Abstract
In a prior study, we showed that the few striatal projection neurons that contain both substance P (SP) and enkephalin (ENK) in rats may preferentially project to the substantia nigra pars compacta. Since striatal neurons that project to the pars compacta are thought to preferentially reside in the striosomal compartment, we investigated if striatal neurons that contain both SP and ENK are preferentially localized to the patch compartment. We used in situ hybridization histochemistry to double-label sections for SP and ENK to identify SP/ENK co-containing neurons, and immunolabeling of adjacent sections for the mu opiate receptor (MOR) to define the striosomal compartment. We found that 32.3% of neurons containing both SP and ENK were localized to the striosomal compartment, which itself only made up 12.8% of the striatum. Our results further showed that the density of neurons co-containing SP and ENK was three-fold higher in striosomes than in the matrix compartment. These results are consistent with the notion that SP/ENK colocalizing neurons preferentially project to pars compacta, and these and our prior results additionally raise the possibility that neurons of this type in the striatal matrix may also project to the pars compacta.
Keywords: Striatum, Substance P, Enkephalin, Projection Neuron, Colocalization
Introduction
Models of mammalian basal ganglia organization have dichotomized striatal projection neurons into two neurochemically and functionally distinct types: neurons containing substance P (SP) that project to the internal pallidal segment (GPi), the substantia nigra pars reticulata (SNr), and/or the substantia nigra pars compacta (SNc), and neurons containing enkephalin (ENK) that project to the external pallidal segment (GPe) [1, 4, 6, 8, 11, 13, 16, 23, 26]. The view that striatal projection neurons consist of two major neurochemically distinct types was based on immunohistochemical and ISHH (in situ hybridization histochemistry) double-label studies that reported low incidence of SP colocalization with ENK in individual striatal perikarya [2, 3, 5, 14, 18, 19] and immunolabeling studies that reported low incidence of SP colocalization with ENK in fibers and terminals in striatal targets [2, 16, 21, 24].
Some recent findings, however, raised the possibility that the dichotomy of striatal projection neurons into two types (an SP+ type and an ENK+ type) might be overly simplified, and that a third neurochemically distinct type that co-contains SP and ENK might be common [7, 12, 14, 27, 31, 33, 35]. In a recent study [36], we used single-cell RT-PCR (scRT-PCR), ISHH, and immunolabeling to address this issue, and found that SP/ENK colocalizing striatal neurons, represent a small fraction of striatal projection neurons in rats (4% in adults and 10% in juveniles). Our results suggested that these neurons may have the SNc as their main project target, based on the presence of SP/ENK colocalizing terminals in SNc but not other striatal target areas. Since striato-SNc neurons reportedly reside in striosomes, which comprise about 10−15% of striatal neurons [15, 16, 17], one would expect SP/ENK colocalizing neurons to be preponderantly found in the striosomes, if they in fact preferentially target SNc. In the present study, we used ISHH double-labeling for SP and ENK in combination with MOR immunolabeling of adjacent sections to define striosomes to examine this issue. We found that SP/ENK neurons are overrepresented in striosomes and may thus be a subset of striato-SNc neurons.
Materials and Methods
Four 4-month old rats were used for the present study. Note that we use primate terminology for the pallidal segments in rat, as per Paxinos and Watson [25], because of the accepted homology of the nonprimate globus pallidus to the primate GPe and of the nonprimate entopeduncular nucleus to the primate GPi. Double-label ISHH was performed on 20μm thick fresh frozen cryostat sections from the four Sprague-Dawley rats. The sections were collected onto precleaned Superfrost®/Plus microscope slides as they were sectioned, dried on a slide warmer and stored at −80°C until used for ISHH. To process the tissue for ISHH, the slides were removed from the −80°C, quickly thawed and dried by a hair dryer. After fixation with 2% paraformaldehyde in saline sodium citrate (2× SSC) for 5 minutes, the sections were acetylated with 0.25% acetic anhydride/0.1M triethanolamine hydrochloride (pH 8.0) for 10 minutes, dehydrated through a graded ethanol series, and air-dried. Digoxigenin-UTP labeled and 35S-UTP labeled cRNA probes (i.e. riboprobes) for preproenkephalin (PPE) and preprotachykinin (PPT), respectively, were transcribed from plasmids with PPE cDNA or PPT cDNA inserts (935bp and 475bp in size, respectively), generously provided by W.S Young of NIMH [30]. For double-labeling, 35S -UTP-labeled PPT riboprobe and digoxigenin-UTP-labeled PPE riboprobe were mixed and applied to individual section. In our prior study [36], we found this combination to be more effective than digoxigenin-PPT and 35S-PPE. For the double-label hybridization, the sections were incubated with digoxigenin (DIG)-labeled and/or 35S -labeled (1×107 dpm/ml) probe(s) in hybridization buffer containing 50% formamide, 4× SSC, 1× Denhardt's solution, 200μg/ml denatured salmon sperm DNA, 250μg/ml yeast tRNA, 10% dextran sulfate, and 20mM DTT at 58°C overnight. After hybridization, the slices were washed at 55°C in 4× SSC, 50% formamide with 2× SSC, 2× SSC, treated with RNase A (20μg/ml) for 30 min at 37°C, and washed in 0.5× SSC at 55°C. Sections were then dehydrated through a graded ethanol series, and air-dried. Digoxigenin labeling was detected using anti-digoxigenin Fab fragments conjugated to alkaline phosphatase (AP), as visualized with nitroblue tetrazolium (NBT) histochemistry (Roche, Indianapolis, IN). Autoradiographic (ARG) labeling was detected by coating slides with Ilford-K5D emulsion (Polysciences, Warrington, PA), keeping them in the dark for 28 days at 4°C, developing them in Kodak D-19 developer (Kodak, Rochester, NY), and fixing them in Kodak fixer. Detection of the radioactive signal was carried out first, followed by digoxigenin detection. Sections were coverslipped with a 1% gelatin-based aqueous solution when both labeling steps were complete.
The distribution and abundance of SP/ENK colocalizing neurons were determined by using Neurolucida-assisted reconstructions of labeled perikarya. For these reconstructions, the contours of the striatum for individual double-labeled sections were outlined at low power using a Neurolucida system (Microbright Field Inc, Vermont) connected to a Nikon Optiphot-2 microscope with a motorized stage, and SP/ENK double-labeled neurons were subsequently plotted as they were encountered during a systematic examination of the striatum using a 20× objective. Neurons with clear DIG signal for ENK overlain with at least 3× above background ARG signal for SP were considered double-labeled, as in our prior study [35]. The reconstructions of striatum with double-labeled perikarya were viewed and printed using NeuroExplorer (MicroBright Field Inc). Canvas software was used to render one of these reconstructions as an illustration for the present paper.
Sections adjacent to those used for the ISHH were immunolabeled for MOR, to identify the striatal patch compartment. The sections used for MOR immunolabeling were air-dried, and fixed by 5 minute immersion in 4% paraformaldehyde, 5 minutes in Bouin's solution and 5 minutes in 80% ethanol. Following rinsing and drying, peroxidase-antiperoxidase single-labeling was carried out on-the-slide, using a commercially available guinea pig polyclonal anti-mu opiate receptor (MOR) antibody (1:1000, Neuromics, Minneapolis, MN), whose specificity has been established previously [9, 34]. Our on-the-slide immunolabeling methods have also been described previously [9, 10, 28]. The distribution of striosomes in the adjacent section was mapped using an overhead projector at the same magnification as the print for each Neurolucida reconstruction. The Neurolucida mapping of SP/ENK neurons and the mapping of striosomes were then aligned for each pair of adjaecent sections, and the abundance of SP/ENK cells in striosome and matrix thereby was determined for each ISHH mapping. Three-four section pairs were typically bilaterally mapped for each rat analyzed. The relative areal extent of striosome and matrix for each MOR-immunolabeled section was determined by digitizing the mapping with a scanner and using NIH Image to measure matrix and striatal area. The regional density of SP/ENK neurons in striosomes and matrix was thereby calculated. A student's t-test was used to assess the statistical significance of the difference between striosomes and matrix in the density of SP/ENK neurons, with a probability of p<0.05 considered significant.
Results
We performed ISHH double-labeling for SP and ENK, using digoxigenin-labeled riboprobes for ENK mRNA and 35S-labeled riboprobes for SP mRNA (Fig. 1). Our ISHH studies revealed a patchy distribution of the SP/ENK neurons in striatum, and clusters of SP/ENK neurons frequently overlapped striosomes (Fig. 1). We counted double-labeled neurons in striatal sections double-labeled by ISHH (DIG for ENK and ARG for SP). Comparison of the SP/ENK Neurolucida mappings to the adjacent MOR-immunnolabeled sections confirmed that apparent SP/ENK clusters commonly coincided with striosomes (Figs. 1, 2). Quantification of SP/ENK neuron frequency in the two compartments revealed that 32.3% of the SP/ENK neurons were in the striosomal compartment, while striosomes made up only 12.8% of the areal extent of the striatum. The regional density of SP/ENK neurons was thus more than three-fold higher in striosomes than in matrix: 7.23 ± 1.45 neurons/mm2 in striosomes versus 2.02 ± 0.24 neurons/mm2 in matrix. This difference was significant (p=0.026) by a student's t-test.
1.
Schematic drawing of a transverse section through the striatum of a 4 month old rat that had been double-labeled by ISHH for SP (PPT) by autoradiographic ISHH and for ENK (PPE) by DIG-ISHH, overlain with the distribution of MOR+ striosomes as visualizrd by immunolabeling of the adjacent section. Perikarya labeled by ISHH for both SP and ENK are shown as dots, while the striosomes are shown as irregular enclosed contours. Abbreviations: AC – anterior commissure; CC – corpus callosum; V – ventricle.
2.
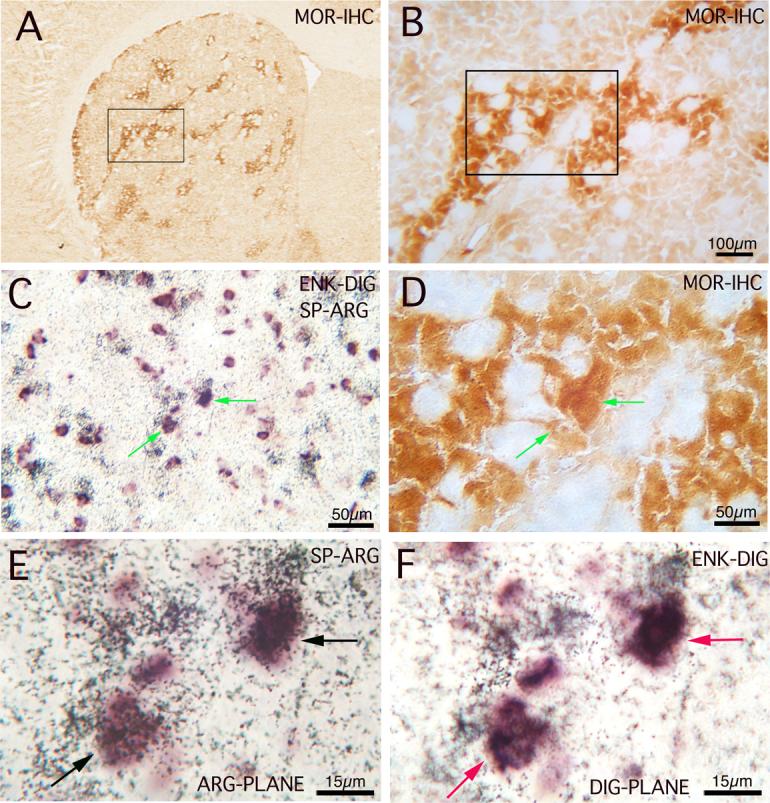
Images of striatum in a 4 month old rat in adjacent coronal sections that had been either immunolabeled for MOR (A, B, D) or double-labeled for SP (PPT) and ENK (PPE), using ARG to visualize the SP and DIG to visualize the ENK (C, E, F). The MOR images show successively higher power views of the region of two double-labeled neurons (arrows) shown in C, E, F, with the box in A showing the field enlarged in B, and the box in B showing the field enlarged in D. C and D are at the same magnification and show the alignment of the two double-labeled neurons with the striosomal compartment. The images E and F show two focal planes of view, one focusing on the ARG silver-grain labeling (E), and one focusing on the DIG labeling (F). E and F are at the same magnification.
Discussion
Our results show that while SP/ENK neurons are not an abundant striatal cell type in rats (10% in juveniles, 4% in adults), they appear to represent many among the striosomal neurons that target the substantia nigra pars compacta. Double-label ISHH is affected by several problems that potentially affect interpretation. First, combining DIG and ARG labeling diminishes the DIG labeling. Secondly, with heavy silver grain ARG labeling over a cell, the underlying DIG labeling of the cell can be obscured, making it more difficult to determine if an ARG-labeled cell is also DIG-labeled. To a large extent, high-power viewing (which we employed) can overcome this difficulty, but reduced DIG labeling when combined with ARG could reduce the frequency of double-labeled cell detected. Notwithstanding these possible problems, the frequencies of SP/ENK co-localizing cells detected by double-label ISHH by us matches that which we have detected by single-cell RT-PCR [36]. Thus, we believe our SP/ENK neuron detection reliably reflects their abundance in rat striatum.
Previously, we observed SP/ENK colocalization in fibers and terminals in the pars compacta and the ventral tegmental area in young rats [35]. Since striato-SNc neurons reportedly reside in striosomes, which comprise about 10−15% of striatal neurons [15, 16, 17], one would therefore expect some or all SP/ENK striatal neurons to reside in striosomes. We did find in the present study that 32.3% of SP/ENK neurons reside in striosomes. Given their frequency in striatum overall and the magnitude of the striosomal compartment, this observation indicates that SP/ENK neurons make up about 10% of the striosomal neurons in adult rats, and thus presumably at least about 10% of striato-SNc neurons. The presence of SP/ENK neurons in the matrix, however, raises the possibility that some extrastriosomal neurons also project to the pars compacta and ventral tegmental area. The SP/ENK neurons projecting to SNc appear to be only one of several neurochemically distinct types projecting to SNc. Other types include neurons co-containing ENK and neurotensin [32], and neurons co-containing SP and dynorphin [2, 29, 36]. How these various types may differ in their functional properties is unknown, but is likely that the perikarya of both ENK/neurotensin and SP/dynorphin striato-SNc neurons make up the remaining projection neurons found in striosomes. Although SP/ENK striatal neurons may be infrequent in rodents, this does not rule out the possibility they are more common in other species, for example primates, in which considerable overlap of SP+ and ENK+ fibers occurs in the ventral tegmental area, SNc and medial SNr [27]. Finally, delineation of striatal projection neurons into neurochemically distinct types does not assume each projects to only one target. For example, direct pathway striatal neurons appear to have a major target (GPi or SNr), and collateral targets as well (GPe, GPi and/or SNr) [4, 11, 20, 22, 23, 27].
Acknowledgements
The authors wish to thank Drs. W.E. Armstrong, and Y. Tong for helpful comments and assistance during the course of this study, and we are grateful to Aminah Henderson for technical assistance. This research was supported by NS-19620 and NS-28721 (A.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD, Reiner A. The extensive co-occurrence of substance P and dynorphin in striatal projection neurons: An evolutionarily conserved feature of basal ganglia organization. J. Comp. Neurol. 1990;295:339–369. doi: 10.1002/cne.902950302. [DOI] [PubMed] [Google Scholar]
- 3.Augood SJ, Westmore K, Emson PC. Phenotypic characterization of messenger mRNA-expressing cells in the neuroleptic-treated rat striatum: a detailed cellular co-expression study. Neuroscience. 1997;76:763–774. doi: 10.1016/s0306-4522(96)00449-6. [DOI] [PubMed] [Google Scholar]
- 4.Beckstead RM, Cruz CJ. Striatal axons to the globus pallidus, entopeduncular nucleus and substantia nigra come mainly from separate cell populations in cat. Neuroscience. 1986;19:147–158. doi: 10.1016/0306-4522(86)90012-6. [DOI] [PubMed] [Google Scholar]
- 5.Besson MJ, Graybiel AM, Quinn B. Coexpression of neuropeptides in the cat's striatum: An immunohistochemical study of substance P, dynorphin B and enkephalin. Neuroscience. 1990;39:33–58. doi: 10.1016/0306-4522(90)90220-x. [DOI] [PubMed] [Google Scholar]
- 6.Bolam JP, Bennett BD. Microcircuitry of the Neostriatum. In: Ariano MA, Surmeier DJ, editors. Molecular and Cellular Mechanisms of Neostriatal Function. Springer-Verlag; Berlin: 1995. pp. 1–19. [Google Scholar]
- 7.Chen Q, Veenman CL, Knopp K,, Yan Z, Medina L, Song WJ, Surmeier DJ, Reiner A. Evidence for the preferential localization of GluR1 subunits of AMPA receptors to the dendritic spines of medium spiny neurons in rat striatum. Neuroscience. 1998;83:749–761. doi: 10.1016/s0306-4522(97)00452-1. [DOI] [PubMed] [Google Scholar]
- 8.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 9.Deng YP, Xie JP, Wang HB, Lei WL, Chen Q, Reiner A. Differential localization of the GluR1 and GluR2 subunits of the AMPA-type glutamate receptor among striatal neuron types in rats. J Chem Neuroanat. 2007;33:167–92. doi: 10.1016/j.jchemneu.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. Differential loss of striatal projection systems in Huntington's disease: a quantitative immunohistochemical study. J Chem Neuroanat. 2004;27:143–64. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Feger J, Crossman AR. Identification of different subpopulations of neostriatal neurones projecting to globus pallidus or substantia nigra in the monkey: A retrograde fluorescence double-labeling study. Neurosci. Lett. 1984;49:7–12. doi: 10.1016/0304-3940(84)90127-7. [DOI] [PubMed] [Google Scholar]
- 12.Fusco FR, Chen Q, Lamoreaux WJ, Figueredo-Cardenas G, Jiao Y, Coffman J, Surmeier DJ, Honig MG, Carlock LR, Reiner A. Cellular localization of huntingtin in striatal and cortical neurons in rats: Lack of correlation with neuronal vulnerability in Huntington's disease. J. Neurosci. 1999;19:1189–1202. doi: 10.1523/JNEUROSCI.19-04-01189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerfen CR. The neostriatal mosaic multiple levels of compartmental organization in the basal ganglia. Ann. Rev. Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 14.Gerfen CR, Young WS., III Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: An in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- 15.Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: Compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc. Natl. Acad. Sci. U.S.A. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–253. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 17.Johnston JG, Gerfen CR, Haber SZ, van der Kooy D. Mechanisms of striatal pattern formation: conservation of mammalian compartmentalization. Dev. Brain Res. 1990;57:93–102. doi: 10.1016/0165-3806(90)90189-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee T, Kaneko T, Taki K, Mizuno N. Preprodynorphin-, preproenkephalin, and preprotachykinin-expressing neurons in the rat neostriatum: An analysis by immunocytochemistry and retrograde tracing. J. Comp. Neurol. 1997;386:229–244. doi: 10.1002/(sici)1096-9861(19970922)386:2<229::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: Sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J. Comp. Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 20.Levesque M, Parent A. The striatofugal fiber system in primates: A reevaluation of its organization based on single-axon tracing studies. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11888–11893. doi: 10.1073/pnas.0502710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marksteiner J, Saria A, Krause JE. Comparative distribution of neurokinin B, substance P- and enkephalin-like immunoreactivities and neurokinin B messenger RNA in the basal forebrain of the rat: evidence for neurochemical compartmentation. Neuroscience. 1992;51:107–120. doi: 10.1016/0306-4522(92)90475-h. [DOI] [PubMed] [Google Scholar]
- 22.Nadjar A, Brotchie JM, Guigoni C, Li Q, Zhou SB, Wang GJ, Ravenscroft P, Georges F, Crossman AR, Bezard E. Phenotype of striatofugal medium spiny neurons in Parkinsonian and dyskinetic nonhuman primates: A çall for a reappraisal of the functional organization of the basal ganglia. J. Neurosci. 2006;26:8653–8661. doi: 10.1523/JNEUROSCI.2582-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parent A, Smith Y, Filion M, Dumas J. Distinct afferents to the internal and external pallidal segments in the squirrel monkey. Neurosci. Lett. 1989;96:140–144. doi: 10.1016/0304-3940(89)90047-5. [DOI] [PubMed] [Google Scholar]
- 24.Parent A. Comparative Neurobiology of the Basal Ganglia. John Wiley & Sons; New York: 1986. [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fourth Edition Academic Press; New York: 1998. [Google Scholar]
- 26.Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res. Rev. 1998;28:235–284. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- 27.Reiner A, Medina L, Haber SN. The distribution of dynorphinergic terminals in striatal target regions in comparison to the distribution of substance P-containing and enkephalinergic terminals in monkeys and humans. Neuroscience. 1999;88:775–793. doi: 10.1016/s0306-4522(98)00254-1. [DOI] [PubMed] [Google Scholar]
- 28.Reiner A, Erichsen JT, Cabot JB, Evinger C, Fitzgerald MEC, Karten HJ. Neurotransmitter organization of the preganglionic projection to the avian ciliary ganglion. Vis. Neurosci. 1991;6:451–472. doi: 10.1017/s0952523800001310. [DOI] [PubMed] [Google Scholar]
- 29.Reiner A, Anderson KD. The patterns of neurotransmitter and neuropeptide co-occurrence among striatal projection neurons: Conclusions based on recent findings. Brain Res. Rev. 1990;15:251–265. doi: 10.1016/0165-0173(90)90003-7. [DOI] [PubMed] [Google Scholar]
- 30.Siegel RE, Young WS. Detection of preprocholecystokinin and preproenkephalin A mRNAs in rat brain by hybridization histochemistry using complementary RNA probes. Neuropeptides. 1985;6:573–580. doi: 10.1016/0143-4179(85)90121-0. [DOI] [PubMed] [Google Scholar]
- 31.Stefani A, Chen Q, Flores-Hernandez J, Jiao Y, Reiner A, Surmeier DJ. Physiological and molecular properties of AMPA/KA receptors expressed by striatal medium spiny neurons. Dev. Neurosci. 1998;20:242–252. doi: 10.1159/000017318. [DOI] [PubMed] [Google Scholar]
- 32.Sugimoto T, Mizuno N. Neurotensin in projection neurons of the striatum and nucleus accumbens, with reference to co-existence with enkephalin and GABA: An immunohistochemical study in the cat. J. Comp. Neurol. 1987;257:383–395. doi: 10.1002/cne.902570307. [DOI] [PubMed] [Google Scholar]
- 33.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svingos AL, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of mu-opioid receptors in rat nucleus accumbens: extrasynaptic plasmalemmal distribution and association with Leu5-enkephalin. J. Neurosci. 1996;16:4162–4173. doi: 10.1523/JNEUROSCI.16-13-04162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vorobjev VS, Sharonova IN, Haas HL, Sergeeva QA. Differential modulation of AMPA receptors by cyclothiazide in two types of striatal neurons. Eur. J. Neurosci. 2000;12:2871–2880. doi: 10.1046/j.1460-9568.2000.00175.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang HB, Laverghetta AV, Foehring R, Deng YP, Sun Z, Yamamoto K, Lei WL, Jiao Y, Reiner A. Single-cell RT-PCR, in situ hybridization histochemical, and immunohistochemical studies of substance P and enkephalin co-occurrence in striatal projection neurons in rats. J Chem Neuroanat. 2006;31:178–99. doi: 10.1016/j.jchemneu.2006.01.003. [DOI] [PubMed] [Google Scholar]



