Abstract
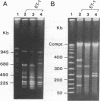
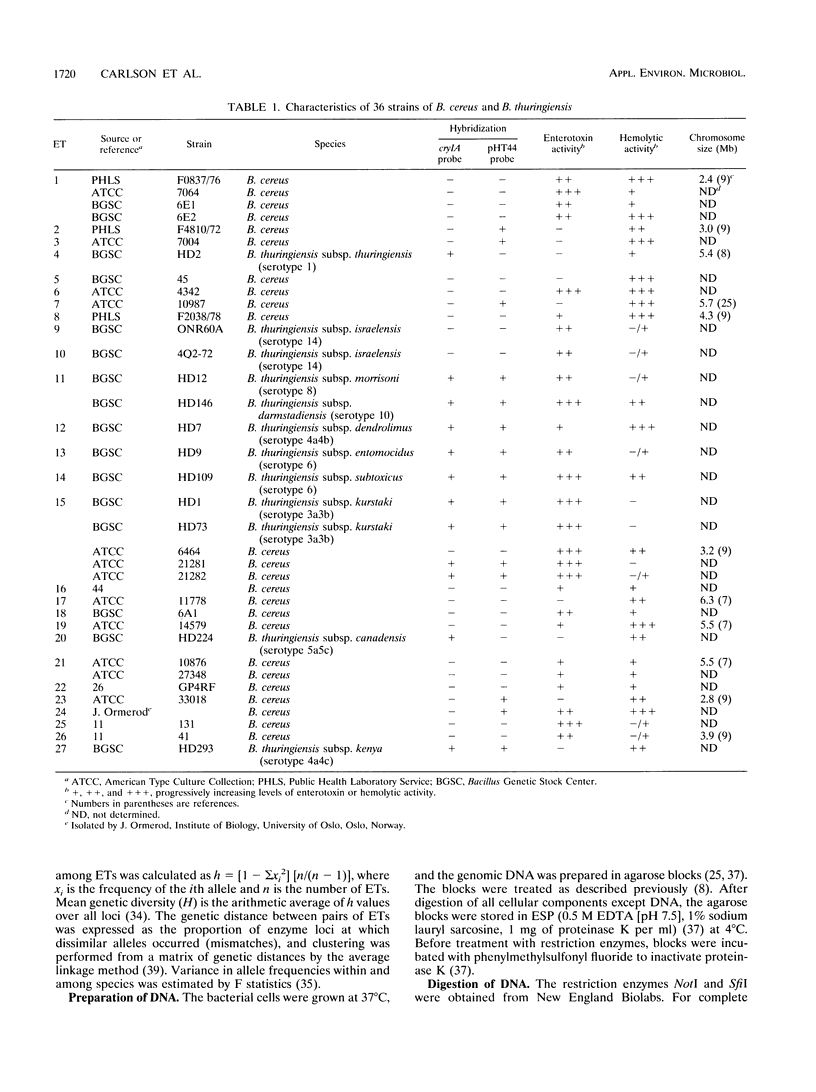
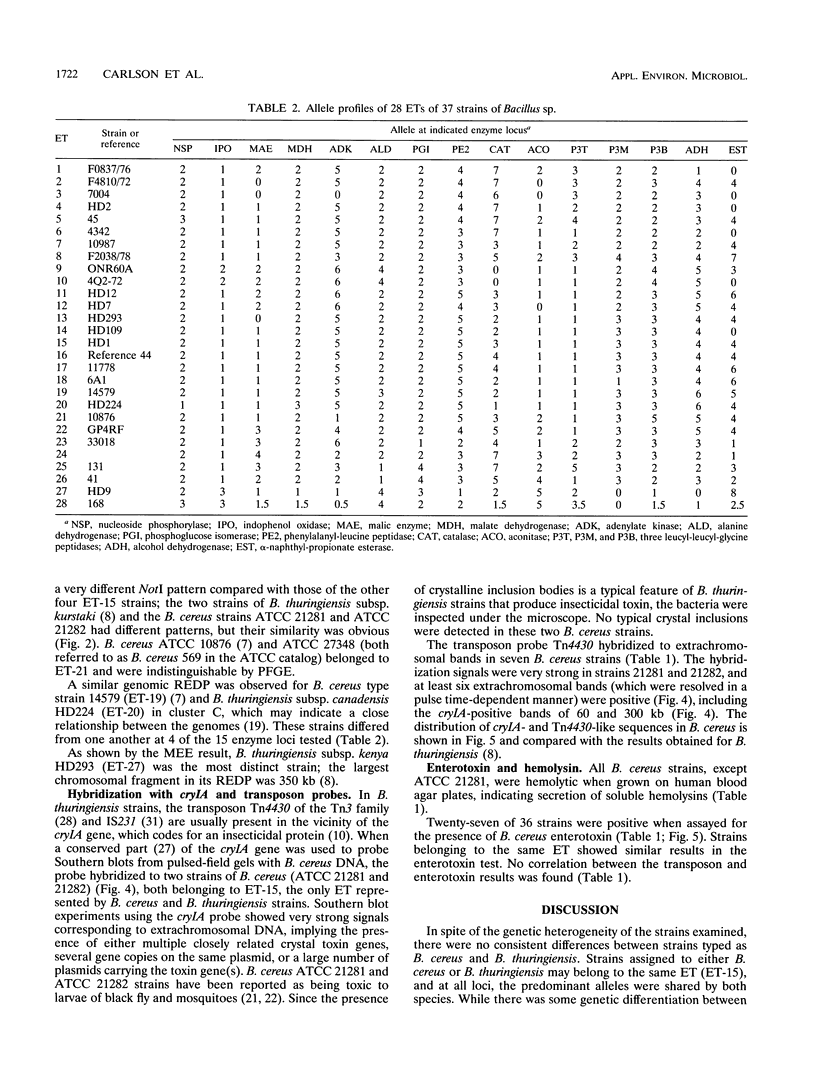
Twenty-four strains of Bacillus cereus were analyzed by pulsed-field gel electrophoresis (PFGE) and compared with 12 Bacillus thuringiensis strains. In addition, the 36 strains were examined for variation in 15 chromosomal genes encoding enzymes (by multilocus enzyme electrophoresis [MEE]). The genome of each strain had a distinct NotI restriction enzyme digestion profile by PFGE, and the 36 strains could be assigned to 27 multilocus genotypes by MEE. However, neither PFGE nor MEE analysis could distinguish between the two species. Two of the B. cereus strains contained extrachromosomal DNA that hybridized to a cryIA insecticidal toxin probe, and seven strains contained DNA with homology to a Tn4430 transposon probe derived from B. thuringiensis. The results strongly indicate that B. cereus and B. thuringiensis should be regarded as one species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Michaux-Charachon S., Jumas-Bilak E., Karayan L., Ramuz M. Presence of one linear and one circular chromosome in the Agrobacterium tumefaciens C58 genome. J Bacteriol. 1993 Dec;175(24):7869–7874. doi: 10.1128/jb.175.24.7869-7874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeit R. D., Arthur M., Dunn R., Kim C., Selander R. K., Goldstein R. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed field electrophoresis to molecular epidemiology. J Infect Dis. 1990 Feb;161(2):230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- Baril C., Richaud C., Baranton G., Saint Girons I. S. Linear chromosome of Borrelia burgdorferi. Res Microbiol. 1989 Oct;140(8):507–516. doi: 10.1016/0923-2508(89)90083-1. [DOI] [PubMed] [Google Scholar]
- Burns D. N., Wallace R. J., Jr, Schultz M. E., Zhang Y. S., Zubairi S. Q., Pang Y. J., Gibert C. L., Brown B. A., Noel E. S., Gordin F. M. Nosocomial outbreak of respiratory tract colonization with Mycobacterium fortuitum: demonstration of the usefulness of pulsed-field gel electrophoresis in an epidemiologic investigation. Am Rev Respir Dis. 1991 Nov;144(5):1153–1159. doi: 10.1164/ajrccm/144.5.1153. [DOI] [PubMed] [Google Scholar]
- Carlson C. R., Grønstad A., Kolstø A. B. Physical maps of the genomes of three Bacillus cereus strains. J Bacteriol. 1992 Jun;174(11):3750–3756. doi: 10.1128/jb.174.11.3750-3756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. R., Kolstø A. B. A complete physical map of a Bacillus thuringiensis chromosome. J Bacteriol. 1993 Feb;175(4):1053–1060. doi: 10.1128/jb.175.4.1053-1060.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansson A., Naidu A. S., Nilsson I., Wadström T., Pettersson H. E. Toxin production by Bacillus cereus dairy isolates in milk at low temperatures. Appl Environ Microbiol. 1989 Oct;55(10):2595–2600. doi: 10.1128/aem.55.10.2595-2600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- González J. M., Jr, Brown B. J., Carlton B. C. Transfer of Bacillus thuringiensis plasmids coding for delta-endotoxin among strains of B. thuringiensis and B. cereus. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6951–6955. doi: 10.1073/pnas.79.22.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J. M., Jr, Carlton B. C. A large transmissible plasmid is required for crystal toxin production in Bacillus thuringiensis variety israelensis. Plasmid. 1984 Jan;11(1):28–38. doi: 10.1016/0147-619x(84)90004-0. [DOI] [PubMed] [Google Scholar]
- González J. M., Jr, Carlton B. C. Patterns of plasmid DNA in crystalliferous and acrystalliferous strains of Bacillus thuringiensis. Plasmid. 1980 Jan;3(1):92–98. doi: 10.1016/s0147-619x(80)90038-4. [DOI] [PubMed] [Google Scholar]
- Harsono K. D., Kaspar C. W., Luchansky J. B. Comparison and genomic sizing of Escherichia coli O157:H7 isolates by pulsed-field gel electrophoresis. Appl Environ Microbiol. 1993 Sep;59(9):3141–3144. doi: 10.1128/aem.59.9.3141-3144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Nozaki R., Aizawa K. Deoxyribonucleic acid relatedness between Bacillus anthracis, Bacillus cereus and Bacillus thuringiensis. Microbiol Immunol. 1978;22(10):639–641. doi: 10.1111/j.1348-0421.1978.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Kinashi H., Shimaji M., Sakai A. Giant linear plasmids in Streptomyces which code for antibiotic biosynthesis genes. 1987 Jul 30-Aug 5Nature. 328(6129):454–456. doi: 10.1038/328454a0. [DOI] [PubMed] [Google Scholar]
- Kolstø A. B., Grønstad A., Oppegaard H. Physical map of the Bacillus cereus chromosome. J Bacteriol. 1990 Jul;172(7):3821–3825. doi: 10.1128/jb.172.7.3821-3825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft J., Berger H., Härtlein M., Müller B., Weidinger G., Goebel W. Cloning and expression in Escherichia coli and Bacillus subtilis of the hemolysin (cereolysin) determinant from Bacillus cereus. J Bacteriol. 1983 Aug;155(2):681–689. doi: 10.1128/jb.155.2.681-689.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J. W., Schnepf H. E., Whiteley H. R. Diversity of locations for Bacillus thuringiensis crystal protein genes. J Bacteriol. 1983 Apr;154(1):419–428. doi: 10.1128/jb.154.1.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lereclus D., Mahillon J., Menou G., Lecadet M. M. Identification of Tn4430, a transposon of Bacillus thuringiensis functional in Escherichia coli. Mol Gen Genet. 1986 Jul;204(1):52–57. doi: 10.1007/BF00330186. [DOI] [PubMed] [Google Scholar]
- Logan N. A., Berkeley R. C. Identification of Bacillus strains using the API system. J Gen Microbiol. 1984 Jul;130(7):1871–1882. doi: 10.1099/00221287-130-7-1871. [DOI] [PubMed] [Google Scholar]
- Mahillon J., Seurinck J., van Rompuy L., Delcour J., Zabeau M. Nucleotide sequence and structural organization of an insertion sequence element (IS231) from Bacillus thuringiensis strain berliner 1715. EMBO J. 1985 Dec 30;4(13B):3895–3899. doi: 10.1002/j.1460-2075.1985.tb04163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G., Goodfellow M., Todd C. A numerical classification of the genus Bacillus. J Gen Microbiol. 1988 Jul;134(7):1847–1882. doi: 10.1099/00221287-134-7-1847. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa K., Sugiyama J., Terada T., Matsusaka N., Sugii S. Improved methods for purification of an enterotoxin produced by Bacillus cereus. FEMS Microbiol Lett. 1991 May 1;64(1):1–5. doi: 10.1016/0378-1097(91)90199-k. [DOI] [PubMed] [Google Scholar]
- Sobral B. W., Honeycutt R. J., Atherly A. G., McClelland M. Electrophoretic separation of the three Rhizobium meliloti replicons. J Bacteriol. 1991 Aug;173(16):5173–5180. doi: 10.1128/jb.173.16.5173-5180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Chang N., Taylor D. E. Pulsed-field gel electrophoresis of Campylobacter jejuni and Campylobacter coli genomic DNA and its epidemiologic application. J Infect Dis. 1991 May;163(5):1068–1072. doi: 10.1093/infdis/163.5.1068. [DOI] [PubMed] [Google Scholar]
- Zahner V., Momen H., Salles C. A., Rabinovitch L. A comparative study of enzyme variation in Bacillus cereus and Bacillus thuringiensis. J Appl Bacteriol. 1989 Sep;67(3):275–282. doi: 10.1111/j.1365-2672.1989.tb02496.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mazurek G. H., Cave M. D., Eisenach K. D., Pang Y., Murphy D. T., Wallace R. J., Jr DNA polymorphisms in strains of Mycobacterium tuberculosis analyzed by pulsed-field gel electrophoresis: a tool for epidemiology. J Clin Microbiol. 1992 Jun;30(6):1551–1556. doi: 10.1128/jcm.30.6.1551-1556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Comfurius P., van Deenen L. L. Complete purification and some properties of phospholipase C from Bacillus cereus. Biochim Biophys Acta. 1971 Apr 13;233(2):474–479. doi: 10.1016/0005-2736(71)90347-6. [DOI] [PubMed] [Google Scholar]