Abstract
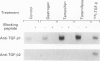
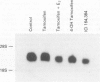
The clinical use of anti-oestrogens in breast cancer therapy has traditionally been restricted to tumours that contain measurable oestrogen receptor protein. However, it is now widely recognised that the clinical response to adjuvant anti-oestrogen therapy appears to be independent of the oestrogen receptor content of the primary tumour. The study reported here was designed to investigate the possibility that human stromal cells can respond to anti-oestrogens by an increased synthesis of the inhibitory growth factor, transforming growth factor beta (TGF-beta). Two established human fetal fibroblast strains were used as models for the breast cancer stromal fibroblasts. These cells were found to respond to the addition of anti-oestrogens by a large increase in their synthesis of biologically active TGF-beta. Despite the application of ligand binding, immunoassay and Northern analysis, no oestrogen receptor or oestrogen receptor mRNA was detected in either of the human fetal fibroblasts strains. These observations may provide a mechanism of action of anti-oestrogens that is independent of the presence of oestrogen receptor in the tumour epithelial cells, and thus provide an explantation for the counter-intuitive results of adjuvant anti-oestrogen action.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giguère V., Yang N., Segui P., Evans R. M. Identification of a new class of steroid hormone receptors. Nature. 1988 Jan 7;331(6151):91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Gleiber W. E., Schiffmann E. Identification of a chemoattractant for fibroblasts produced by human breast carcinoma cell lines. Cancer Res. 1984 Aug;44(8):3398–3402. [PubMed] [Google Scholar]
- Haggie J. A., Sellwood R. A., Howell A., Birch J. M., Schor S. L. Fibroblasts from relatives of patients with hereditary breast cancer show fetal-like behaviour in vitro. Lancet. 1987 Jun 27;1(8548):1455–1457. doi: 10.1016/s0140-6736(87)92206-9. [DOI] [PubMed] [Google Scholar]
- Horgan K., Jones D. L., Mansel R. E. Mitogenicity of human fibroblasts in vivo for human breast cancer cells. Br J Surg. 1987 Mar;74(3):227–229. doi: 10.1002/bjs.1800740326. [DOI] [PubMed] [Google Scholar]
- Iqbal M. J., Corbishley T. P., Wilkinson M. L., Williams R. A microassay for the determination of binding parameters of estrogen and androgen receptors employing affinity immobilization on Cibacron blue 3GA-Sepharose 6B. Anal Biochem. 1985 Jan;144(1):79–85. doi: 10.1016/0003-2697(85)90086-7. [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Dillard P. J., Sporn M. B., Roberts A. B. Complementary deoxyribonucleic acid cloning of a messenger ribonucleic acid encoding transforming growth factor beta 4 from chicken embryo chondrocytes. Mol Endocrinol. 1988 Dec;2(12):1186–1195. doi: 10.1210/mend-2-12-1186. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Collins M. M., Rowsby L., Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977 Nov;75(2):305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Kondaiah P., Van Obberghen-Schilling E., Ludwig R. L., Dhar R., Sporn M. B., Roberts A. B. cDNA cloning of porcine transforming growth factor-beta 1 mRNAs. Evidence for alternate splicing and polyadenylation. J Biol Chem. 1988 Dec 5;263(34):18313–18317. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Krycève-Martinerie C., Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984 Oct;121(1):184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Dotzlaw H. Variant estrogen receptor mRNA species detected in human breast cancer biopsy samples. Mol Endocrinol. 1989 Apr;3(4):687–693. doi: 10.1210/mend-3-4-687. [DOI] [PubMed] [Google Scholar]
- Pircher R., Lawrence D. A., Jullien P. Latent beta-transforming growth factor in nontransformed and Kirsten sarcoma virus-transformed normal rat kidney cells, clone 49F. Cancer Res. 1984 Dec;44(12 Pt 1):5538–5543. [PubMed] [Google Scholar]
- Skidmore J., Walpole A. L., Woodburn J. Effect of some triphenylethylenes on oestradiol binding in vitro to macromolecules from uterus and anterior pituitary. J Endocrinol. 1972 Feb;52(2):289–298. doi: 10.1677/joe.0.0520289. [DOI] [PubMed] [Google Scholar]
- Sutherland R. L. Estrogen antagonists in chick oviduct: antagonist activity of eight synthetic triphenylethylene derivatives and their interactions with cytoplasmic and nuclear estrogen receptors. Endocrinology. 1981 Dec;109(6):2061–2068. doi: 10.1210/endo-109-6-2061. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Masui T., Harris C. C., Sporn M. B. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C. K., Murphy L. C., Sutherland R. L. Microsomal binding sites for nonsteroidal anti-estrogens in MCF 7 human mammary carcinoma cells. Demonstration of high affinity and narrow specificity for basic ether derivatives of triphenylethylene. J Biol Chem. 1984 Apr 10;259(7):4223–4229. [PubMed] [Google Scholar]
- ten Dijke P., Hansen P., Iwata K. K., Pieler C., Foulkes J. G. Identification of another member of the transforming growth factor type beta gene family. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4715–4719. doi: 10.1073/pnas.85.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]