar]
- 17.Gazzinelli RT, Giese NA, Morse HC. In vivo treatment with interleukin 12-protects mice from immune abnormalities observed during murine acquired immunodeficiency syndrome (MAIDS) J Exp Med. 1994;180:2199. doi: 10.1084/jem.180.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin-12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tahara H, Zeh HJ, Storkus WJ, Pappo I, Watkins SC, Guhler U, Wolf SF, Robbins PD, Lotze MT. Fibroblasts genetically engineered to secrete interleukin-12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 1994;54:182. [PubMed] [Google Scholar]
- 20.Noguchi Y, Richards EC, Chen YT, Old LJ. Influence of interleukin-12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci USA. 1995;92:2219. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubhard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin-12 against murine tumors. J Exp Med. 1993;178:1223. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiher RD, Storkus WJ. Recombinant IL-12 administration induces tumor regression in association with IFN-γ production. J Immunol. 1994;153:1697. [PubMed] [Google Scholar]
- 23.Martinotti A, Stoppacciaro A, Vagliani M, Melani C, Spreafico F, Wysocka M, Panniani G, Trinchieri G, Colombo MP. CD4 T cells inhibit in vivo the CD8-mediated immune response against murine colon carcinoma cells transduced with interleukin-12 genes. Eur J Immunol. 1995;25:137. doi: 10.1002/eji.1830250124. [DOI] [PubMed] [Google Scholar]
- 24.Boussiotis VA, Gribben JG, Freeman GJ, Nadler LM. Blockade of the CD28 costimulatory pathway: a means to induce tolerance. Curr Opin Immunol. 1994;6:797. doi: 10.1016/0952-7915(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 25.Freeman GJ, Gray GS, Gimmi CD, Lombard DB, Zhou LJ, White M, Fingeroth JD, Gribben JG, Nadler LM. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991;174:625. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 27.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledhetter JA. Binding of the B cell activation antigen 87 to CD28 costimulates T cell proliferation and interleukin-2 mRNA accumulation. J Exp Med. 1991;173:721. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guinan EC, Gribben JG, Boussiotis VA, Freeman GJ, Nadler LM. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84:3261. [PubMed] [Google Scholar]
- 29.Baskar S, Glimcher L, Nabavi N, Jones RT, Ostrand-Rosenberg S. Major histocompatibility complex class II+B7-1+ tumor cells are potent vaccines for stimulating tumor rejection in tumor-bearing mice. J Exp Med. 1995;181:619. doi: 10.1084/jem.181.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by Bp7-transfected melanoma cells. Science. 1993;259:368. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 31.Kubin M, Kamoun M, Trinchieri G. Interleukin-12 synergizes with B7-CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180:211. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy EE, Temes G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O’Garra A. B7 and interleukin-12 cooperate for proliferation and interferon-γ production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Plaen E, Lurquin C, Van Pel A, Mariamé B, Szikora J-P, Wölfel T, Sibille C, Chomez P, Boon T. Immunogenic (tum−) variants of mouse tumor P815: cloning of the gene of tum− antigen P19A and identification of the tum− mutation. Proc Natl Acad Sci USA. 1988;85:2274. doi: 10.1073/pnas.85.7.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boon T, Cerottini J, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 35.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91:6458. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins PF, El-Gamil M, Kawakami Y, Stevens E, Yannelli JR, Rosenberg SA. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994;54:3124. [PubMed] [Google Scholar]
- 39.Smith GL, Levin JZ, Palese P, Moss B. Synthesis and cellular location of the ten influenza polypeptides individually expressed by recombinant vaccinia viruses. Virology. 1987;160:336. doi: 10.1016/0042-6822(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 40.Blasco R, Moss B. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene. 1995;158:157. doi: 10.1016/0378-1119(95)00149-z. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan EL. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457. [Google Scholar]
- 42.Karupiah G, Blanden RV, Ramshaw IA. Interferon-γ is involved in the recovery of athymic nude mice from recombinant vaccinia virus/linterleukin-2 infection. J Exp Med. 1990;172:1495. doi: 10.1084/jem.172.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gajewski T, Renauld J, Van Pel A, Boon T. Costimulation with B7-1, IL-6, and IL-12 is sufficient for primary generation of murine antitumor cutotoxic T lymphocytes in vitro. J Immunol. 1995;154:5638. [PubMed] [Google Scholar]
- 44.Orange JS, Salazar-Mather TP, Opal SM, Spencer RL, Miller AH, McEwen BS, Biron CA. Mechanism of interleukin-12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coutelier JP, Van Broeck J, Wolf SF. Interleukin-12 gene expression after viral infection in the mouse. J Virol. 1995;69:1955. doi: 10.1128/jvi.69.3.1955-1958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang Y, Graham B. Interleukin-12 increases the immunogenicity of RSV vaccination. IL-12 in Infection (Abstr) 1995 [Google Scholar]
- 47.Jenkins MK, Johnson JG. Molecules involved in T cell costimulation. Curr Opin Immunol. 1993;5:361. doi: 10.1016/0952-7915(93)90054-v. [DOI] [PubMed] [Google Scholar]
- 48.Hodge JW, Abrams S, Schlom J, Kantor JA. Induction of antitumor immunity by recombinant vaccinia viruses expressing B7-1 or B7-2 costimulatory molecules. Cancer Res. 1994;54:5552. [PubMed] [Google Scholar]
- 49.Walter H, Schepensn S, Van Wauwe J, De Boer M. Ligation of CD28 on resting T cells by its ligand B7 results in the induction of both Th1- and Th2-type cytokines. Eur Cytokine Netw. 1994;5:13. [PubMed] [Google Scholar]
- 50.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enchancing the expression of Bcl-xL. Immunity. 1995;3:87. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 51.Kundig TM, Bachmann MF, Lefrancois L, Puddington L, Hengartner H, Zinkernagel RM. Nonimmunogenic tumor cells may efficiently restimulate tumor antigen-speclfic cytotoxic T cells. J Immunol. 1993;150:4450. [PubMed] [Google Scholar]
- 52.Ressing ME, Sette A, Brandt RM, Ruppert J, Wentworth PA, Hartman M, Oseroff C, Grey HM, Melief CJ, Kast WM. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol. 1995;154:5934. [PubMed] [Google Scholar]
- 53.Chen W, Peace DJ, Rovira DK, You SG, Cheever MA. T cell immunity to the joining region of p210BCR-ABL protein. Proc Natl Acad Sci USA. 1992;89:1468. doi: 10.1073/pnas.89.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 55.Townsend A, Ohlen C, Rogers M, Edwards J, Mukherjee S, Bastin J. Source of unique tumour antigens. Nature. 1994;371:662. doi: 10.1038/371662a0. [DOI] [PubMed] [Google Scholar]


