Abstract
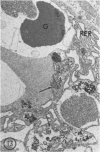
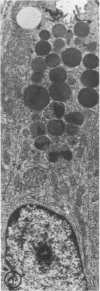
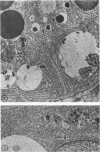
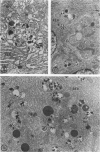
The effects of methylmercury on the intestinal epithelium were studied in 14 adolescent male Macaca mulatta monkeys weighing 3 to 5 kg. They were divided into three groups: two controls received daily applesauce vehicle without methylmercury. Nine chronic low-dose animals received 0.2 to 1.0 mg of methylmercury per day for 80 to 491 days. Three acute high-dose animals received 2.0 mg methylmercury for 17 to 18 days, when they became terminally ill. Light and electron microscopic observations were made on samples of duodenum and ileum following perfusion and immersion fixation in a glutaraldehyde-paraformaldehyde fixative. Numerous uniquely structured inclusions were prominent in the Paneth cells of the chronic low-dose animals and some necrotic Paneth cells were seen, especially in the most chronic and higher dosed animals of the group. Acute high-dose treatment produced some inclusions in the Paneth cells similar to those of the chronic low-dose group, but degenerative and necrotic cells were more frequently seen. These alterations were not seen in other intestinal epithelial cells. Paneth cells are selectively altered. These findings suggest that a function of Paneth cells may be to eliminate metals from the body.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fowler B. A. The morphologic effects of dieldrin and methyl mercuric chloride on pars recta segments of rat kidney proximal tubules. Am J Pathol. 1972 Oct;69(1):163–178. [PMC free article] [PubMed] [Google Scholar]
- Fowler B. A. Ultrastructural evidence for nephropathy induced by long-term exposure tosmall amounts of methyl mercury. Science. 1972 Feb 18;175(4023):780–781. doi: 10.1126/science.175.4023.780. [DOI] [PubMed] [Google Scholar]
- Gritzka T. L., Trump B. F. Renal tubular lesions caused by mercuric chloride. Electron microscopic observations: degeneration of the pars recta. Am J Pathol. 1968 Jun;52(6):1225–1277. [PMC free article] [PubMed] [Google Scholar]
- Halbhuber K. J., Stibenz H. J., Halbhuber U., Geyer G. Autoradiographische Untersuchungen über die Verteilung einiger Metallisotope im Darm von Laboriumstieren. Ein Beitrag zur Ausschiedungsfunktion der Panethsachen Körnerzellen. Acta Histochem. 1970;35(2):307–319. [PubMed] [Google Scholar]
- Holmes E. J. Neoplastic Paneth cells; their occurrence in 2 adenomas and one carcinoma of the colon. Cancer. 1965 Nov;18(11):1416–1422. doi: 10.1002/1097-0142(196511)18:11<1416::aid-cncr2820181105>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Klein R., Herman S. P., Bullock B. C., Talley F. A. Methyl mercury intoxication in rat kidneys. Functional and pathological changes. Arch Pathol. 1973 Aug;96(2):83–90. [PubMed] [Google Scholar]
- Lewin K. The Paneth cell in disease. Gut. 1969 Oct;10(10):804–811. doi: 10.1136/gut.10.10.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet N. K., Body R. L. Mercury burden of human autopsy organs and tissues. Arch Environ Health. 1974 Jul;29(1):18–24. doi: 10.1080/00039896.1974.10666520. [DOI] [PubMed] [Google Scholar]
- Nabarra B., Galle P., Andrianarison I., Lebois E. Apport du micro-analyseur à sonde électronique dans l'étude des lésions rénales provoquées par l'intoxication aiguë mercurielle. Kidney Int. 1973 Sep;4(3):208–215. doi: 10.1038/ki.1973.102. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Phillips F. S., Sternberg S. S. The lethal actions of antitumor agents in proliferating cell systems in vivo. Am J Pathol. 1975 Oct;81(1):205–218. [PMC free article] [PubMed] [Google Scholar]
- Prick J. J., Sonnen A. E., Slooff J. L. Organic mercury poisoning. I. Proc K Ned Akad Wet C. 1967;70(2):152–169. [PubMed] [Google Scholar]
- Shaw C. M., Mottet N. K., Body R. L., Luschei E. S. Variability of neuropathologic lesions in experimental methylmercurial encephalopathy in primates. Am J Pathol. 1975 Sep;80(3):451–470. [PMC free article] [PubMed] [Google Scholar]
- Staley M. W., Trier J. S. Morphologic heterogeneity of mouse Paneth cell granules before and after secretory stimulation. Am J Anat. 1965 Nov;117(3):365–383. doi: 10.1002/aja.1001170305. [DOI] [PubMed] [Google Scholar]
- TRIER J. S. STUDIES ON SMALL INTESTINAL CRYPT EPITHELIUM. I. THE FINE STRUCTURE OF THE CRYPT EPITHELIUM OF THE PROXIMAL SMALL INTESTINE OF FASTING HUMANS. J Cell Biol. 1963 Sep;18:599–620. doi: 10.1083/jcb.18.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner P. G. Cytology of intestinal epithelial cells. Int Rev Cytol. 1968;24:233–343. doi: 10.1016/s0074-7696(08)61401-1. [DOI] [PubMed] [Google Scholar]
- Verity M. A., Brown W. J. Hg2 plus-induced kidney necrosis. Subcellular localization and structure-linked lysosomal enzyme changes. Am J Pathol. 1970 Oct;61(1):57–74. [PMC free article] [PubMed] [Google Scholar]
- Ware R. A., Burkholder P. M., Chang L. W. Ultrastructural changes in renal proximal tubules after chronic organic and inorganic mercury intoxication. Environ Res. 1975 Aug;10(1):121–140. doi: 10.1016/0013-9351(75)90078-x. [DOI] [PubMed] [Google Scholar]