Abstract
Purpose
Fatty replacement of bone marrow resulting from radiation therapy can be seen on T1-weighted magnetic resonance (MR) images. We evaluated the radiographic appearance of the vertebral bodies in children treated with proton craniospinal irradiation (CSI) to illustrate the distal edge effect of proton radiotherapy.
Methods
The study cohort consists of 13 adolescents between the ages of 12–18 who received CSI with proton radiotherapy at Massachusetts General Hospital. Ten of these patients had reached maximal or near-maximal growth. Proton beam radiation for these ten patients was delivered to the thecal sac and exiting nerve roots only, whereas the remaining three patients had a target volume that included the thecal sac, exiting nerve roots and entire vertebral bodies. Median CSI dose was 27 [range 23.4–36] Cobalt Gray Equivalents (CGE) given in 1.8 CGE fractions. MR images of the spine were obtained after completion of radiotherapy.
Results
MR images of patients who received proton radiotherapy to the thecal sac only demonstrate a sharp demarcation of hyperintense T1-weighted signal in the posterior aspects of the vertebral bodies, consistent with radiation-associated fatty marrow replacement. MR images of the patients prescribed proton radiotherapy to the entire vertebral column had corresponding hyperintense T1-weighted signal involving the entire vertebral bodies.
Conclusion
The sharp delineation of radiation-associated fatty marrow replacement in the vertebral bodies demonstrates the rapid decrease in energy at the edge of the proton beam. This provides evidence for a sharp fall-off in radiation dose and supports the premise that proton radiotherapy spares normal tissues unnecessary irradiation.
Keywords: proton radiotherapy, craniospinal irradiation, late effects, medulloblastoma, dosimetry
Introduction
As advances in the treatment of pediatric central nervous system (CNS) tumors generate longer survival, the late effects of therapy are becoming increasingly important. Many pediatric brain tumors are sensitive to radiation therapy, but the entrance and exit doses of conventional photon radiotherapy pose a risk of injury to the developing normal brain and other structures outside the targeted radiation field. Proton radiotherapy possesses a particular clinical advantage over standard photon radiotherapy because its physical characteristics allow the maximum dose to be deposited in the target tissue, sparing the exit dose to normal surrounding tissues and reducing the entrance dose in many circumstances.1
Pediatric CNS tumors that have a propensity for subarachnoid dissemination require craniospinal irradiation (CSI) to reduce the risk of recurrence along the neuraxis.2 CSI delivered with proton radiotherapy dramatically reduces the amount of normal tissue irradiated compared to photon radiotherapy and thereby reduces the acute and late toxicities of therapy.3 When planning a proton craniospinal radiation field for a pediatric patient, the radiation dose is usually prescribed to the entire vertebral body to prevent unbalanced vertebral body growth. This technique takes into account the fact that the developing vertebral body has several ossification centers that fuse at different stages during childhood and puberty.4 Radiation given to just a portion of the vertebral body can impair growth in that portion of the vertebral body and lead to scoliosis or kyphosis.5 However, for an adolescent who has reached maximal or near-maximal growth as determined by hand radiographs and growth curves, asymmetric vertebral growth is of minimal or no concern, so it is not necessary to irradiate the entire vertebral body. Accordingly, in adolescents, the proton beam can be optimized to deliver the full dose of radiation to the thecal sac and exiting nerve roots only, with only incidental entrance dose to the proximal tissue consisting of bone, muscle, and skin.
The following report recounts our experience at the Massachusetts General Hospital treating adolescents with CSI to the thecal sac only and demonstrates the benefit of proton radiotherapy by providing an image of the clear demarcation of radiation-induced fatty replacement of the bone marrow at the edge of the proton beam.6 We present visual evidence of the physical properties of proton radiotherapy, where the energy of the proton beam quickly falls just distal to the target and spares normal tissues unnecessary radiation.
Methods
We reviewed our institutional pediatric database for patients between the ages of 12 and 18 who received CSI with proton radiotherapy. We identified thirteen patients who received CSI and had a diagnosis of medulloblastoma (n = 6), germinoma (n = 4), pineoblastoma (n = 2), and primitive neuroectodermal tumor (n = 1). Based on clinical suspicion of growth maturation, nine of these adolescents had x-rays of the hand to assess their potential for further growth. Seven patients had bone ages that demonstrated maximal or near-maximal growth. Three others were determined to have reached maximal growth based on parent height, achieved height, and growth curves. For these ten patients, radiation planning with proton beams for the spinal portion of CSI included only the thecal sac and exiting nerve roots. The remaining three patients had not yet reached maximal growth, so proton radiotherapy was delivered to the entire vertebral bodies in addition to the thecal sac and exiting nerve roots. The median CSI dose was 27 [range 23.4–36] Cobalt Gray Equivalents (CGE) given in 1.8 CGE fractions using a 235 MeV proton beam. Patients received an additional boost to the site of the surgical tumor bed or residual tumor (median dose 54 [range 41.4–57.6] CGE given in 1.8 CGE fractions). Seven patients received concurrent chemotherapy consisting of vincristine (n = 3), etoposide (n = 3), and carboplatinum and vincristine (n = 1). We obtained all MR images of the spine that were acquired after completion of proton radiotherapy.
Results
MR images of the spine after radiotherapy were available for review in nine of the ten patients who were treated to the thecal sac only (every effort was made to obtain images for the remaining patient, but this patient had not yet had an MRI of the spine at his home institution). All nine MR images demonstrate a sharp demarcation of hyperintense T1-weighted signal in the posterior aspects of the vertebral bodies along the entire spinal column, consistent with radiation-associated fatty infiltration. These hyperintense regions roughly coincide to the regions where the proton beam radiation dose is greater than 10 CGE. Patients whose proton radiotherapy was prescribed to the full vertebral body (n=3) had corresponding hyperintense T1-weighted changes throughout the entire vertebral body. Figure 1 shows representative T1-weighted MR images before and after radiation therapy for the patients treated to the thecal-sac only and a planning CT image with the proton dose distribution. Figure 2 shows analogous images from a patient who received the full dose of radiation to the entire vertebral body. These marrow changes were seen as early as 10 days after radiotherapy and have persisted on images 21 months after radiotherapy.
Figure 1. 14 year-old girl with supratentorial PNET: CSI prescribed to thecal sac and exiting nerve roots only.
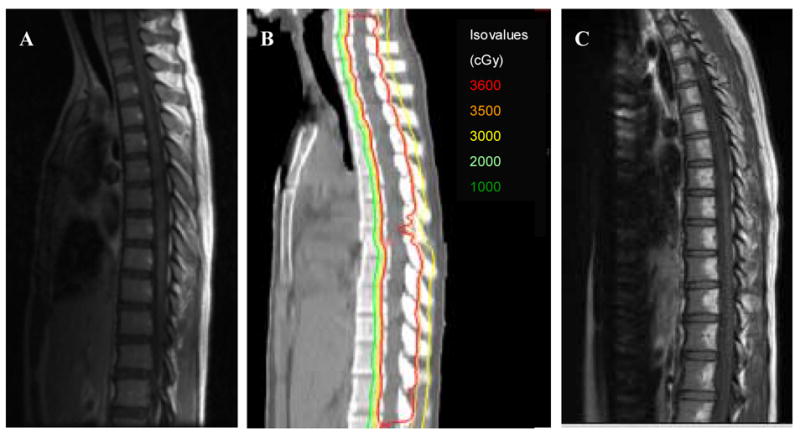
a) T1-weighted MRI one week before radiation treatment
b) CT-Proton radiotherapy treatment plan
c) T1-weighted MRI showing hyperintense fatty changes in posterior aspect of vertebral bodies 1 month after completion of proton radiotherapy
Figure 2. 7 year-old boy with medulloblastoma: CSI prescribed to thecal sac and entire vertebral body.

a) T1-weighted MRI one week before radiation treatment
b) CT-Proton radiotherapy treatment plan
c) T1-weighted MRI showing hyperintense fatty changes throughout entire vertebral bodies 1 month after completion of proton radiotherapy
Discussion
Craniospinal irradiation is a critical component of curative treatment for several pediatric brain tumors, including medulloblastoma, primitive neuroectodermal tumors, and some germ cell tumors, as it reduces the risk for CNS recurrence in tumors that may seed along the neuraxis.7 However, treatment with conventional photon CSI can produce both acute and long-term severe complications during and after therapy because a substantial amount of normal tissue receives the exit dose from photons. During radiation treatment and for several weeks thereafter, patients receiving photon radiotherapy CSI can experience severe morbidity from anorexia, weight loss, nausea, vomiting, laryngitis, pharyngitis, esophagitis, gastritis, and bone marrow suppression and immunosuppression (which can lead to infections or a delay in chemotherapy administration).8 The possible late effects of photon CSI include stunted growth of vertebral bodies and hypoplasia of paraspinal muscles and connective tissue resulting in decreased stature, primary hypothyroidism, hypothalamic-pituitary dysfunction,9 cardiomyopathy, restrictive lung disease, small and large bowel enteritis, primary ovarian failure, decreased marrow reserve, and induction of secondary malignancies.
Dosimetric studies show that proton radiotherapy CSI greatly reduces the amount of radiation to surrounding normal structures while adequately treating the target tissues.3 Here, we present the experience at the Massachusetts General Hospital of treating only the thecal sac in ten patients and offer a representative image showing that only the posterior portion of the vertebral bodies received a significant radiation dose as physiologically demonstrated by the fatty marrow replacement.6 These sharply delineated changes in the bone marrow correspond to the rapid decrease in radiation dose just distal to the target as a result of the Bragg peak effect of proton radiotherapy.1 Radiation doses as low as 1600 Gy have been shown to produce the T1-hyperintense signal characteristic of radiation-induced fatty marrow infiltration,6 so the MR images presented here confirm that all structures anterior to the sharp delineation of fatty marrow replacement were spared significant radiation, including the remainder of the bone marrow in the vertebral bodies, thyroid, esophagus, lungs, heart, bowels, and gonads. The risk of secondary malignancy remains in the tissues irradiated, but due to the dramatically decreased volume of normal tissue that is irradiated with proton CSI compared to photon CSI, the reduction in the secondary cancer risk is estimated to be up to 15-fold.10 Treating only the thecal sac reduces the radiation-associated marrow suppression in the vertebral bodies. This allows the chemotherapy regimen to proceed in the short-term and maintains bone marrow reserves for further therapy. Partial marrow regeneration is thought to occur in pediatric patients 1–3 years after radiotherapy, which may be seen as decreased T1-weighted signal intensity in a mottled or peripheral band pattern on MR imaging.11 We will continue to monitor the appearance of MR images in these patients in addition to their hematopoietic recovery and organ function.
Conclusions
Treatment with proton radiotherapy can reduce both the acute and late toxicities of therapy by minimizing the dose to normal structures while maintaining excellent coverage of the target volume. In adolescents who have reached maximal growth, the advantages of proton CSI can be enhanced by optimizing the proton beam to treat only the thecal sac and exiting nerve roots. Dosimetric planning studies for CSI show that proton radiotherapy is more conformal than conventional 3-D photon radiotherapy or intensity-modulated radiotherapy.1, 3, 12 The images presented here are unique because they visually demonstrate the physiologic correlate to the dosimetry studies through radiographic evidence of fatty marrow replacement in the regions that received a radiation dose approximately greater than 10 CGE. Prospective studies of the acute and late toxicities of proton radiotherapy are currently underway at our institution that will provide the clinical correlate to the superior dose distribution of proton radiotherapy CSI.
Acknowledgments
We would like to thank Robert Schneider, C.M.D. and Judith Adams, C.M.D. for radiation treatment plans and images for this report.
Footnotes
Conflicts of Interest Notification: None of the authors has a conflict of interest with the information presented in this report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yock TI, Tarbell NJ. Technology insight: Proton beam radiotherapy for treatment in pediatric brain tumors. Nat Clin Pract Oncol. 2004;1:97–103. doi: 10.1038/ncponc0090. quiz 101 p following 111. [DOI] [PubMed] [Google Scholar]
- 2.Oyharcabal-Bourden V, Kalifa C, Gentet JC, et al. Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: a French Society of Pediatric Oncology Study. J Clin Oncol. 2005;23:4726–4734. doi: 10.1200/JCO.2005.00.760. [DOI] [PubMed] [Google Scholar]
- 3.St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727–734. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA, et al. American Academy of Orthopaedic Surgeons., National Institutes of Health (U.S.) Howey-in-the-Hills, Florida, May 1997. 1. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1998. Skeletal growth and development : clinical issues and basic science advances : workshop. [Google Scholar]
- 5.Arkin AM, Simon N. Radiation scoliosis; an experimental study. J Bone Joint Surg Am. 1950;32A:396–401. [PubMed] [Google Scholar]
- 6.Yankelevitz DF, Henschke CI, Knapp PH, et al. Effect of radiation therapy on thoracic and lumbar bone marrow: evaluation with MR imaging. AJR Am J Roentgenol. 1991;157:87–92. doi: 10.2214/ajr.157.1.1904679. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch M. Medulloblastoma: staging and treatment outcome. Int J Radiat Oncol Biol Phys. 1988;14:1103–1107. doi: 10.1016/0360-3016(88)90385-9. [DOI] [PubMed] [Google Scholar]
- 8.Chang EL, Allen P, Wu C, et al. Acute toxicity and treatment interruption related to electron and photon craniospinal irradiation in pediatric patients treated at the University of Texas M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 2002;52:1008–1016. doi: 10.1016/s0360-3016(01)02717-1. [DOI] [PubMed] [Google Scholar]
- 9.Constine LS, Woolf PD, Cann D, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 10.Miralbell R, Lomax A, Cella L, et al. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54:824–829. doi: 10.1016/s0360-3016(02)02982-6. [DOI] [PubMed] [Google Scholar]
- 11.Cavenagh EC, Weinberger E, Shaw DW, et al. Hematopoietic marrow regeneration in pediatric patients undergoing spinal irradiation: MR depiction. AJNR Am J Neuroradiol. 1995;16:461–467. [PMC free article] [PubMed] [Google Scholar]
- 12.Kirsch DG, Tarbell NJ. New technologies in radiation therapy for pediatric brain tumors: the rationale for proton radiation therapy. Pediatr Blood Cancer. 2004;42:461–464. doi: 10.1002/pbc.10471. [DOI] [PubMed] [Google Scholar]


