Abstract
Flow-cytometric conditions for detection of lysosomal-associated membrane proteins (LAMPs) on the surface of recently degranulated cells were optimized for rhesus macaques and used to investigate the functional properties of rhesus cytomegalovirus (rhCMV)-specific CD8+ T lymphocytes with regards to cytotoxicity and interferon (IFN)-γ secretion in six asymptomatic CMV-seropositive rhesus macaques. Unlike humans, the rhesus macaque LAMP-1 protein CD107a underwent little or no endocytosis over a six to 18 hour stimulation period. Following in vitro stimulation, rhCMV-specific CD8+ T lymphocytes were heterogeneous with regards to the composition of cells positive for CD107a and/or IFN-γ, time to reach peak degranulation, and kinetics of IFN-γ secretion relative to degranulation. Responder CD8+ T lymphocytes that underwent degranulation without IFN-γ production (CD107a+IFN-γ−) were predominantly composed of terminally differentiated effectors (CD28−CD45RA+). Moreover, they had significantly lower frequencies of effector memory (CD28−CD45RA−) cells compared to the IFN-γ-secreting cells that did or did not undergo degranulation (CD107a+IFN-γ+ or CD107a−IFN-γ+). The perforin content of effector CD8+ T lymphocytes was significantly greater than that of effector memory CD8+ T lymphocytes in rhesus macaques, suggesting that they were more cytolytic. Our findings suggest that the composition of rhCMV-specific CD8+ T lymphocytes with regards to CD107a+IFN-γ− responders may be an important determinant of their ability to control CMV replication.
Keywords: Rhesus CMV, CD8+ T lymphocytes, SIV, CD107a, Degranulation, CTL
Introduction
Cytotoxic CD8+ T lymphocytes (CTL) are essential for the immune control of several viral pathogens. CTL recognize cells presenting viral peptides bound to surface MHC class I molecules. Upon TCR engagement with peptide-MHC class I complexes, activated CD8+ T lymphocytes exhibit several effector functions, including cytokine production and cytotoxicity. Cytotoxicity is mediated either by release of pre-formed cytolytic granules, or less frequently by a granule-independent pathway involving Fas/FasL interaction (Kagi et al., 1994; Barry and Bleackley, 2002). Lytic granules consisting of perforin and granzymes are contained in membrane-bound lysosomes coated with lysosomal-associated membrane proteins (LAMPs) (Peters et al., 1991). During the process of degranulation, the membrane of the secretory lysosomes fuses with the plasma membrane of the activated CD8+ T lymphocyte and the lysosomal granules are then released into the immunological synapse between the CD8+ T lymphocyte and its target (Fukuda, 1991). LAMPs, therefore, are not usually present on the surface of T cells but are exposed only during active degranulation. This property has recently been exploited for flow-cytometric identification of CTL at the single-cell level.(Betts et al., 2003)
Simultaneous characterization of cytolytic and cytokine-secreting effector functions at the single-cell level have shed light on the functional heterogeneity of virus-specific CD8+ CTL in mice and humans (Betts et al., 2004; Wolint et al., 2004; Lacey et al., 2005; Betts et al., 2006; Lacey et al., 2006; Mongkolsapaya et al., 2006). In this study, we have optimized the flow-cytometric technique for detection of degranulating CD8+ T lymphocytes in rhesus macaques, and used it to characterize the functional properties of rhesus cytomegalovirus (rhCMV)-specific CD8+ T lymphocytes. Our data show that while anti-human CD107a and CD107b antibodies can reliably detect degranulating T lymphocytes in rhesus macaques, the endocytosis properties of rhesus CD107a differ appreciably from their human counterpart. This difference led to modifications in the technique for detecting LAMPs in rhesus macaques and facilitated optimal detection of concurrent cytokine secretion in activated T lymphocytes. Concurrent detection of degranulation and IFN-γ secretion revealed heterogeneity in the composition of rhCMV-specific CD8+ T lymphocytes with regards to the proportion of cells exhibiting either one or both functions. Activated CD8+ T lymphocytes displaying evidence of degranulation without IFN-γ production differed in their memory phenotype from cells producing IFN-γ with or without degranulation, suggesting that these represent a functionally distinct population. These data reinforce the importance of using multiple effector functions to evaluate virus-specific CD8+ T lymphocytes.
Materials and methods
Animals
T cell responses to superantigen (SAg) and rhCMV CD8+ T cell epitopes were analyzed in six SIV-negative, CMV-seropositive rhesus macaques housed in the breeding colony at the New England Primate Research Center (Table I). All animals were maintained in accordance with federal and institutional guidelines of animal care (Anonymous, 1996).
Table I. rhCMV-specific CD8+ T lymphocyte responses in six CMV-seropositive SIV-negative rhesus macaques.
| Animal I.D. | Age in years | Target specificity of rhCMV-specific CD8+ T cells | Frequency of rhCMV-specific CD8+ T cells (%)d | ||
|---|---|---|---|---|---|
| rhCMV protein | a.a. residue | Peptide nomenclature | |||
| Mm105.93 | 13 | IE1a | 73-87 | IE1#19 | 1.2 ± 0.8 |
| IE1 | 329-336 | IE1#83L | 4.0 ± 2.3 | ||
| IE2 b | 463-477 | IE2#117 | 6.0 ± 4.4 | ||
| Mm212.87 | 19 | IE2 | 186-196 | IE2#46F | 1.5 ± 0.8 |
| IL10c | 121-135 | IL10#31 | 2.2 ± 0.8 | ||
| Mm247.87 | 19 | IE1 | 329-336 | IE1#83L | 4.2 ± 2.2 |
| Mm361.95 | 11 | IE1 | 245-259 | IE1#62 | 17.1 ± 4.4 |
| IE1 | 329-336 | IE1#83L | 3.3 ± 0.6 | ||
| IE2 | 199-213 | IE2#51 | 0.3 ± 0.1 | ||
| Mm111.91 | 15 | IE2 | 551-565 | IE2#139 | 0.8 ± 0.7 |
| Mm543.91 | 15 | IE1 | 217-231 | IE1#55 | 17.7 ± 10.6 |
| IE2 | 407-421 | IE2#103 | 2.9 ± 0.8 | ||
immediate early 1 protein.
immediate early 2 protein.
interleukin-10 protein.
mean ± standard deviation of two to five replicates.
Antibodies
Monoclonal antibodies (MAb) used for costimulation in the intracellular cytokine staining assay consisted of purified, azide-free anti-human CD28 MAb (clone CD28.2, BD Biosciences, San Jose, CA) and anti-CD49d MAb (clone 9F10, BD Biosciences), cross-linked with affinity-purified F(ab')2 fragments of goat anti-mouse IgG (Kierkegaard and Perry Lab, Inc., Gaithersburg, MD). The following directly conjugated anti-human MAb reagents were obtained from BD Biosciences: anti-CD3 FITC and APC (clone SP34), anti-CD3 PB and APC-Cy7 (clone SP34-2), anti-CD4 PE and PerCP (clone L200), anti-CD8 PerCP (clone SK1) and Alexa Fluor 700 (clone RPA-T8), anti-CD107a FITC and PE (clone H4A3), anti-CD107b FITC (clone H4B4), anti-CD63 FITC (clone H5C6), anti-CD95 APC (clone DX2), anti-CD28 FITC and PE (clone 28.2), anti-CD45RA PerCP and PE-Cy7 (clone L48), anti-IFN-γ APC (clone B27) and PE-Cy7 (clone 4S.B3), and anti-TNF-α FITC (clone Mab11). Anti-CD28 ECD (clone 28.2) was obtained from Beckman Coulter (Fullerton, CA). Anti-perforin FITC (clone Pf-344) was obtained from Mabtech (Mariemont, OH). Anti-granzyme B PE (clone GB12) was obtained from Caltag Labs (Burlingame, CA).
Stimulating antigens
Staphylococcus enterotoxin A (SEA) and enterotoxin B (SEB) were used together or alone at a final concentration of 100 ng/ml (Sigma, St. Louis, MO) for superantigen (SAg) stimulation. rhCMV-specific CD8+ T lymphocytes were stimulated with their cognate peptides (Table I). CD8+ T lymphocyte epitopes in the rhCMV immediate early 1 (IE1), immediate early 2 (IE2), phosphoprotein 65 (pp65), and interleukin-10 (IL10) proteins were mapped by the IFN-γ ELISPOT and intracellular cytokine staining assay using 8 to 15 amino acid (aa) long overlapping peptides (Table I and unpublished data). With the exception of IE1#83L and IE2#46F, epitopes were mapped to the 15 aa level. Peptides were synthesized by F-moc chemistry at the Massachusetts General Hospital peptide core facility (Charlestown, MA) using an automated peptide synthesizer (MBS 396, Advanced Chemtech). Individual lyophilized peptides were resuspended at 100 mg/ml in 100% DMSO (Sigma) and used at 1-2 μg/ml with a final DMSO concentration of <0.05%.
Isolation of PBMC
Blood collected in vacutainer tubes containing the anticoagulant heparin was subjected to centrifugation over a Ficoll Lymphocyte Separation Medium (MP Biomedicals, Aurora, OH) density gradient, washed twice in phosphate-buffered saline (PBS) and resuspended in RPMI 1640 medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Sigma), 10 mM HEPES (Mediatech, Inc.; Herndon, VA), 2 mM L-glutamine (Invitrogen), 50 U penicillin/ml and 50 μg streptomycin/ml (Invitrogen) (henceforth referred as R-10 medium). Assays were performed either on freshly isolated peripheral blood mononuclear cells (PBMC) used within 2 to 4 hours of cell preparation, or on PBMC cryopreserved in 90% FCS and 10% DMSO.
Intracellular cytokine staining (ICS) assay
PBMC were stimulated with the appropriate antigen in the presence of purified anti-CD28 and anti-CD49d costimulatory antibodies for all four-color flow cytometry experiments as previously described (Kaur et al., 2002). In experiments that included CD28 as a cell surface marker for delineating the memory phenotype of rhCMV-specific CD8+ T lymphocytes, only anti-CD49d was used for costimulation during the period of in vitro stimulation. For optimal stimulation, costimulatory antibodies were cross-linked in 12 × 75-mm polystyrene tubes precoated with 2.5 μg/ml of affinity purified F(ab')2 fragments of goat anti-mouse IgG suspended in 50 mM Tris pH 8.6 and kept overnight at 4°C. 1 × 106 PBMC were stimulated with SAg or rhCMV peptides for six hours or overnight (14-18 hours). Brefeldin A (Golgiplug, 1 μg/ml; BD Biosciences) was added one hour after the start of stimulation. In some experiments, brefeldin A was replaced with monensin (Golgistop, 2μM; BD Biosciences) for the duration of stimulation. PBMC were stimulated in tubes incubated at a 5° slant above horizontal at 37°C in a humidified 5% CO2 atmosphere. At the end of the stimulation period, cells were washed with PBS and incubated with 0.02% EDTA solution in DPBS (Sigma) for 15 min at 37°C. Subsequent washes were in PBS/2% FCS. Cells were washed and surface stained with two or more of the fluorochrome-conjugated antibodies to CD3, CD4, CD8, CD45RA, CD95 and CD28 at 4°C for 30 min. After surface staining, cells were washed and fixed using 100 μl of Fixation Medium A (Caltag) at RT for 15 min. After being washed, cells were incubated with 100 μl of Permeabilization Medium B (Caltag) and simultaneously stained with fluorochrome-conjugated antibodies specific for intracellular markers: IFN-γ, TNF-α, or perforin at RT for 30 min. After a final wash, cells were resuspended in 1% paraformaldehyde in PBS. Four-parameter flow cytometry samples were acquired on a FACSCalibur flow cytometer (BD Biosciences), while seven-color polychromatic flow cytometry samples were acquired on a LSRII flow cytometer (BD Biosciences). A minimum of 200,000 lymphocyte events was collected for analysis. Flow cytometry data files were analyzed using FlowJo software version 8.1.1 (Tree Star, San Carlos, CA). Flow cytometry gates to identify positive cytokine signal were based on unstimulated control tubes. Responses towards stimulating antigen were calculated after subtraction of background responses in unstimulated (medium alone) PBMC. The surface markers CD95, CD28 and CD45RA were used to characterize the memory phenotype of IFN-γ-secreting or degranulating CD8+ T cell populations. The Boolean gate platform was used to create the full array of possible combinations amongst all CD95+ memory cells. These equated to four phenotype patterns when using CD28 and CD45RA.
Flow cytometric detection of degranulation
In initial experiments, the protocol used for identification of degranulating CD8+ T lymphocytes in humans (Betts et al., 2003) was followed. Thus, fluorochrome conjugated antibodies to the LAMP proteins CD107a, CD107b, and CD63 were added at the beginning of stimulation along with monensin. At the end of the stimulation period, cells were washed, surface stained with antibodies to CD3, CD4, and CD8 as described previously, and fixed in 1% paraformaldehyde. Flow cytometry gates for positive CD107a and CD107b expression were based on unstimulated control tubes (Fig. 1). Modifications to this protocol for detecting degranulating cells in rhesus macaques are described under the results section.
Figure 1. Expression of LAMPs on T lymphocytes in rhesus macaques.
PBMC were stimulated for six hours with (A) superantigen (SAg) and (B) rhCMV peptide IE1#83L in the presence of monensin and antibodies to CD107a (FITC), CD107b (PE) or CD63 (FITC) antibodies. Lymphocytes gated on CD8+ and CD4+ T cells are shown. Numbers denote frequency of the gated population expressing the corresponding LAMP.
Statistical analysis
Statistical analysis was performed using PRISM version 4.0 (GraphPad Software Inc., San Diego, CA). Correlation between surface CD107a and perforin expression was analyzed by the nonparametric Spearman Rank correlation test. The nonparametric Wilcoxon signed rank test was used for all paired comparisons. These included comparison of surface CD107a expression under different experimental conditions, and comparison of granzyme B and perforin content in effector and effector memory CD8+ T lymphocytes. Differences in the memory phenotype of the three responder populations, CD107a+IFN-γ−, CD107a−IFN-γ+ and CD107a+IFN-γ+, were analyzed by the repeated measures ANOVA and the Bonferroni post-hoc test.
Results
LAMPs are expressed on the surface of degranulating CD8+ T lymphocytes in rhesus macaques
We first tested whether antibodies to three human LAMP proteins, CD107a, CD107b and CD63 recognize their rhesus counterparts. CD107a and CD107b molecules were detected on the surface of rhesus macaque CD8+ and CD4+ T lymphocytes stimulated with SAg, but not on resting T lymphocytes (Fig. 1A). Unlike CD107a and CD107b, the LAMP protein CD63 was consistently detected at a high level on the surface of unstimulated CD8+ T lymphocytes (Fig.1A) and thus, was excluded from further experiments. Although SAg stimulation resulted in some upregulation of CD107 on CD4+ T cells, stimulation with a CD8-specific rhCMV peptide resulted in surface expression of CD107a and CD107b exclusively on CD8+ T lymphocytes (Fig. 1B). Stimulation with an unrelated peptide did not mobilize CD107a to the cell surface (data not shown). Thus, surface expression of CD107 molecules was specific and confined to responding antigen-specific T lymphocytes.
In humans, the appearance of CD107a and CD107b on the surface of CD8+ T lymphocytes was associated with a concurrent loss of intracellular perforin, consistent with its association with degranulation (Betts et al., 2003). In order to verify that this also applies to LAMPs on rhesus macaque CD8+ T lymphocytes, we simultaneously measured surface CD107a and intracellular perforin. Resting CD8+ T lymphocytes contained intracellular perforin but did not express surface CD107a (data not shown). In SAg-stimulated cells, cells expressing surface CD107a contained little or no intracellular perforin, while perforin-positive cells remained CD107a-negative (Fig. 2A). Kinetic analysis showed a reciprocal relationship between CD107a and perforin (Fig. 2B-C). Surface expression of CD107a on CD8+ T lymphocytes was detected as early as one hour after stimulation and increased over a four-hour stimulation period (Fig. 2B). A concurrent decline in perforin-positive CD8+ T lymphocytes (Fig. 2B) and an inverse correlation between surface CD107a and intracellular perforin (Rho=0.83, P-Value=0.015, Spearman Rank Correlation test; Fig. 2C) was observed. Thus, similar to humans, measurement of surface CD107a is a sensitive marker for identifying recently degranulated cytolytic T cells in rhesus macaques.
Figure 2. Surface expression of CD107a is associated with decline in intracellular perforin.
(A) Representative contour plot of SEB-stimulated CD8+ T lymphocytes at four hours post-stimulation. (B) Kinetics of surface CD107a expression and intracellular perforin following SEB stimulation. The percent maximal response at each time point is shown. Mean values of two experiments shown. Error bars show standard error of mean (SEM). (C) Inverse correlation between surface CD107a expression and intracellular perforin. Rho and P-Values calculated by the nonparametric Spearman Rank correlation test.
Optimal conditions for detection of degranulating CD8+ T cells in rhesus macaques
Although human CD107a and CD107b molecules are encoded on different chromosomes, they are coordinately expressed on activated T cells in humans, and both proteins are rapidly endocytosed following externalization (Fukuda, 1991; Betts et al., 2003). Due to the rapid endocytosis, optimal detection of degranulated CD8+ T lymphocytes in humans requires the presence of anti-CD107a and anti-CD107b MAb along with monensin throughout the stimulation period (Betts et al., 2003). Monensin neutralizes the acidic pH of endosomes and thereby prevents degradation of fluorochrome conjugates of endocytosed anti-CD107 MAbs (Betts and Koup, 2004). We investigated whether similar experimental conditions are required for optimal detection of degranulating T cells in rhesus macaques.
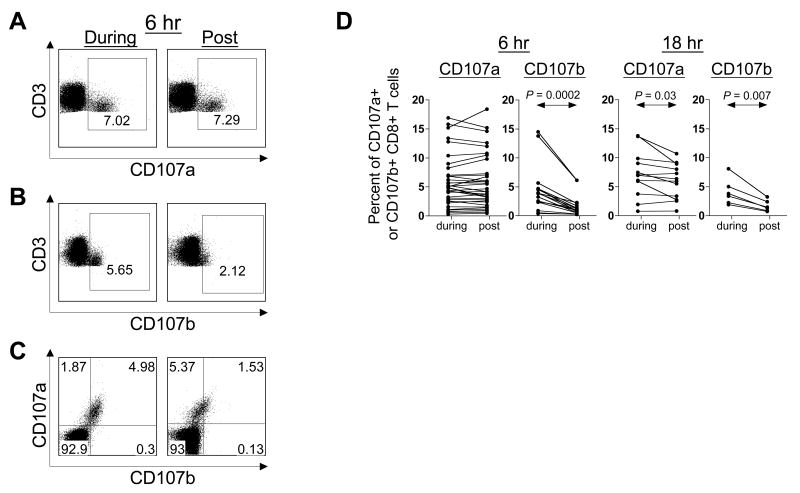
In contrast to humans, the presence of anti-CD107a MAb throughout the stimulation period (“during”), or its addition at the end of a six-hour stimulation period (“post”), did not alter detection of surface CD107a on activated CD8+ T lymphocytes (Fig. 3A). This did not hold true for CD107b, which similar to humans, was rapidly endocytosed as evidenced by a two- to three-fold reduction in surface CD107b signal when anti-CD107b MAb was added at the end of stimulation (Fig. 3B). Consistent with the observation of differential endocytosis of CD107a and CD107b in rhesus macaques, these molecules were coordinately expressed only when both antibodies were present throughout (“during”) the stimulation period (Fig. 3C). If the antibodies were added at the end of the stimulation period (“post”), the frequency of CD107a+ cells far exceeded the frequency of CD107b+ cells (Fig. 3C). Repeated analysis of SAg and rhCMV peptide stimulation in six rhesus macaques confirmed a significant reduction in surface CD107b, but not CD107a, by addition of antibodies at the end (“post”) of a six-hour stimulation period (Fig. 3D).
Figure 3. Optimal conditions for detecting degranulating CD8+ T lymphocytes in rhesus macaques.
PBMC were stimulated with SEB and stained with antibodies to (A) CD107a (PE), (B) CD107b (FITC), or (C) both CD107a and CD107b, present throughout the stimulation period (“during”), or added at the end of the stimulation period (“post”). Gated CD8+ T lymphocytes are shown. (D) Comparison of surface LAMP expression when antibodies were added “during” or “post”. Data following 6 or 18 hours of SEB and rhCMV-CD8 peptide stimulation are shown. P-Values were calculated by the nonparametric Wilcoxon Signed Rank test.
We next asked whether CD107a underwent endocytosis, albeit at a slow rate. Compared to six hours, a greater and significant reduction of surface CD107a was observed if the antibody was added at the end of an 18-hour stimulation period (Fig. 3D). However, the extent of endocytosis of CD107a (maximum 1.4-fold reduction) continued to be considerably lower than that of CD107b. A two- to three-fold reduction in surface CD107b at the end of 18 hours was similar to that observed at six hours. These results suggest that in rhesus macaques, endocytosis of CD107a exposed on the surface of recently degranulated T cells starts late and occurs slowly, while endocytosis of CD107b occurs rapidly and reaches near maximal levels by the end of six hours. Thus, unlike their human counterparts, rhesus macaque CD107a and CD107b molecules are differentially endocytosed in CD8+ T lymphocytes. Moreover, measurement of CD107a alone accounts for all CD107b-positive cells, although the converse is not true (Fig. 3C). Hence, future experiments to identify degranulating T lymphocytes in rhesus macaques were performed only with the anti-CD107a antibody.
Monensin impairs detection of intracellular IFN-γ and TNF-α in rhesus macaque T cells
In the previous experiments, monensin was included whenever the anti-LAMP antibodies were present during the stimulation period. Since monensin also blocks protein transport from the endoplasmic reticulum to the Golgi complex, we examined whether its use was sufficient for simultaneous detection of intracellular cytokines in activated rhesus macaque CD8+ T lymphocytes. Monensin does not completely block secretion of TNF-α from activated human T cells (O'Neil-Andersen and Lawrence, 2002). Hence, a combination of monensin and brefeldin A are used for concurrent detection of degranulation and TNF-α secretion in human T cells (Betts and Koup, 2004).
Following a six to 18 hour period of stimulation with SEB, the use of monensin resulted in a significantly lower frequency of both IFN-γ- and TNF-α-secreting cells compared to brefeldin A (Fig. 4A-C). The blunting effect of monensin on intracellular IFN-γ and TNF-α signal was more pronounced at the end of 18 hours (Fig. 4B). In contrast to intracellular cytokines, detection of surface CD107a did not significantly differ between brefeldin A and monensin (Fig. 4A-B, bottom panel and Fig 4D). These data are consistent with the delayed kinetics of endocytosis of CD107a in rhesus macaques.
Figure 4. Differential effects of monensin and brefeldin A on detection of CD107a, IFN-γ and TNF-α.
PBMC from rhesus macaques were stimulated with SEB in the presence of monensin or brefeldin A for (A) six hours or (B) 18 hours and analyzed by four-color flow cytometry. Numbers denote frequency of gated CD8+ T lymphocytes. Comparison of (C) IFN-γ production and (D) CD107a expression of CD8+ T lymphocytes stimulated with SEB (left panels) or cognate rhCMV peptides (right panels) for six hours in the presence of brefeldin A (BFA) or monensin (Mon). P-Values were calculated by the nonparametric Wilcoxon Signed Rank test. (E - J) Effect of addition of different concentrations of monensin to 1 μg/ml of BFA on CD107a and IFN-γ detection in CD8+ T cells stimulated with SEB or rhCMV peptides in two macaques. Data after six hours of stimulation are shown. Concentrations of monensin ranging between 0.2 and 2 μM were used.
We next asked whether addition of monensin to brefeldin A would enhance detection of surface CD107a in experiments that combined intracellular cytokine staining with CD107a staining. Titration experiments were performed by addition of monensin at concentrations ranging between 2 μM and 0.2 μM to 1 μg/ml of brefeldin A. Data on SEB- and rhCMV peptide-stimulated PBMC in two macaques after a six hour stimulation period are shown (Fig. 4E to J). Consistent with our previous experiment, intracellular IFN-γ detection in the presence of monensin alone was always lower than that with brefeldin A alone while CD107a was unchanged or slightly higher with brefeldin A alone (Fig. 4E to J). On addition of monensin to brefeldin A in the titration experiments, surface expression of CD107a either remained unchanged (Fig. 4F-G), or in some instances, resulted in a small increment (19-36%) in CD107a detection (Fig. 4E, H, I, J). Since the increment of CD107a detection by addition of monensin was minimal when present, future experiments combining IFN-γ and CD107a staining were performed with only brefeldin A as the secretory inhibitor for intracellular cytokine staining and monensin was not added.
Heterogeneity in degranulation and IFN-γ secretion in rhCMV-specific CD8+ T lymphocytes
It is now well recognized that measurement of IFN-γ secretion alone does not provide a complete assessment of the number and function of virus-specific CD8+ T lymphocytes (De Rosa et al., 2004; Betts et al., 2006). Having established optimal conditions for detection of degranulating and IFN-γ-secreting CD8+ T lymphocytes in rhesus macaques, we next evaluated the functional composition of rhCMV-specific CD8+ T lymphocytes targeting individual epitopes. First, the kinetics of degranulation and IFN-γ production in rhCMV-specific and SAg-stimulated CD8+ T lymphocytes were evaluated in three healthy CMV-seropositive rhesus macaques (Mm247.87, Mm212.87, and Mm105.93; Table I) using four-color flow cytometry.
Three responding CD8+ T cell populations consisting of I) cells degranulating but not secreting IFN-γ (CD107a+IFN-γ−), II) cells secreting IFN-γ without degranulation (CD107a−IFN-γ+), and III) degranulated cells concurrently secreting IFN-γ (CD107a+IFN-γ+) were detected (Fig. 5A). The relative proportion of these three populations was dynamic and changed with the duration of stimulation (Fig. 5B). In the first hour after SAg or rhCMV peptide stimulation, 70-85% of the responding CD8+ T lymphocytes had degranulated but not produced IFN-γ (Fig. 5B). In these experiments, brefeldin A was added from the beginning of stimulation. Thus, the relative scarcity of IFN-γ-secreting CD8+ T cells at this early time-point was not due to the absence of a secretory inhibitor in the first hour of stimulation. By two hours, all three responding populations were detected, and the relative proportions of each varied with animal and stimulating antigen (Fig. 5B and data not shown). By four hours, the pattern of responding CD8+ T cells was relatively stable and dominated by population III (CD107a+IFN-γ+; Fig. 5B and data not shown). When the kinetics of surface CD107a were compared with intracellular IFN-γ, three patterns (denoted as A, B and C) were evident (Fig. 5C). In pattern A illustrated by Mm247.87 PBMC stimulated with SAg, responding CD8+ T cells had degranulated to near maximal levels by two hours post-stimulation (Fig. 5C). IFN-γ production was <40% of its maximal value at two hours, and peaked later at 4-6 hours post-stimulation (Fig. 5C). In pattern B illustrated by rhCMV IE1#83L-specific CD8+ T lymphocytes in Mm247.87, both IFN-γ production and degranulation peaked early and simultaneously in the first two hours post-stimulation (Fig. 5C). Pattern C illustrated by rhCMV IE1#83L-specific CD8+ T lymphocytes in Mm105.93 resembled pattern B with regards to the kinetics of degranulation and IFN-γ production being concordant. However, peak levels were reached only after six hours of stimulation (Fig. 5C). The patterns were reproducible in separate experiments performed one to four months apart (Fig. 5C). It is noteworthy that the kinetic experiments revealed differences in the rapidity of degranulation and IFN-γ production between different responding CD8+ T lymphocytes which are not apparent by examination of single time-points four to six hours post stimulation (Fig. 5B-C). Whether the differences in kinetics of degranulation relative to IFN-γ secretion have a bearing on their functional properties remains to be determined.
Figure 5. Heterogeneity in IFN-γ-secreting and cytolytic ability of CD8+ T lymphocytes.
(A) Gating strategy showing responding subsets of SEB-stimulated CD8+ T lymphocytes. Three responding subsets shown; CD107a+IFN-γ− (I), CD107a−IFN-γ+ (II), and CD107a+IFN-γ+ (III). (B) Representative data on kinetic analysis of responding CD8+ T lymphocyte subsets, and (C) Differential kinetics of surface CD107a expression and IFN-γ production. (D) Composition of eleven rhCMV-specific CD8+ T lymphocytes responses in six rhesus macaques at the end of 18 hours of stimulation. All data obtained by four-color flow cytometry. Mean values are shown. Error bars represent SEM of two to four independent experiments performed one to four months apart.
Heterogeneity with regards to IFN-γ secretion and degranulation was further examined in all but one specificity of rhCMV-specific CD8+ T lymphocytes in six rhesus macaques at the end of an 18-hour stimulation period (Table I and Fig. 5D). One epitope (rhCMV IE1#83L) was recognized by three animals, Mm247.87, Mm105.93, and Mm361.95 (Table I and Fig. 5D). Variations in the proportion of CD107a+IFN-γ−, CD107a+IFN-γ+, and CD107a−IFN-γ+ among responding CD8+ T lymphocytes were apparent between animals responding to the same stimulus (rhCMV IE1#83L) and in the same animal for different antigen specificities, as illustrated by Mm105.93 (Fig. 5D).
Memory phenotype of responding CD8+ T lymphocytes subsets
Heterogeneity in composition of IFN-γ-secreting and degranulating cells could be related to differences in the memory phenotype of different rhCMV-specific CD8+ T lymphocyte populations. We next examined whether the three subsets of rhCMV-specific CD8+ T lymphocytes identified on the basis of co-expression of surface CD107a and IFN-γ secretion differed in their memory phenotype. rhCMV-specific (n=12) CD8+ T lymphocyte responses were analyzed in six rhesus macaques (Table II) by seven-color flow cytometry at the end of an 18 hour stimulation period. The memory phenotype of rhCMV-specific and total CD8+ T lymphocytes were analyzed by surface expression of CD95, CD28, and CD45RA. As previously described in rhesus macaques (Pitcher et al., 2002), naïve CD8+ T lymphocytes were phenotypically homogenous and consisted of CD95−CD28+CD45RA+ cells, while CD95+ memory cells displayed phenotypic heterogeneity (Fig. 6A). Based on co-expression of CD95 and CD28, memory CD8+ T lymphocytes in rhesus macaques have been broadly designated as central memory (CD95+CD28+) and effector memory (CD95+CD28−) cells (Pitcher et al., 2002). In humans, CD27−CD28−CCR7− memory CD8+ T lymphocytes that express CD45RA have been shown to be functionally different from those that are CD45RA− in having increased cytolytic activity and poor proliferative ability, and are thus commonly referred to as “terminally differentiated effectors” (Hamann et al., 1997; Sallusto et al., 1999). In this study, four phenotypically distinct populations of memory (CD95+) CD8+ T lymphocytes could be identified based on co-expression of CD28 and CD45RA. These included a CD95+CD28−CD45RA− population designated as effector memory (EM), a CD95+CD28−CD45RA+ population designated as terminally differentiated effectors (Eff), a CD95+CD28+CD45RA− population designated as central memory 1 (CM1) cells, and a minor (<5%) CD95+CD28+CD45RA+ population designated as central memory 2 (CM2) (Fig. 6A). While the designations of EM, Eff and the dominant central memory (CM1) T lymphocytes conforms to previous phenotypic definitions of memory CD8+ T lymphocytes (Hamann et al., 1997; Sallusto et al., 1999; Pitcher et al., 2002), the minor CD95+CD28+CD45RA+ population, which hitherto has not been reported, was arbitrarily designated as CM2 in this study.
Table II. Differences in memory phenotype between the functional subsets of rhCMV-specific CD8+ T lymphocytes.
| % CD8+ T cells that are | ||||
|---|---|---|---|---|
| CD95+28−45RA−(EM) | CD95+28−45RA+(Eff) | CD95+28+45RA−(CM1) | CD95+28+45RA+(CM2) | |
| Total CD8+ T cells (n=6) | 24.9±12.9 | 38.5±11.4 | 11.7±4.0 | 1.8±1.3 |
| rhCMV-specific CD8+ (n=12) | ||||
| CD107a+IFNγ− | 18.2±13.9* | 61.7±16.3# | 3.9±3.4 | 1.2±0.9 |
| CD107a−IFNγ+ | 34.8±18.9 | 48.0±21.9 | 5.4±4.5 | 1.3±1.1 |
| CD107a+IFNγ+ | 36.8±19.0 | 44.6±24.1 | 5.4±6.5 | 1.5±2.5 |
P-value <0.01 (repeated measures ANOVA and Bonferroni post-hoc test) for difference between CD107a+IFNγ− rhCMV-specific responders and either CD107a−IFNγ+ or CD107a+IFNγ+ rhCMV-specific responders.
P-value <0.01 (repeated measures ANOVA and Bonferroni post-hoc test) for difference between CD107a+IFNγ− rhCMV-specific responders and CD107a−IFNγ+ rhCMV-specific responders.
Figure 6. Memory phenotype of responding CD8+ T lymphocyte subsets of rhCMV-specific CD8+ T lymphocytes analyzed by seven-color flow cytometry.
(A) Delineation of memory populations of CD8+ T lymphocytes based on concurrent measurement of CD95, CD28 and CD45RA. Overlay plots of four subsets of CD95+ memory cells are shown. (B) Memory phenotype of CD107a+IFN-γ− (I), CD107a−IFN-γ+ (II), and CD107a+IFN-γ+ (III) functional of rhCMV-specific CD8+ T lymphocytes at the end of an 18-hour stimulation period. P-Values were determined by repeated measures ANOVA and the Bonferroni post-hoc test. The frequency of CM1 and CM2 cells were significantly lower (P <0.05) than the Eff and EM populations for all three responding subsets (not shown on graph). The flow cytometry panel consisted of CD3 PB, CD8 Alexa700, CD95 APC, CD28 FITC, CD45RA PerCP, CD107a PE, and IFN-γ PE-Cy7 antibodies.
rhCMV-specific CD8+ T lymphocytes were predominantly effector memory (CD95+CD28−CD45RA−) and/or terminally differentiated effector (CD95+CD28−CD45RA+) cells (Table II). Differences in memory phenotype between the degranulating alone and IFN-γ-secreting subsets of rhCMV-specific CD8+ T lymphocytes were observed (Table II and Fig. 6B). The CD107a+IFN-γ− subset of rhCMV-specific CD8+ T lymphocytes had significantly higher frequencies of effector as compared to effector memory cells (Fig. 6B). In contrast, the two IFN-γ-secreting subsets of rhCMV-specific CD8+ T lymphocytes had comparable frequencies of effector and effector memory cells (Fig. 6B). As a result, CD107a+IFN-γ− rhCMV-specific CD8+ T lymphocytes contained significantly higher frequencies of effectors and significantly lower frequencies of effector memory cells compared to the dual functional CD107a+IFN-γ+ or the single functional CD107a−IFN-γ+ rhCMV-specific CD8+ T cell population (Table II).
In humans, memory CD8+ T lymphocytes (including CMV-specific CD8+ T cells) with a terminally differentiated effector phenotype (CD28-CD45RA+) have been shown to be cytolytic and have a high granzyme B and perforin content compared to effector memory cells (Baars et al., 2000; Appay et al., 2002). Since the enrichment of effectors in the CD107a+IFN-γ− subset of rhCMV-specific CD8+ T lymphocytes suggested a higher cytolytic capability of this population, we investigated whether the cytolytic granule content of effectors differed from that of effector memory cells in rhesus macaques. The intracellular granzyme B and perforin content of different memory populations (CD95+) of CD8+ T lymphocytes was examined in nine rhCMV-seropositive rhesus macaques (Fig. 7). Central memory CD8+ T lymphocytes consistently had low (<5%) to undetectable intracellular granzyme B and perforin (Figure 7A and data not shown). The cytolytic granule content of effectors and effector memory CD8+ T lymphocytes varied considerable between animals (Fig. 7B). The frequency and content of perforin granules, but not granzyme B, were found to be significantly higher in the effector as compared to effector memory CD8+ T lymphocytes (Fig. 7B-C). These data suggest that similar to humans, a terminally differentiated effector phenotype of CD8+ T lymphocytes in rhesus macaques may represent CD8+ T cells with greater cytolytic ability.
Figure 7. Cytolytic granule content of effector (CD95+CD28-CD45RA+) and effector memory (CD95+CD28- 45RA-) CD8+ T lymphocytes in rhesus macaques.
(A) Overlay histograms showing granzyme B (left) and perforin (right) content of different memory populations of CD8+ T lymphocytes. (B) Comparison of the frequency of granzyme B+ and perforin+ cells between effector (Eff) and effector memory (EM) CD8+ T lymphocytes. (C) Comparison of the median channel fluorescence (MCF) of granyme B and perforin content between effectors (Eff) and effector memory (EM) CD8+ T lymphocytes. P-Values were calculated by the non-parametric Wilcoxon signed rank test. The eight-color flow cytometry panel consisted of CD3 APC-Cy7, CD4 PerCP, CD8 Alexa700, CD95 APC, CD28 ECD, CD45RA PE-Cy7, perforin FITC, and granzyme B PE antibodies.
Discussion
In this study the flow-cytometric technique used for detection of degranulating cells in humans was adapted to rhesus macaques, and used to evaluate the IFN-γ-secreting and cytolytic properties of rhCMV-specific CD8+ T lymphocytes in immunocompetent CMV-seropositive animals. CD8+ T lymphocytes recognizing at least twelve rhCMV epitopes in six macaques were analyzed at the single-cell level. Following a 6-18 hour period of in vitro stimulation, rhCMV-specific CD8+ T lymphocytes were composed of a dominant population that had secreted IFN-γ and simultaneously undergone degranulation, and two minor populations that showed evidence of one of either two effector functions. We show that the proportion of each of the three responder populations was heterogeneous, varying between different rhCMV-specific CD8+ T lymphocyte populations in the same animal, as well as varying between responder CD8+ T lymphocytes recognizing the same rhCMV epitope in different animals. We also show that the memory phenotype of the three responding populations of rhCMV-specific CD8+ T lymphocytes was not uniform. Responder CD8+ T lymphocytes that underwent degranulation without IFN-γ secretion were predominantly composed of terminally differentiated effector type (CD28−CD45RA+) cells. In contrast, the two IFN-γ-secreting populations consisted of comparable frequencies of effector memory (CD28−CD45RA−) and effector cells, regardless of the presence or absence of concurrent degranulation. Since effector CD8+ T lymphocytes in rhesus macaques were found to have significantly greater perforin content compared to effector memory CD8+ T lymphocytes, these findings raise the possibility that the composition of rhCMV-specific CD8+ T lymphocytes with regards to CD107a+IFN-γ− responders may be an important determinant of their ability to control CMV replication.
Using a combination of the CD107 mobilization assay and intracellular cytokine staining, there have been limited reports of heterogeneity in the degranulating and cytokine producing properties of human CD8+ T lymphocytes responding to epitopes in HIV, CMV, and dengue virus (Betts et al., 2004; Lacey et al., 2005; Lacey et al., 2006; Mongkolsapaya et al., 2006). Similar to our observation in rhCMV-specific CD8+ T lymphocytes, human CMV-specific CD8+ T lymphocytes targeting different epitopes showed variation in their degranulating and cytokine producing capacity (Lacey et al., 2005; Lacey et al., 2006). Interestingly, IFN-γ-secreting CD8+ T lymphocytes recognizing epitopes in the human CMV IE1 protein contained fewer cells with degranulating ability compared to pp65-specific CD8+ T lymphocytes (Lacey et al., 2005; Lacey et al., 2006). In this study, the analysis of rhCMV-specific CD8+ T lymphocytes was confined to those recognizing epitopes in the immediate early 1 and 2 proteins, and hence a comparison with pp65-specific CD8+ T lymphocyte response cannot be made. However, it is noteworthy that unlike human CMV-CD8+ T cells (Lacey et al., 2006), there did not appear to be a defect in degranulating ability of IE-specific rhCMV-specific CD8+ T cells. The functional implication of the differential degranulating ability of IE1-specific and pp65-specific CD8+ T lymphocytes in humans is not clear, particularly in light of a recent report on the efficacy of IE1-specific but not pp65-specific CTL, to protect heart and lung transplant recipients against CMV disease (Bunde et al., 2005). It is of interest that unlike IE1-specific CTL in humans which showed low levels of degranulation (Lacey et al., 2005; Lacey et al., 2006), rhCMV IE1-specific CTL showed degranulation in the majority of responding cells.
It is not know whether the cells that mobilize CD107 with or without cytokine production differ in their cytolytic potential. In this study, we observed that CD107a+IFN-γ−, but not CD107a+IFN-γ+, rhCMV-specific CD8+ T lymphocytes were enriched for memory cells with a terminally differentiated effector phenotype (CD28−CD45RA+). Since viable cell sorting of CD107a+IFN-γ− and CD107a+IFN-γ+ cells is not possible in rhesus macaques, we compared the cytolytic granule content of effector and effector memory CD8+ T lymphocytes in rhesus macaques. Although both memory subsets contained variable levels of granzyme B and perforin, the effectors contained a significantly higher proportion of perforin+ cells. The observation that CD107a+IFN-γ− cells were enriched for effectors raises the possibility that rhCMV-specific CD8+ T lymphocytes undergoing degranulation without cytokine production may be more cytolytic than the dominant dual effector population. Data from humans and mice support this possibility. Memory CD8+ T lymphocytes (including CMV-specific CD8+ T lymphocytes) of a differentiated effector phenotype (CD28−CD27−CD45RA+) in humans have been shown to be cytolytic and have a higher perforin content compared to those with an effector memory (CD28−CD27+CD45RA−) phenotype (Baars et al., 2000; Appay et al., 2002). In mice, lymphocytic choriomeningitis virus-specific CD8+ T lymphocytes with an effector phenotype were found to be more cytotoxic than those with an effector memory phenotype, even though both memory subsets showed similar CD107 mobilization (Wolint et al., 2004). The recent report of CD45RAbright and CD45RAdim subsets of CD107a-positive HIV-specific CD8+ T lymphocytes that are differentially lost in progressive HIV infection (Jones et al., 2006) is further evidence for the presence of distinct phenotypic and functional populations of degranulating cells within antigen-specific CD8+ T lymphocytes.
It is noteworthy that studies that have used granzyme B or perforin ELISPOT, or the Lysispot assay to measure cytolytic function at the single-cell level, have shown a marked discordance between the frequencies of IFN-γ-secreting and cytolytic cells for SIV-, HIV-, and human CMV-specific CD8+ T lymphocytes (Snyder et al., 2003; Kleen et al., 2004; Calarota et al., 2006; Snyder-Cappione et al., 2006). In most instances, the frequency of IFN-γ-secreting cells has far exceeded the frequency of cytolytic cells. The reason for the discrepancy from flow cytometric-based assays where the majority of responding CD8+ T lymphocyte tend to be CD107a+IFN-γ+, may lie in the fact that cytolytic granule content is not taken into consideration when CD107 mobilization alone is used as a surrogate marker of cytotoxicity. Even though CD107a mobilization on the surface of activated CD8+ T lymphocytes or natural killer cells directly correlates with the extent of cytotoxicity evaluated by a traditional chromium release assay (Betts et al., 2003; Rubio et al., 2003; Alter et al., 2004), studies in mice have elegantly demonstrated that cytolytic ability is dependent on the granule content, and CD107a mobilization alone is not a measure of cytolytic ability (Wolint et al., 2004).
The kinetic experiments confirmed the onset of degranulation within one hour of stimulation, results that are similar to those described previously for activated CD8+ T lymphocytes in humans and mice (Betts et al., 2003; Wolint et al., 2004) and for human NK cells (Alter et al., 2004). In this study the analysis of kinetics of degranulation and IFN-γ secretion following SEB and rhCMV peptide stimulation in three animals (three SEB responders and six rhCMV-specific CD8+ T lymphocytes) illustrated an interesting finding. Virus-specific CD8+ T lymphocytes displaying similar co-expression profiles of CD107a and IFN-γ at the end of 6-18 hours of in vitro stimulus, could differ with regards to the rapidity to reach peak degranulation and the rate at which IFN-γ secretion “caught up” with degranulation (Figure 5C). The differential kinetics of degranulation and IFN-γ secretion may be related to the differences in memory composition of the different responding populations. Effectors degranulate at a faster rate compared to effector memory cells while the rate of cytokine production is comparable in both (Bachmann et al., 1999). If the rapidity of degranulation relative to cytokine secretion is a determinant of effective immune control, kinetic experiments may be required to tease out functional differences between two seemingly similar antigen-specific responses. Additionally, the dynamics of degranulation and IFN-γ kinetics also demonstrate that the duration of antigen stimulation can significantly affect the composition of virus-specific CD8+ T lymphocytes and thus comparative studies need to take the duration of stimulus into consideration.
This study is the first detailed analysis of functionality of rhCMV-specific CD8+ T lymphocytes recognizing several different epitopes in rhesus macaques, an animal model that is valuable for studies on AIDS pathogenesis including immunopathogenesis of CMV infection. Unlike humans in whom both pp65 and IE are immunodominant, (Sylwester et al., 2005) the rhCMV-specific CD8+ T lymphocyte response is characterized by a dominant response to the IE1 and IE2 proteins, with pp65 being recognized to a lesser degree both in terms of magnitude and frequency of responders (Kaur A; unpublished data). Consistent with the memory phenotype of human CMV-specific CD8+ T lymphocytes (Gillespie et al., 2000; Champagne et al., 2001; Gamadia et al., 2001; Appay et al., 2002; Ellefsen et al., 2002; Wills et al., 2002), rhCMV-specific CD8+ T lymphocytes as a whole had lower frequencies of central memory cells (CD28+CD45RA−) and higher frequencies of effector memory (CD28−CD45RA+) and effector cells (CD28−CD45RA+) compared to the total CD8+ T lymphocyte population. The concurrent use of multiple surface markers has added to the complexity and phenotypic heterogeneity of memory T lymphocyte populations within previously defined categories. Thus, a hitherto unreported minor population of CD28+CD45RA+ memory CD8+ T lymphocytes (designated as CM2) was also identified; but its functional significance remains unknown.
Finally, results of our optimization experiments revealed a difference in endocytosis of LAMP proteins, specifically CD107a, between rhesus macaques and humans. Variability in cytoplasmic tail sequences which are involved in endocytosis (Chang et al., 2002) may account for this difference from human CD107a/b as well as from macaque CD107b. Our finding of absent to marginal endocytosis of CD107a during the usual in vitro stimulation period has important technical implications. Since the presence of monensin during stimulation was found to significantly decrease IFN-γ and TNF-α signal by intracellular cytokine staining, a non-requirement of monensin for CD107a detection would be expected to facilitate concurrent detection of IFN-γ and TNF-α without undue loss of sensitivity. In practice, we found variability in the requirement of monensin for optimal detection of surface CD107a in rhesus macaques. In some instances, addition of monensin to brefeldin A resulted in up to a 30% increase in CD107a detection without appreciably compromising IFN-γ detection. Doses as low as 0.2 μM of monensin (one-tenth the recommended dose) were sufficient to observe this effect. Thus, the requirement for addition of low doses of monensin to brefeldin A for optimal detection of CD107a and IFN-γ may need to be determined on a case by case basis. In conclusion, the establishment of optimal conditions for flow-cytometric detection of degranulation in rhesus macaques will facilitate studies on simultaneous evaluation of multiple effector functions of antigen-specific CD8+ T lymphocytes in the rhesus macaque animal model of AIDS.
Acknowledgments
We would like to thank Sarah Pryputniewicz and Melissa Kasheta for assistance with rhCMV epitope mapping, and Michelle Connole and Jackie Gillis for acquisition of flow cytometry samples. This study was supported by National Institutes of Health grant AI 43890 (A.K.). K.S.C. performed the experiments, analyzed the data and wrote the manuscript. A.K. supervised the study and wrote the manuscript. The authors declare no competing financial interests.
Abbreviations
- APC
allophycocyanin
- APC-Cy7
allophycocyanin-Cy7
- Alexa700
Alexa Fluor 700
- BFA
brefeldin A
- CTL
cytotoxic T lymphocyte
- CM
central memory
- ECD
energy coupled dye
- Eff
effectors
- ELISPOT
enzyme-linked immunospot
- EM
effector memory
- FITC
fluorescein isothiocynate
- ICS
intracellular cytokine staining
- IE1
immediate early 1 protein
- IE2
immediate early 2 protein
- IFN-γ
interferon-gamma
- IL10
interleukin-10 protein
- LAMP
lysosomal associated membrane protein
- Mon
monensin
- PB
Pacific Blue
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- PE-Cy7
phycoerythrin-Cy7
- PerCP
peridinin chlorophyll protein
- pp65
phosphoprotein
- rhCMV
rhesus cytomegalovirus
- SAg
superantigen
- SEA
staphylococcus enterotoxin A
- SEB
staphylococcus enterotoxin B
- TNF-α
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Anonymous. The Institute of Laboratory Animal Resources. National Research Council: Guide for the Care and Use of Laboratory Animals. 1996. pp. 86–123. [Google Scholar]
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Baars PA, Ribeiro Do Couto LM, Leusen JH, Hooibrink B, Kuijpers TW, Lens SM, van Lier RA. Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+CD45RA+CD27- human T cells. J Immunol. 2000;165:1910–7. doi: 10.4049/jimmunol.165.4.1910. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol. 1999;29:291–9. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2:401–9. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Price DA, Brenchley JM, Lore K, Guenaga FJ, Smed-Sorensen A, Ambrozak DR, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004;172:6407–17. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, Proesch S, Reinke P, Volk HD, Lehmkuhl H, Kern F. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–6. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarota SA, Otero M, Robinson TM, Dai A, Lewis MG, Boyer JD, Weiner DB. Independence of granzyme B secretion and interferon- gamma production during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:1441–50. doi: 10.1086/503364. [DOI] [PubMed] [Google Scholar]
- Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- Chang MH, Karageorgos LE, Meikle PJ. CD107a (LAMP-1) and CD107b (LAMP-2) J Biol Regul Homeost Agents. 2002;16:147–51. [PubMed] [Google Scholar]
- De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, Evans TG, Koup R, Miller CJ, Roederer M. Vaccination in humans generates broad T cell cytokine responses. J Immunol. 2004;173:5372–80. doi: 10.4049/jimmunol.173.9.5372. [DOI] [PubMed] [Google Scholar]
- Ellefsen K, Harari A, Champagne P, Bart PA, Sekaly RP, Pantaleo G. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur J Immunol. 2002;32:3756–64. doi: 10.1002/1521-4141(200212)32:12<3756::AID-IMMU3756>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991;266:21327–30. [PubMed] [Google Scholar]
- Gamadia LE, Rentenaar RJ, Baars PA, Remmerswaal EB, Surachno S, Weel JF, Toebes M, Schumacher TN, ten Berge IJ, van Lier RA. Differentiation of cytomegalovirus-specific CD8(+) T cells in healthy and immunosuppressed virus carriers. Blood. 2001;98:754–61. doi: 10.1182/blood.v98.3.754. [DOI] [PubMed] [Google Scholar]
- Gillespie GM, Wills MR, Appay V, O'Callaghan C, Murphy M, Smith N, Sissons P, Rowland-Jones S, Bell JI, Moss PA. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74:8140–50. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N, Eggena M, Baker C, Nghania F, Baliruno D, Mugyenyi P, Ssali F, Barugahare B, Cao H. Presence of distinct subsets of cytolytic CD8+ T cells in chronic HIV infection. AIDS Res Hum Retroviruses. 2006;22:1007–13. doi: 10.1089/aid.2006.22.1007. [DOI] [PubMed] [Google Scholar]
- Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–30. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Kaur A, Hale CL, Noren B, Kassis N, Simon MA, Johnson RP. Decreased frequency of cytomegalovirus (CMV)-specific CD4+ T lymphocytes in simian immunodeficiency virus-infected rhesus macaques: inverse relationship with CMV viremia. J Virol. 2002;76:3646–58. doi: 10.1128/JVI.76.8.3646-3658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleen TO, Asaad R, Landry SJ, Boehm BO, Tary-Lehmann M. Tc1 effector diversity shows dissociated expression of granzyme B and interferon-gamma in HIV infection. Aids. 2004;18:383–92. doi: 10.1097/00002030-200402200-00003. [DOI] [PubMed] [Google Scholar]
- Lacey SF, La Rosa C, Zhou W, Sharma MC, Martinez J, Krishnan A, Gallez-Hawkins G, Thao L, Longmate J, Spielberger R, Forman SJ, Limaye A, Zaia JA, Diamond DJ. Functional comparison of T cells recognizing cytomegalovirus pp65 and intermediate-early antigen polypeptides in hematopoietic stem-cell transplant and solid organ transplant recipients. J Infect Dis. 2006;194:1410–21. doi: 10.1086/508495. [DOI] [PubMed] [Google Scholar]
- Lacey SF, Martinez J, Gallez-Hawkins G, Thao L, Longmate J, Haq W, Spielberger R, Forman SJ, Zaia JA, Diamond DJ. Simultaneous reconstitution of multiple cytomegalovirus-specific CD8+ cell populations with divergent functionality in hematopoietic stem-cell transplant recipients. J Infect Dis. 2005;191:977–84. doi: 10.1086/428136. [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A, Khemnu N, Tangthawornchaikul N, Chotiyarnwong P, Sae-Jang K, Koch M, Jones Y, McMichael A, Xu X, Malasit P, Screaton G. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol. 2006;176:3821–9. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- O'Neil-Andersen NJ, Lawrence DA. Differential modulation of surface and intracellular protein expression by T cells after stimulation in the presence of monensin or brefeldin A. Clin Diagn Lab Immunol. 2002;9:243–50. doi: 10.1128/CDLI.9.2.243-250.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschopp J, Slot JW, Geuze HJ. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099–109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Snyder JE, Bowers WJ, Livingstone AM, Lee FE, Federoff HJ, Mosmann TR. Measuring the frequency of mouse and human cytotoxic T cells by the Lysispot assay: independent regulation of cytokine secretion and short-term killing. Nat Med. 2003;9:231–5. doi: 10.1038/nm821. [DOI] [PubMed] [Google Scholar]
- Snyder-Cappione JE, Divekar AA, Maupin GM, Jin X, Demeter LM, Mosmann TR. HIV-specific cytotoxic cell frequencies measured directly ex vivo by the Lysispot assay can be higher or lower than the frequencies of IFN-gamma-secreting cells: anti-HIV cytotoxicity is not generally impaired relative to other chronic virus responses. J Immunol. 2006;176:2662–8. doi: 10.4049/jimmunol.176.4.2662. [DOI] [PubMed] [Google Scholar]
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–85. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJ, Carmichael AJ. Identification of naive or antigen-experienced human CD8(+) T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8(+) T cell response. J Immunol. 2002;168:5455–64. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J Exp Med. 2004;199:925–36. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]