Abstract
To assess the potential of native Envelope glycoprotein (Env) trimers as neutralizing antibody vaccines, we immunized guinea pigs with three types of VLPs and soluble gp120. Particles included “SOS-VLPs” (bearing disulfide-shackled functional trimers), “UNC-VLPs” (bearing uncleaved nonfunctional Env), and “naked VLPs” (bearing no Env). The SOS-VLPs were found to have a density of about 27 native trimers per particle, about twice that of live inactivated HIV-1 preparations. As immunogens, UNC- and SOS-VLP rapidly elicited anti-gp120 antibodies. Gp120-specific antibodies were focused on the V3 loop and the gp120 coreceptor binding site. Reactivity to the gp41 immunodominant domain was absent in SOS-VLP sera, presumably because gp120-gp41 association is stabilized. Gp120 immune sera were less focused on the V3 loop, and reacted with the receptor binding sites of gp120. Some Env-VLP sera neutralized primary isolates at modest titers. Neutralization activity was found to be affected by the cell lines used. Depending on the assay particulars, non-Env specific antibodies in VLP sera could enhance infection, or nonspecifically neutralized. However, we found that the TZM-BL neutralization assay was clear of these effects. We also report a native trimer shift assay that eliminates nonspecific effects and confirm the neutralization activity. Overall, our results suggest that a major focus of VLP sera was against components of particles other than Env trimers, including nonfunctional gp120/gp41 monomers. To make progress toward a more effective VLP vaccine, we will need to find ways to refocus the attention of B cells on native trimers.
Keywords: HIV, vaccine, antibody, neutralizing, neutralization, VLPs, gp120, gp41, Env
INTRODUCTION
An effective vaccine is desperately needed to prevent the spread of human immunodeficiency virus type 1(HIV-1) (Garber, Silvestri, and Feinberg, 2004; Koff, Kahn, and Gust, 2007; Tramont and Johnston, 2003). It is likely that a potent and broadly reactive neutralizing antibody (nAb) response will be a crucial component of vaccine immunity to HIV-1. However, despite the considerable ingenuity of vaccine researchers, progress toward an immunogen able to elicit such as response has been minimal.
NAbs are thought to target envelope glycoprotein (Env) spikes on HIV-1 surfaces, thereby inhibiting entry into susceptible cells. Functional Env spikes consist of noncovalently-associated trimers of gp120/gp41 heterodimers (Earl, Doms, and Moss, 1990). The gp120 moiety mediates binding to target cell receptors, triggering gp41-mediated fusion and entry. As the presumptive targets of neutralization, logic has fueled the hypothesis that authentic Env trimers might be able to elicit nAbs in a vaccine setting (Emini and Koff, 2004; Koff, Kahn, and Gust, 2007; Tramont and Johnston, 2003). However, a major stumbling block for vaccinologists has been that authentic trimers are technically challenging to produce in a purified form. A number of groups have expressed soluble recombinant trimers that resemble the native structure to varying degrees (Beddows et al., 2006; Beddows et al., 2005; Binley et al., 2000; Chakrabarti et al., 2002; Earl et al., 2001; Farzan et al., 1998; Li et al., 2006; Sanders et al., 2002b; Srivastava et al., 2002; VanCott et al., 1997; Yang et al., 2000). Challenges include optimizing the inefficient gp120/gp41 cleavage process (Yamshchikov et al., 1995) and stabilizing the weak inter-subunit associations within trimers (Staropoli et al., 2000). Gp120/gp41 processing can be enhanced by optimizing the substrate sequence and by co-expressing furin (Binley et al., 2002; McKenna et al., 2003; Yamshchikov et al., 1995). Gp120-gp41 association can be stabilized by appropriately positioned cysteine mutations to form an intermolecular disulfide bond (Binley et al., 2000). These advances led to a recombinant Env glycoprotein, termed gp140SOS (Binley et al., 2000). The later observation that gp140SOS trimers readily dissociate into monomers presented a further challenge (Schulke et al., 2002). One solution involved introducing a helix-breaking mutation in gp41, to make mutant trimers termed gp140SOSIP (Beddows et al., 2006; Beddows et al., 2005; Sanders et al., 2002b). An alternative solution may be to express trimers in situ on membrane surfaces, perhaps imparting stability without the need for additional mutations.
Env can be presented on membranes in several ways, including liposomes, inactivated viruses and virus-like particles (VLPs) or pseudovirions (Grovit-Ferbas et al., 2000; Grundner et al., 2002; Race et al., 1995; Rossio et al., 1998). Particulate vaccines have a long history of success for a variety of diseases, including those caused by rotavirus (Conner et al., 1996), norwalk virus (Harrington et al., 2002), tick-borne encephalitis virus (Aberle et al., 1999) and HCV (Issel et al., 1992). Successful clinical trials of human papilloma VLPs have led to recent FDA approval (Evans et al., 2001; Koutsky et al., 2002). There are, of course, even better known examples of particulate vaccines, of which Salk’s inactivated polio vaccine and the hepatitis B vaccines are perhaps the best examples (Salk, 1977; Valenzuela et al., 1982). The success of these immunogens may derive from an ability to prime T-cells (Haffar et al., 1991; Wagner et al., 1998), and to rapidly induce high titer Ab responses, even sometimes in the absence of adjuvant (Lorin et al., 2004; Wagner et al., 1998). Another key factor in their success may derive from the authentic presentation of surface structures (Aberle et al., 1999; Beddows et al., 2005; Edinger et al., 2000).
Particulate vaccine candidates for HIV-1 have been described by many groups (Buonaguro et al., 2005; Evans et al., 2005; Grovit-Ferbas et al., 2000; Haffar et al., 1991; Hammonds et al., 2003; Katz and Moss, 1997; Lifson et al., 2004; McBurney, Young, and Ross, 2007; McKenna et al., 2003; Montefiori et al., 2001; Vzorov, Lea-Fox, and Compans, 1999; Wagner et al., 1996; Yao et al., 2000), in some cases progressing to clinical trials (Persson et al., 1998; Tramont and Johnston, 2003). Overall, however, the approach has been under-researched, perhaps in part due to a perceived lack of improvement in nAb induction, compared to other vaccine approaches (Daniel et al., 1994; Polacino et al., 1999; Race et al., 1995; Richmond et al., 1998; Verrier et al., 2000). Underpinning this lack of success are several challenges that may need to be overcome before VLPs can realize their full potential as vaccines.
A key technical issue with VLP or other membranous vaccines is their tendency to elicit “anti-cell” antibodies against non-Env membrane proteins. These antibodies can have unpredictable side effects in neutralization assays that vary from neutralization (Arthur et al., 1992; Chan et al., 1992) to enhancement (Giannecchini et al., 2001; Verrier et al., 2000). One possible explanation for these effects is that anti-cell Abs affect the viability of target cells. Making the situation even more complex, it has been suggested that enhancing Abs may even mask the effect of other, neutralizing Abs (Giannecchini et al., 2001; Hammonds et al., 2005). As a result, it has often been difficult to unequivocally interpret neutralization data in particle and cell-based vaccine studies (Buonaguro et al., 2005; Giannecchini et al., 2001; Hammonds et al., 2005; LaCasse et al., 1999; Langlois et al., 1992; Poon et al., 2005a; Poon et al., 2005b).
A second challenge for VLP vaccines is that native trimers may not be the only form of Env present on their surfaces. Possible non-trimeric forms of Env include gp41 stumps arising from gp120 shedding (Chertova et al., 2002; McKeating, McKnight, and Moore, 1991; Moore et al., 2006), gp120/gp41 monomers (Moore et al., 2006), and uncleaved gp160 resulting from inefficient processing. These forms of Env do not resemble functional trimers and may elicit non-neutralizing antibody responses.
A third potential challenge of particulate vaccines is that many commonly used adjuvants contain detergent surfactants, exemplified by Ribi adjuvant, containing Tween 80. Surfactants stabilize oil-in-water emulsions to enhance the delivery of proteins to the membranes of immune cells over a large surface area. However, they may intercalate with or destabilize the lipid membranes of VLPs, causing Env to be released (Moore et al., 2006), and as a consequence, trimers may partially or completely fall apart.
Despite these challenges, VLPs remain among the few vaccine candidates able to present truly authentic trimers, and therefore deserve further investigation. In this pilot study, we evaluated the immunogenicity of VLPs that had been adjusted to try to overcome some of the abovementioned pitfalls. Env proteins were truncated to improve expression and to help ensure proper gp120/gp41 cleavage. A gp120-gp41 disulfide bond was introduced to prevent gp120 shedding. Furthermore, we employed various neutralization assay formats to analyze VLP immune sera, to help identify neutralization amid any background nonspecific activity.
RESULTS
Production of VLP immunogens
In this pilot study, we opted for moderate Env expression by plasmid pCAGGS, together with pNL4-3.Luc.R-E- to express VLPs bearing functional Env trimers (Binley et al., 2003). To stabilize gp120-gp41 association and eliminate the potential problem of gp120 shedding that might occur with “WT-VLPs”, we generated “SOS-VLPs” that introduce a gp120-gp41 disulfide bond (Abrahamyan et al., 2003; Binley et al., 2003; Binley et al., 2000), and UNC-VLPs that abolish gp120-gp41 processing (Fig. 1). Collectively, we refer to these as “Env-VLPs, distinct from “naked” VLPs, lacking Env.
Figure 1. Depiction of various forms of Env on VLP surfaces.
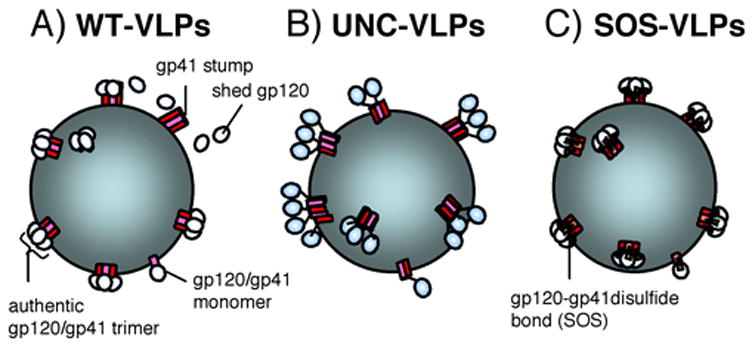
A) WT-VLPs. Env is proteolytically processed into gp120/gp41 trimers. Gp120 shedding leaves gp41 stumps behind. Gp120/gp41 monomers are a putative minor species (Moore et al., 2006), B) UNC-VLPs. Env is uncleaved, eliminating gp120 shedding. However, Env conformation is inauthentic (Binley et al., 2000), C) SOS-VLPs; Env is proteolytically processed and gp120 shedding is eliminated by a gp120-gp41 disulfide bond (the SOS mutation), while retaining function. As for WT-VLPs, gp120/gp41 monomers are a putative minor species.
We compared full-length and truncated gp160 forms of Env on VLPs. Gp160ΔCTWT was completely dependent on the expression of structural proteins by pNL4-3.Luc.R-E- for secretion into supernatant (Fig. 2A, lanes 1–3), but full-length gp160 was secreted into the supernatant, even when pNL4-3.Luc.R-E- was not co-expressed (not shown), perhaps indicating spontaneous vesicle formation or an increase in cell lysis. In addition, gp160ΔCT exhibited higher expression and gp120/gp41 cleavage than the full-length form, as observed previously (Binley et al., 2003; Yamshchikov et al., 1995; Yao et al., 2000). Gp160ΔCTSOS was also fully cleaved and reducible by DTT (Fig. 2A, lanes 4 and 5). Since gp160ΔCT is also fusogenic (Abrahamyan et al., 2005; Binley et al., 2003; Crooks et al., 2005; Edwards et al., 2002), we reasoned that this is an authentic form of Env suitable for testing as an immunogen. In contrast to WT- and SOS-VLPs, the Env in UNC-VLPs was uncleaved, as expected (data not shown and ref. (Moore et al., 2006)).
Figure 2. Production of VLPs bearing authentic Env trimers.

A) Secretion of WT and SOS gp160ΔCT expressed by pCAGGS into transfection supernatant with or without co-transfection of pNL4-3.Luc.R-E- to induce particle budding, as indicated. Supernatants were metabolically labeled with 35S and immunoprecipitated with mAbs 2G12 and b12 and resolved in reducing and nonreducing SDS-PAGE, B) Electron microscopy of WT-VLP particles budding from 293T cells. Nascent particles are indicated by arrows, C) Unlabeled SOS-VLPs were pelleted from transfection supernatants and analyzed by reducing SDS-PAGE and Western blot, probing with anti-gp120 mAbs PA1 and B12 or anti-p24 serum, HIVIG or anti-gp120 mAbs B12 and PA1, as indicated, D) Negative stain electron micrograph of an SOS-VLP particle. Arrows indicate trimers. Bar=50 nM. Inset: model of an Env trimer, reproduced with permission of Dr. P.D. Kwong (Kwong et al., 2000).
Electron microscopic analysis of 293T cells co-transfected with pCAGGS gp160ΔCTWT and pNL4-3.Luc.R-E- revealed efficient particle budding (Fig. 2B). SDS-PAGE and Western blotting of VLP preparations provided further evidence that gp160ΔCT was associated with particles (Fig. 2C). The Gag to Env ratio appeared to be similar in WT-, SOS- and UNC-VLPs and live activated HIV preparations ADA and MN in Western blots (data not shown). Concentrated VLPs preparations (1,000x) typically contain 5μg gp120 per ml (determined by comparison to a known JR-FL gp120 reference in Western blot) and 500ug total protein per ml, measured spectrophotometrically. Again, the 1000x concentrated live inactivated virus preparations were very similar. High-resolution negative stain electron microscopy revealed trimers on the surfaces of WT-VLPs (Fig. 2D), similar to those observed on inactivated SIV (Zanetti et al., 2006; Zhu et al., 2003; Zhu et al., 2006). The average spike density on several VLP electron micrographs was 27 (range 15–37), approximately twice the number previously counted on live inactivated HIV (range 7–14) (Chertova et al., 2002; Zhu et al., 2003; Zhu et al., 2006).
Analysis of Env oligomers
Analysis of Env liberated from WT- and SOS-VLPs in BN-PAGE revealed a major band of approximately 400kDa, corresponding to authentic trimers (Zanetti et al., 2006; Zhu et al., 2006), as well as a gp120/gp41 monomer band (Fig. 3A; (Moore et al., 2006)). This contrasts with soluble gp140SOS that readily dissociates into monomers (Schulke et al., 2002), suggesting that expression in membranes lends some stability to Env trimers, as we initially hypothesized. The dominance of trimer band in both WT and SOS-VLPs suggests that gp120 shedding from WT-VLPs is not a major problem (Chertova et al., 2002; Zhu et al., 2003; Zhu et al., 2006). However, probing similar native PAGE blots with gp41 antibodies reveals gp41 stumps in WT that are not seen with SOS (Moore et al., 2006), suggesting that some gp120 shedding may occur (data not shown). The consistent production of gp41 immunodominant loop antibodies in natural infection suggests that gp41 stumps are a common antigen that might be best eliminated from immunogens, perhaps by the SOS mutation.
Figure 3. Native PAGE of VLP-derived Env proteins.

WT-VLPs, UNC-VLPs and SOS-VLPs were compared in Bis-Tris gels in A) native (blue-native) B) denaturing but nonreducing and C) reducing conditions. Ferritin was used as a molecular weight marker with bands at 439 and 220 kDa, as indicated. The same samples were analyzed by SDS-PAGE in D) nonreducing and E) reducing conditions.
In contrast to WT- and SOS-VLPs, UNC-VLP-derived Env separated as several species that appear to be monomers, dimers, trimers and tetramers (Fig. 3A) (Earl, Doms, and Moss, 1990; Moore et al., 2006; Staropoli et al., 2000; Zhang et al., 2001). Denaturing but not reducing conditions caused WT and SOS trimers to dissociate into monomers (Fig. 3B), but UNC oligomers remained largely unchanged (Fig. 3B). Denaturing and reducing conditions eliminated oligomers in all preparations (Fig. 3C). SDS-PAGE gels run in parallel indicated that little, if any uncleaved Env existed in WT-VLPs (Fig. 3D) and confirmed that reducing conditions disrupted the gp120-gp41 SOS bond (compare Fig. 3D and E). Here, the gp120 from WT- and SOS-VLP appeared as a doublet. We previously proposed that the two forms of gp120 arise from trimers and putative monomers of gp120/gp41 present on virion surfaces (Moore et al., 2006).
Immunogenicity of VLPs in guinea pigs
In this pilot study, we wished to identify an immunization algorithm suitable for further development. Thus, we evaluated multiple concepts relating to immunogens and the end stage serum analysis. We immunized a total of 17 guinea pigs with recombinant JR-FL gp120, UNC-VLPs, SOS-VLPs, and naked-VLPs. Animals were immunized as outlined in Fig. 4A. Each was immunized 3 times at 6 week intervals with bleeds collected 1 or 2 weeks thereafter. Although VLPs can induce potent Ab responses without adjuvants (Lorin et al., 2004; Wagner et al., 1998), to ensure maximal Ab titers, we included adjuvants in our regimes (Hammonds et al., 2005). One consideration in selecting adjuvants was that those that are emulsion-based could disrupt membranes. We therefore selected immunostimulatory DNA CpG as our main adjuvant, which is unlikely to affect VLP membranes. However, the CpG formulations used were optimized for rabbits and mice, and it was not known if they would be effective in guinea pigs, for which a commercially available CpG product was not available. Therefore, in a few animals (P1, P13 and P14), CpG was supplemented with Ribi Ras3c or QS-21, as indicated (Fig. 4).
Figure 4. Guinea pig serum Ab binding titers against Env.
A) Guinea pigs were immunized with JR-FL gp120, SOS-VLPs, UNC-VLPs, or naked VLPs, formulated in ImmunEASY CpG and in some cases Ribi or QS21, as indicated. Mature IC50 titers at the end of immunizations against JR-FL gp120 in intact and denatured (dgp120) forms and the gp41 “AVERY” peptide are shown. Progressively darker cells are used to visually differentiate binding potency. B) Kinetics of gp120 serum titer maturation during immunization in animals S2, S11 and P18. Arrows indicate days of immunization.
Binding titers to gp120 and a gp41 peptide
We measured serum gp120 titers over the course of immunizations. Of the 3 gp120-immunized control animals, P1 had the highest titer (Fig. 4A), perhaps owing to the QS21 adjuvant. In the Env-VLP-immunized animals, we observed high anti-Env titers, comparable to the A62 human plasma and gp120-immune sera (Fig. 4A). The kinetics of the responses to Env-VLPs was rapid: high titers were generated even after a single inoculation (compare animals S2 and S11 in Fig. 4B). The animals that received VLPs formulated in Ribi or QS21 as well as CpG generated titers similar to others, suggesting that CpG was sufficient for maximum titers. As expected, naked-VLPs did not elicit significant gp120 titers (Fig. 4A, B).
To evaluate the conformation dependency of antibody binding, we measured serum titers against denatured and reduced gp120 (dgp120). In most Env-VLP sera, dgp120 titers were <10% of those against intact gp120 (exceptions were S8 and S11), but in gp120-immunized animals, they were even lower. In contrast, a HIV-1 seropositive donor plasma exhibited similar titers to dgp120 and intact gp120. Taken together, this suggests that Env conformation was well preserved during adjuvant formulation and delivery and that Ribi or QS-21 formulation had similar effects on antigens compared to CpG alone.
To determine the effect of the SOS mutation on gp41 exposure, we measured antibody responses against a peptide derived from the immunodominant epitope of gp41 (the “AVERY” peptide). Most Env-VLP sera had very low titers. Exceptions were animals S7 and S8 that had been immunized with UNC-VLPs. As anticipated, the naked-VLP- and gp120 sera did not react with the peptide. In contrast, the HIV-1 seropositive plasma exhibited a high titer, in line with the use of similar peptides for HIV-1 diagnosis (Fig. 4A).
Mapping the specificity of immune sera
To map the immune sera, we used virus capture competitions and peptide ELISAs.
i) Inhibition of virus capture by mAbs
To determine the effect of sera on mAb-mediated particle capture (Derby et al., 2006), graded dilutions of sera were mixed with WT-VLPs bearing VSV-G, which were then added to mAb-coated microplates. This assay has the advantage of employing particulate (and therefore native) Env rather than gp120 or other soluble forms of Env. Using two different V3 mAb prototypes, LE311 and 39F, we found that SOS-VLP and UNC-VLP sera contained high titers of anti-V3 Abs (Fig. 5A, B), as did the HIV+ plasma control. Comparison of these titers to the IC50s of mAb self-competition allowed an estimate of the quantity of V3 blocking antibody (Crooks et al., 2005), which ranges from about 5–100μg/ml in these sera. In comparison, the gp120 sera showed only moderate titers of V3 competing Abs. As expected, the naked VLP sera did not exhibit detectable competition.
Figure 5. Mapping of guinea pig sera.
A) The ability of guinea pig sera and HIV+ human plasma A62 to inhibit virus capture by various mAbs. Reciprocal IC50 inhibition titers are shown. The IC50 of each mAb to self-inhibit capture is also shown (given in μg/ml). Epitopes are given in parentheses as the V3 loop, CD4 binding site (CD4bs), CD4-induced epitope (CD4i), the mannose epitope of 2G12 on gp120 (mann.), or the gp41 cluster I region. B) Representative inhibition curves of LE311 and 15e mAb capture by sera as indicated. C) Midpoint titers against peptides spanning the V3 loop of JR-FL gp120. Progressively darker cells are used to differentiate binding potency. n.d.=not done.
Focusing next on the CD4 binding site-overlapping mAbs 15e and b12, we found that serum P1 effectively inhibited virus capture with a potency corresponding to approximately 10–60 μg/ml CD4 binding site-overlapping IgG equivalents. The S1 gp120 serum, VLP sera S6, S11 and the A62 plasma moderately inhibited capture (Fig. 5A, B), while most others were weak or completely ineffective. Inhibition of the two CD4 binding site mAbs by each serum was largely consistent.
We next examined inhibition by antibodies that bind to the “CD4-induced” epitope, that overlaps the coreceptor binding site. The A62 HIV+ plasma and most of the Env-VLP sera effectively blocked X5 capture. Moderate blocking was observed for sera S6, and P14, and weak blocking was observed with S1, S2 and the naked VLP sera. The very high titer of the HIV+ plasma reflects recent findings that these antibodies are common in natural infection (Decker et al., 2005).
2G12-blocking activity was very low in all guinea pig sera and the HIV+ plasma control, consistent with the rare and unusual nature of this specificity. MAb 7B2 was next used to measure activity to the immunodominant loop of gp41. Activity was high in sera S7, S8 and S12, but weak in most others. For sera S7 and S8, this result is consistent with reactivity to the immunodominant peptide in Fig. 4A. UNC oligomers do not have the same exclusivity for binding only nAbs as SOS and WT trimers (Moore et al., 2006). 7B2 blocking by the S12 serum is anomalous and difficult to explain, given that this site is occluded on SOS-VLPs (Moore et al., 2006). The HIV-1 seropositive plasma also reacted strongly with the gp41 immunodominant loop, consistent with Fig 4A. Another guinea pig recently immunized with WT-VLPs also developed 7B2-blocking activity (data not shown), perhaps arising from exposure of gp41 stumps following gp120 shedding.
ii) Peptide ELISA
We next assessed serum binding to a panel of overlapping peptides spanning the V1, V2 and V3 loops (Fig. 5C). Some sera had weak activity against the V1 loop, particularly SOS-VLP sera S12, P13 and P15 (not shown). The V1 loop has, in fact, emerged as a common specificity in gp120-immune sera (Derby et al., 2006; Garrity et al., 1997; He et al., 2002; Li et al., 2006). However, none of the sera reacted with the V2 loop (not shown). The A62 plasma did not react effectively with any of these peptides.
A major focus of many ENV-VLP sera appeared to be the N-terminus of the V3 loop, specifically peptides 8838 (TRPNNNTRKSIHI) and 8839 (NNNTRKSIHIGPGRAF) (Fig. 5C). The A62 plasma and several V3 loop-specific mAbs, including LE311 and 39F (not shown) also recognized these peptides. Although no consistent patterns separated the immunogen groups, the gp120 sera had lower V3 peptide titers, in line with weaker V3 mAb capture inhibition (compare Figs 5A and C). The reactivity of other Env-VLP sera to V3 peptides was generally stronger, but quite variable. Interestingly, V3 mAb competition (Fig. 5A) was not always associated with V3 peptide reactivity, suggesting that some binding depends on conformations that are not well approximated by peptides.
Collectively, our mapping efforts indicate that SOS-VLPs and UNC-VLPs elicit high titers of V3 loop-specific Abs and often quite high titers of CD4i-overlapping Abs, but weak CD4 binding site overlapping antibodies. In contrast, gp120 sera exhibited moderate V3 and CD4i titers and moderate to high titers of CD4bs-overlapping Abs. It is possible that gp120 and Env-VLP sera differ in that the gp120 might exist in a native conformation, while forms of Env presented on the VLPs might resemble “triggered” forms of Env, in which the V3 loop and coreceptor binding sites have become more exposed. The SOS mutation did, however, appear to largely prevent exposure of the gp41 immunodominant epitope, as was intended. Finally, regarding adjuvants, there was generally insufficient evidence so far to suggest that QS21 or Ribi added much beyond that achieved by CpGs. A possible exception was that the gp120 response in P1 was stronger and more focused on the CD4 binding site.
Analysis of neutralization activity
Reports on particle immunogens for HIV-1 have variably described neutralization or enhancement of infection by immune sera (Arthur et al., 1992; Buonaguro et al., 2005; Haffar et al., 1991; Hammonds et al., 2005; McBurney, Young, and Ross, 2007; Poon et al., 2005a; Poon et al., 2005b; Verrier et al., 2000). This may be in part related to the generation of antibodies against non-Env membrane proteins that can have unpredictable effects on infection. Indeed, it is often unclear whether “neutralization” is mediated by Abs directed to Env or to other membrane proteins (“anti-cell” antibodies). With this in mind, we evaluated neutralization in several formats, incorporating controls to help distinguish anti-Env activity from nonspecific effects.
We first measured neutralization against the index primary virus, JR-FL, in a luciferase assay using canine CF2 target cells (Fig. 6A). Some SOS- and UNC-VLP sera (S6, S11, S12, P14 and P15) enhanced infection by 2–3 fold (Fig. 6A, column 1), even at high dilutions (exemplified by serum P15 in Fig. 7A), while other Env-VLP sera and the gp120 sera had no effect. In contrast, the naked VLP sera exhibited apparent “neutralization”. In the following two sections, we separately address the activity of gp120/Env-VLP sera and naked VLP sera in the CF2 neutralization assay.
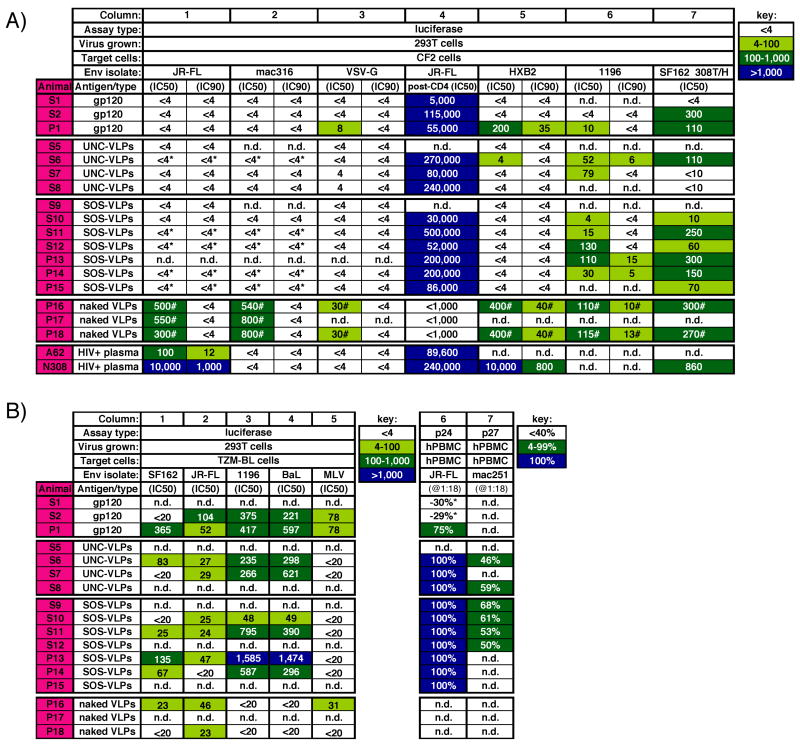
Figure 6. Serum neutralizing activity in various assays.
The neutralization activity of guinea pig sera and HIV+ plasmas A62 and N308 was measured using A) CF2 cells or B) TZM-BL cells (columns 1–5) or PBMCs (columns 6 and 7) as targets. In the CF2 assay, JR-FL, SIVmac316, HXB2 and SF162 308T/H Envs were gp160ΔCT and the 1196 Env was a full-length gp160. The virus producer cells (293T cells or human PBMC, hPBMC) and the target cells in which neutralization was measured are indicated. * denotes enhancement in the presence of serum. # indicates “neutralization” that is likely to be the result of toxicity against the target cells or other nonspecific factors. Progressively darker cells are used to differentiate binding potency. n.d.=not done.
Figure 7. The nature of Env-VLP sera neutralization and enhancement.
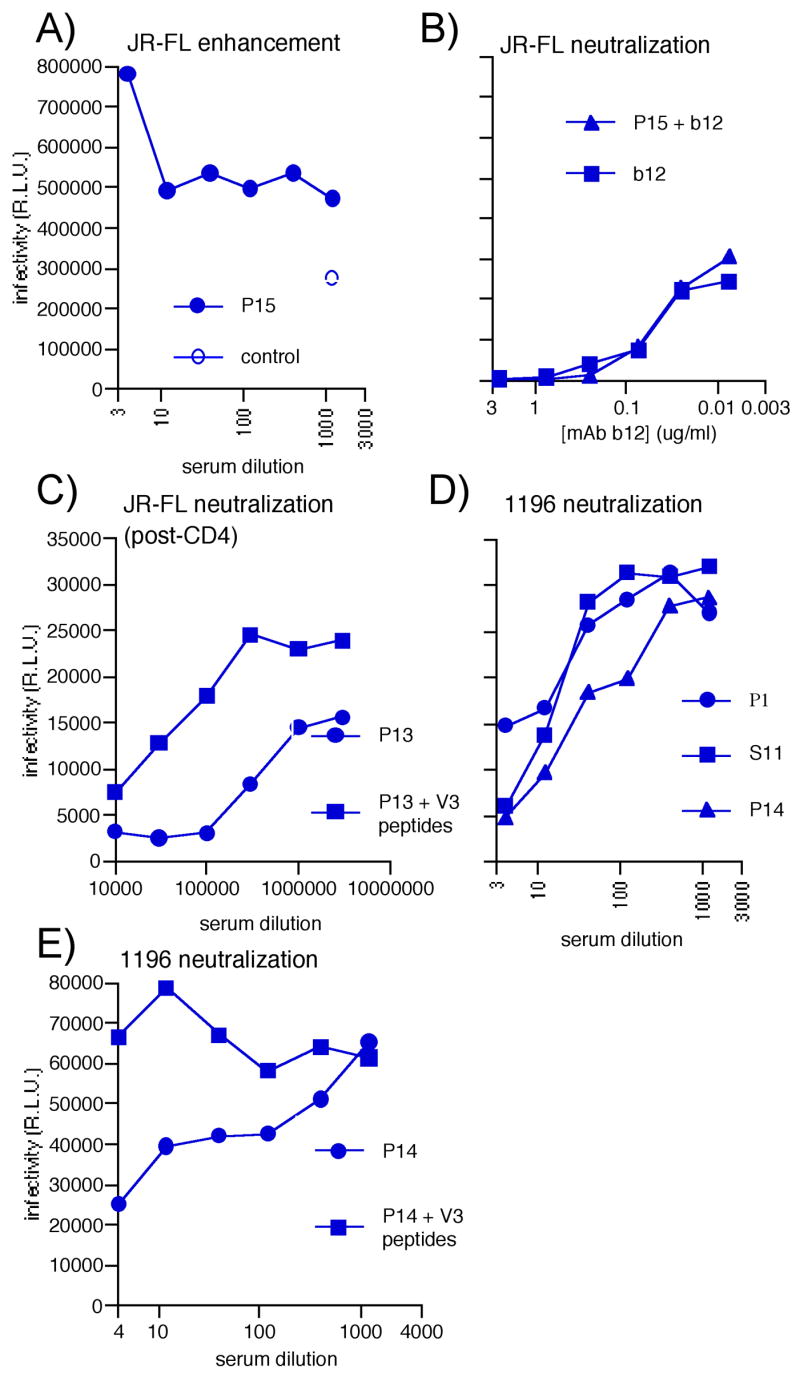
A) SOS-VLP serum P15 enhancement of JR-FL virus infection in the CF2 assay. B) The effect of spiking P15 serum with the neutralizing mAb b12 compared to b12 mAb alone on JR-FL infection. Here, the P15 serum starting dilution was 1:4 and the b12 starting concentration was 2.5μg/ml in both samples. The P15 serum and b12 mAb were diluted in parallel. C) Post-CD4 activity of P13 against JR-FL, with and without interference by fixed 20μg/ml concentrations of V3 peptides, TRPNNNTRKSIHIGP (8837) and NNTRKSIHIGPGRAF (8838). D) Representative neutralization curves against virus 1196 in the CF2 assay. E) Neutralization by the P14 serum against 1196 in the presence or absence of interfering V3 peptides 8837 and 8838, as above. The effect of the peptides alone on infection was negligible (not shown).
Neutralization activity of the gp120 and Env-VLP sera
Enhancement could arise either from antibodies directed to Env that somehow activate fusion or from antibodies directed to membrane components that somehow facilitate virus attachment. To investigate, we tested the effect of sera on SIVmac316 infection (Fig. 6A, column 2). This virus was made by transfection of the same 293T cells as the HIV pseudoviruses and, therefore, would be expected to bear the same set of cellular membrane proteins. Similar enhancement of this virus was noted with many of the sera, as it was for JR-FL, suggesting that the enhancement stemmed from Abs directed to non-Env membrane components of VLPs. In contrast, the control HIV+ plasmas A62 and N308 neutralized JR-FL, but not SIVmac316.
It has been suggested that enhancing Abs may mask neutralization (Giannecchini et al., 2001; Langlois et al., 1992). Indeed, in a previous study, removal of anti-cell antibodies against FIV particle vaccine by adsorption against producer cells was reported to uncover neutralizing activity (Giannecchini et al., 2001). To test this concept, we adsorbed SOS-VLP serum P15 against 293T cells and removed ~90% of the anti-cell reactivity, as assessed by FACS (data not shown), while retaining strong gp120 titers (diluted only 2–3 fold during adsorption). However, this did not uncover any neutralization (not shown).
To further determine whether enhancing Abs mask neutralization, we spiked the JR-FL-enhancing serum P15 with neutralizing mAb b12. Comparing neutralization by b12 alone to a b12/serum mixture (Fig. 7B) showed that the serum did not reverse b12 neutralization. Thus, infection by a virus that has been neutralized was not rescued by factors that otherwise enhance infection. In further neutralization assays using a VSV-G pseudotyped virus, the Env-VLP sera showed no enhancement (Fig. 6A, column 3), perhaps because VSV-G entry is amphotropic and therefore influenced by different factors compared to HIV and SIV.
Though we did not see convincing neutralization against the index virus, JR-FL, ELISAs had suggested that sera contained potent anti-Env reactivity (Fig. 4). To assess neutralization in alternative conditions, we measured “post-CD4” activity (Crooks et al., 2005) by pre-incubating JR-FL particles with sCD4 and graded dilutions of sera and then measuring infection of CF2 cells expressing only CCR5. We found that all Env-VLP and gp120 sera neutralized effectively in this format (Fig. 6A, column 4), as did both HIV+ plasmas. However, as expected, naked-VLP sera did not. Enhancement was not observed in the post-CD4 format, providing further evidence that factors that enhancing effects are negated when the virus is neutralized. With reference to our previous analysis (Crooks et al., 2005), post-CD4 neutralization suggested the presence of Abs that recognize the V3 loop and/or CD4-induced epitope(s), consistent with our mapping analysis (Fig. 5). The contribution of V3 Abs to neutralization was investigated in “peptide interference” neutralization assays (Beddows et al., 2005; Li et al., 2006; Selvarajah et al., 2005). Here, V3 peptides strongly inhibited post-CD4 neutralization by serum P13 (Fig. 7C), suggesting a major role for V3 Abs in post-CD4 neutralization.
Although we were encouraged by the strong serum activity in the post-CD4-format, it is important to point out that this assay dramatically expands access to epitopes that are normally cryptic in native trimers and become exposed only fleetingly during infection, as evidenced by the generally weak to modest activity of these types of antibodies in traditional neutralization assays (Bou-Habib et al., 1994; Crooks et al., 2005; Decker et al., 2005). Indeed, most HIV+ plasmas contain high concentrations of CD4i Abs without strong neutralizing titers. Therefore, the significance of post-CD4 activity should not be over-interpreted.
Given the lack of neutralization against the JR-FL index virus in the standard format, we tested some more sensitive isolates. The P1 serum strongly neutralized HXB2, a highly sensitive X4 isolate (Fig. 6A, column 5). Perhaps underlying this activity, mapping had indicated high levels of CD4bs-overlapping Abs in this serum (Fig. 5A). However, no Env-VLP other serum neutralized HXB2, probably because the predominant V3 Abs (Fig. 5) do not cross-react with this isolate.
The gp120 serum P1 weakly neutralized SF162, as did SOS-VLP sera S12 and P15, but no others (not shown). We also observed weak neutralization against ADA and BaL (not shown), but moderate neutralization against the 1196 isolate (Fig. 6A, column 6, and Fig. 7D). Epitope interference assays suggested that the 1196 neutralization was V3-mediated (Fig. 7E), consistent with the greater susceptibility of this virus to V3-directed neutralization (Binley et al., 2004).
Collectively, our neutralization data so far suggested that Env-VLP sera neutralize via anti-V3 Abs and the gp120 serum P1 neutralizes via CD4 binding site overlapping Abs. The lack of SF162 neutralization via the Env-VLP sera may stem from differences in the V3 loop between JR-FL and SF162. When we substituted a residue in SF162 to match the N-terminus of the V3 to JR-FL, the mutant virus was significantly more sensitive to Env-VLP sera (Fig. 6A, column 7). The SF162 variant was slightly less sensitive to the neutralizing N308 HIV+ plasma, revealing that the mutation did not globally increase neutralization sensitivity.
Analysis of neutralization by naked VLP sera
In light of the absence of JR-FL neutralization by gp120 and Env-VLP sera, it was not immediately clear why naked VLP sera potently “neutralized” (Fig. 6A, column 1). Evidence that the neutralization was non-specific came from the observation that the sera also neutralized SIVmac316 (Fig. 6A, column 2). In addition, interference of P18 neutralization of the 1196 isolate using V3 peptides had no effect (not shown), consistent with nonspecific neutralization.
Another method to help distinguish nonspecific activity from genuine neutralization is that the former may not embody the sigmoidal dose-response typical of neutralization. We and others have observed that IC90 titers typically occur at approximately a 10 fold higher Ab concentration than the IC50 (Binley et al., 2004). In contrast, the “neutralization” naked VLP sera took the form of “shallow” curves, with IC50 titers of ~1:500 in many cases, that never reached an IC90 (at a dilution of 1:20 or greater), suggesting nonspecific neutralization (Fig. 8A). Different isolates were affected to varying degrees by these non-specific antibodies (Fig. 6A, column 5) in a way we do not yet understand.
Figure 8. Analysis of nonspecific neutralization in naked VLP sera.

A) The nonspecific neutralizing effect of P18 on 1196 infection, compared to a control with no serum. B) The effect of P18 on b12 mAb neutralization of JR-FL. In the spiking experiment, P18 at a final dilution of 1:4 was mixed with 2.5μg/ml b12 and the mixture was serially diluted and added to the virus. C) Reactivity of guinea pig serum P12 with 293T, U87 and CF2 cell lines measured by FACS. The pre-bleeds were used at a 1:50 dilution and final bleed were used at a dilution of 1:250.
To determine whether non-specific neutralization influences genuine neutralization, we evaluated the effect of spiking a naked VLP serum (P18) with the b12 mAb at 2.5μg/ml. Here, b12 neutralization could be distinguished only when its concentration was sufficient to reach an IC90 (compare Figs. 8A, B).
One possible explanation as to why naked VLP sera nonspecifically neutralized while Env-VLP sera generally enhanced might be that the specificity or titer of anti-cell Abs in Env-VLP sera may differ from those in naked VLP sera. FACS analysis revealed that naked VLP serum P18 bound more potently to 293T cells than serum P12 (median fluorescence intensities at a 1:2,000 serum dilution were 717 and 181, respectively). These differences could underlie the differential effects of these sera in neutralization assays, where nonspecific neutralization might occur above a threshold of anti-cell Abs.
It is possible that the cells in which virus was produced and/or the target cells in which virus infection is measured might influence neutralization. We therefore assessed serum binding to various cell lines by FACS. We found that SOS-VLP serum P12 reacted extremely strongly with 293T cells, the same cell line used to produce VLPs (Fig. 8C) and almost as strongly against U87 cells (a human glioma cell line). However, its reactivity against canine CF2 cells used in the neutralization assays described above was much lower, though still significantly positive. This suggests that a fraction of the anti-cell activity in sera is cell line-specific.
Alternative neutralization assays
To investigate the effect of differential cell reactivity on neutralization, alternative neutralization assays were performed at a separate site (D.C.M.). In an assay measuring infection of 293T cell-produced virus in TZM-BL target cells (Fig. 6B columns 1–5), the nonspecific neutralizing activity of naked VLP sera was much lower and reactivity against the MuLV control virus was also low (Fig. 6B, column 5). Serum titers against SF162, JR-FL, 1196, or BaL pseudotyped viruses were, in general, higher (Fig. 6B, columns 1–4), than the corresponding titers using CF2 target cells (Fig. 6A and data not shown). Serum P1 was very potent against SF162 (Fig. 6B, column 1), consistent with the presence of CD4 binding site-overlapping antibodies. Neutralizing activity against JR-FL, though higher than before, remained relatively modest (Fig. 6B, column 2). Neutralization of 1196 and BaL was more impressive. Titers against 1196 were, in many cases, more than ten fold higher than in the initial CF2 assay (compare Fig. 6A, column 6 to Fig. 6B, column 3). It is possible that Ribi and QS21 adjuvants given to animals P13 and P14 contributed to especially high titers.
The above results were surprising, considering the greater overall anti-cell reactivity that would be expected against the human HeLa-based TZM-BL target cells. In addition to the cell line, differences in protocol may contribute to this effect. In the TZM-BL assay, but not the CF2 assay, sera, virus and cells are co-incubated during the entire infection period. We have found that this tends to increase neutralization IC50 titers (not shown). Another possibility may relate to coreceptor density. The CF2 cells in the initial assay express high levels of CCR5, to assist in efficient infection. This may decrease the window of opportunity for antibodies that neutralize via binding the V3 or coreceptor binding sites (Crooks et al., 2005). The lower CCR5 density on the TZM-BL cells could therefore contribute to the greater neutralization potencies. Finally, in the CF2 assay, we used a short spinoculation step to increase overall infection levels that was not used in the second assay. However, spinoculation did not significantly affect the activity of neutralizing mAbs (not shown).
In further neutralization assays employing PBMC-produced JR-FL primary viruses, also assayed on PBMC, we observed complete neutralization with most VLP sera at a 1:18 dilution, but little or no neutralization by the gp120 sera (Fig. 6B, column 6). We also observed strong activity against SIVmac251 in this format (Fig. 6B, column 7). It appears that, as in the CF2 assay toxicity adversely affected neutralization measurement. Taken together, these results highlight the unpredictable effects of anti-cell antibodies. Given that the rules governing these effects are far from clear, our results provide a cautionary note, suggesting that particular care should be taken when examining sera generated against membranous antigens.
Measurement of trimer binding in blue native PAGE
In circumstances when neutralization assays are compromised by high background activity, BN-PAGE offers an independent way examine trimer binding. We recently reported that a shift in the migration of a VLP-derived trimer band in BN-PAGE, associated with a depletion of unliganded trimer correlates with mAb neutralization (Crooks et al., 2005; Moore et al., 2006). Here, we adapted the method to measure neutralization by polyclonal sera and plasmas. We first tested the ability of the guinea pig sera to bind to JR-FL Env trimers in the presence or absence of additional neutralizing mAb b12 (Fig. 9A). We found that Env-VLP sera depleted the unliganded trimer to 43–96% of the control density, suggesting weak neutralization. Tellingly, the naked-VLP serum, P16 did not deplete unliganded trimers significantly, suggesting that nonspecific effects are inconsequential in this native PAGE assay. In experiments in which VLP sera and b12 were mixed together, the effect of b12 to deplete unliganded trimers was not affected (Fig. 9A). The shifted trimers were somewhat diffuse compared to when b12 was used alone, perhaps indicating multiple forms of trimer-Ab complexes.
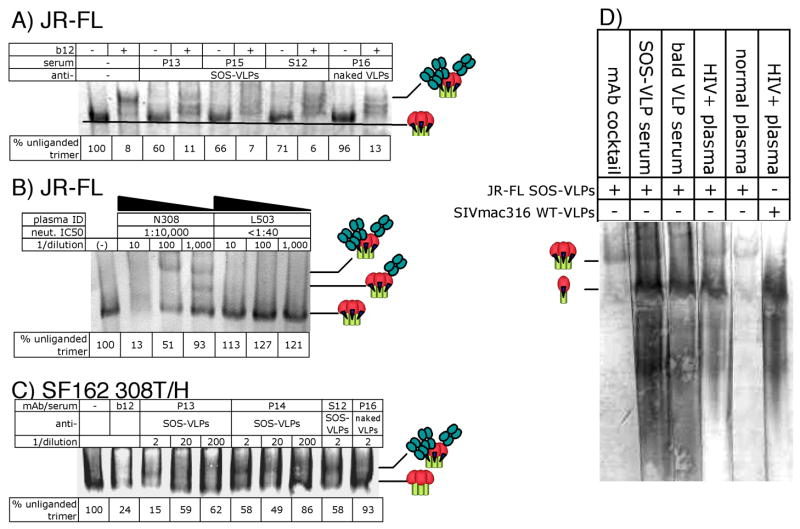
Figure 9. Blue-native PAGE trimer shifts distinguish neutralization from nonspecific effects.
A) JR-FL 140T SOS-VLPs were incubated with VLP sera and/or mAb b12 Fab, as indicated, at a final dilution of 1:3 for sera and 20μg/ml for mAb b12. Samples were resolved by BN-PAGE and Env proteins were visualized by Western blot. The bands are identified in cartoon form as unliganded and liganded trimers. In liganded complexes, for clarity, the antibodies are depicted as Fabs. The density of the unliganded trimer band relative to that in the untreated VLP control (taken as 100%) are given in the bar below the gel. B) SOS-VLP trimer shift assays were performed with graded dilutions of the neutralizing N308 or non-neutralizing L503 plasmas at the dilutions indicated. C) Trimer shifts using sera at a 1:2 dilution were performed using the neutralization-sensitive isolate SF162 308T/H. MAb b12 IgG was used at 30 μg/ml. D) SOS-VLPs or SIVmac316 VLPs were probed with a gp120 mAb cocktail (Crooks et al., 2005; Moore et al., 2006), VLP sera (P15 or P18), HIV-infected plasma (N308) or HIV negative plasma.
For reference purposes, we analyzed the ability of the neutralizing plasma N308 and the non-neutralizing plasma L503 to shift JR-FL trimers. Like b12, N308 effectively depleted unliganded trimers (Fig. 9B). At low dilution, this was accompanied by a smear, that resolved into distinct shifted bands at higher dilutions, suggesting differing numbers of neutralizing Abs attached to trimers. In contrast, the non-neutralizing plasma L503 did not detectably deplete unliganded trimers, even at the lowest dilution, confirming that neutralization can be measured unequivocally by BN-PAGE, as defined by a loss of unliganded trimer, accompanied by a gain in higher molecular weight liganded forms.
We next determined whether Env-VLP sera could mediate more effective trimer shifts of isolates they effectively neutralized. In Fig. 9C, mAb b12 and the Env-VLP sera P13, P14 and S12 all shifted SF162 308T/H trimers to varying degrees. P13 was more effective than P14 and S12, as reflected in neutralization assays (Fig. 6A, column 7). On the other hand, the naked VLP serum P16 did not shift trimers, consistent with its lack of neutralization activity.
Strong reactivity of VLP sera and HIV plasmas to a non-Env component of VLPs
We examined the overall antibody reactivity with VLPs by comparing neutralizing mAbs, VLP sera, HIV patient sera and HIV negative sera as probes for VLPs in native PAGE/Western blot, revealing some remarkable findings (Fig. 9D). The neutralizing mAbs bound to the strong trimer band, and the weaker monomer band ((Moore et al., 2006). In contrast, the SOS-VLP serum bound extremely strongly to a ~150 kDa band and much less to the trimer, consistent with the idea that gp120/gp41 monomers are a prime target of B cells. Surprisingly, the naked VLP serum also bound extremely well to a band of the same size as the monomer. Since the naked VLP sera do not react with Env (Fig. 4), this indicates reactivity to a non-Env component of VLPs. A HIV positive patient serum gave similar results to the SOS-VLP sera: weak trimer binding, but strong reactivity to a band around the size of the monomer. In contrast, a HIV negative serum was essentially unreactive. Probing SIV-VLPs, we also observed HIV plasma reactivity to the ~150kDa protein, suggesting that VLPs and HIV infection elicit strong responses to a non-Env protein (Gag or host protein) component of VLPs. Overall, this further suggests that non-trimer components of particles are preferred targets for B cells both in immunizations and natural infection.
DISCUSSION
Here we showed that VLPs are a versatile vaccine platform, providing a complete framework to assess VLP-Env trimers as immunogens and a powerful end-stage system for analyzing sera (Crooks et al., 2005; Derby et al., 2006). Our initial vaccine approach emerged from the idea that earlier particulate HIV vaccine candidates may have failed to elicit broadly neutralizing antibodies because the Env trimers are unstable. In particular, gp120 shedding might leave behind non-functional depleted trimers or gp41 stumps (Chertova et al., 2002; Grovit-Ferbas et al., 2000; Hammonds et al., 2003; McKeating, McKnight, and Moore, 1991; Moore et al., 2006). To address this problem, we introduced a disulfide bridge to stabilize gp120-gp41 (SOS). To further optimize VLP immunogens, we truncated the gp41 cytoplasmic tail to increase expression and gp120/gp41 cleavage, with only a mild effect on neutralization sensitivity (Abrahamyan et al., 2005; Binley et al., 2003; Edwards et al., 2002; Gabuzda et al., 1992; Mammano et al., 1997). Since SOS-VLPs retain fusion competency (Abrahamyan et al., 2003; Binley et al., 2003; Crooks et al., 2005; Moore et al., 2006), we reasoned that they were a viable starting point for testing the ability of authentic trimers to elicit neutralizing antibodies (Abrahamyan et al., 2003; Binley et al., 2003).
Nonfunctional gp120/gp41 monomers may be a prime Ab target on VLPs
In spite of very high binding titers to monomeric gp120 and neutralization activity against sensitive primary isolates, SOS-VLP sera did not neutralize the JR-FL parent virus or most other neutralization-refractive isolates. This mirrors the majority of vaccine studies in which immune sera neutralize only V3-sensitive primary isolates like BaL, SF162 and 1196 (Koff, Kahn, and Gust, 2007). Indeed, the neutralizing activity of VLP sera appeared to stem at least in part from V3 antibodies. The fact that the V3 loop is cryptic on trimers of neutralization-resistant viruses like JR-FL (Bou-Habib et al., 1994) raises the question: where do these anti-V3 Abs come from? Since our control immunogens, UNC-VLPs also elicited strong responses to the V3 loop, we suggest that, in each case, B cells responded to forms of Env other than functional trimers, perhaps gp120/gp41 monomers (SOS-VLPs) or unprocessed gp120-gp41 (UNC-VLPs). Thus, although SOS-VLPs eliminate gp120 shedding, the SOS mutation is only a partial solution toward presenting trimeric Env on VLPs for vaccine purposes.
Previous analyses have suggested that gp120/gp41 monomers, unlike trimers, these are highly accessible to V3 Abs (Burrer et al., 2005; Moore et al., 2006; Nyambi et al., 1998; Poignard et al., 2003) and may be a primary target for B cells. We hypothesize that monomers act as decoys, distracting the attention of B cells from the more compact trimers, in what might be considered an “immunogenic hierarchy”. Evidence of such hierarchies in studies of immune responses to HIV-1 have in fact been widely reported (Cleveland et al., 2000; Garrity et al., 1997; Jelonek et al., 1996; Liu et al., 2006). Perhaps at odds with this concept, affinity selection in germinal centers dictates that higher affinity B cell blasts proliferate at the expense of lower affinity ones. Thus, antibody responses to multiple separate proteins might be expected to develop independently and equivalently, as if they were given alone, as for the trivalent measles, mumps, and rubella vaccine (Usonis et al., 2005). However, the situation with VLPs is in fact quite different, because trimers and monomers are linked on the same particle, and therefore may be considered as a single target. Thus, B cells would be selected against only the most accessible foreign target(s), the V3 loop, of the most accessible structure (the monomer), at the expense of other epitopes, in this case, the trimer.
We previously showed that monomer is only a minor component of particles, compared to the predominant trimer (Fig. 3 and ref (Moore et al., 2006)). This raises the question why a minor species would dominate the B cell response. The answer may simply be that form rather than quantity dictates the antibody response. We previously showed that non-neutralizing antibodies capture HIV with high efficiency, even though they do not bind efficiently to trimers, suggesting that monomer binding mediates the capture. BN-PAGE further confirmed the more promiscuous binding properties of monomers (Moore et al., 2006). Thus, the monomer may dominate as an antigen, simply because it is a particularly accessible target.
Of particular significance to the present study, a previous report compared the immunogenicity of UNC and “SOSIP” mutants of a soluble gp140 glycoprotein and gp120 monomer in rabbits (Beddows et al., 2006). Sera generated against SOSIP stabilized trimers potently neutralized the parental JR-FL isolate. This activity was not due to V3 Abs, but its precise specificity remains unclear. Although the neutralization was type-specific, because the JR-FL isolate is refractive to neutralization, the results with SOSIP suggest a possible step in the right direction for HIV vaccine research.
Both SOSIP gp140 and SOS-VLPs vaccine approaches emerged from the early observation that SOS gp140 readily dissociates into monomers and a need to stabilize them as trimers (Schulke et al., 2002). The relative success of SOSIP gp140 raises questions about what might underlie the quite different findings of the present study. At odds with the idea that particles present native forms of Env, and therefore may be particularly effective immunogens, the results of these two studies might be interpreted to suggest that the reverse is, in fact, true. More likely, however, the differences probably arise from the fact that monomeric forms of Env were completely eliminated in SOSIP gp140 immunizations, but not in SOS-VLPs (Moore et al., 2006). However, since VLP trimers are recognized exclusively by neutralizing Abs (Moore et al., 2006), unlike even the most advanced soluble trimers (Dey et al., 2006), they may still have an advantage for presenting truly native trimers, if the right formulation can be found.
Factors affecting the quality of the immune response
We were cautious that commonly used adjuvants such as alum and Ribi might disrupt particle membranes, perhaps affecting Env conformation. Therefore, we selected immunostimulatory CpG as our primary adjuvant. Our mapping data indicate, however, that sera from the few animals that received VLPs in emulsion adjuvants differed little from those who received CpG alone. There is a suggestion, however, that the additional adjuvants lead to higher neutralization titers against certain viruses (exemplified by the activities of sera P13 and P14 in Fig. 6). Therefore, adjuvants may play a significant role in getting the most out of immunogens. In contrast to Env-VLP sera, naked VLP sera all showed potent nonspecific neutralization in certain neutralization assays. The lack of Env on these particles may have driven a potent anti-cell Ab response. Further studies should help us to fully understand these effects.
The route of immunization could affect the stability of VLPs after immunization, perhaps also affecting the quality of the antibody response. Intranasal administration, for example, has been shown to be effective (McBurney, Young, and Ross, 2007). However, since the nAb titers in other studies have not been dramatically improved, the more fundamental problem of non-functional forms of Env may need to be solved before the extent of the advantages offered by varying route and adjuvant can be fully appreciated. Another, perhaps more significant factor is the choice of model species for vaccation trials, based partly on the notion that some species may simply not have the repertoire to generate nAbs (Koff, Kahn, and Gust, 2007). Indeed, we have recently begun VLP immunizations in macaques that suggest a greater focus on the CD4bs and improved neutralization activity. Developments in mapping technology, we attempted here, should in future help us to make more informed adjustments to our vaccines.
The effects of “anti-cell” antibodies
A drawback of membranous immunogens is that they induce Abs against non-Env components, exemplified by the strong reactivity of VLP and HIV patient sera to a non-Env VLP band in BN-PAGE (Fig. 9D). Antibodies against non-Env VLP surface components can enhance or non-specifically “neutralize” infection in a largely unpredictable manner (Arthur et al., 1992). We incorporated controls at various levels in neutralization assays to identify any nonspecific activity. However, the cleanest method to assess neutralization was the native trimer shift assay (Fig. 9A–C) that should help in assessing the progress of any membranous HIV vaccine.
The differences between IC50s in assays of the two laboratories that tested VLP sera were somewhat surprising. Perhaps related to this, a recent proficiency test of the TZM-BL neutralization assay in multiple laboratories (D.C.M., unpublished) has illuminated unexpected discrepancies that appear to implicate pseudovirus stock preparation as an important variable. Further proficiency testing is now underway to understand this issue toward the ultimate goal of standardizing neutralization assays.
The future of HIV-VLP immunogens
In natural infection, the delay in development of nAbs (Deeks et al., 2006), despite the early development of gp120 binding antibodies suggests that nonfunctional forms of Env provide the virus with a valuable fitness advantage by diverting antibody responses from functional targets. To make progress, we may need to get beyond these defenses (Burton and Parren, 2000). It may be possible to refocus Ab responses against trimers (Moore et al., 2006) by modifying or blocking epitopes on nonfunctional Env (Cole et al., 2004; Garrity et al., 1997; Keller and Arora, 1999; Selvarajah et al., 2005; Srivastava et al., 2003). However, it could be argued that trimers are inherently poor immunogens, and that removal of irrelevant forms of Env may not change that fact. On the other hand, the development of cross-neutralizing activity in a subset of infected HIV patient sera, however, suggests that, eventually, B cells can in fact respond to trimers and the neutralizing activity of SOSIP gp140 sera provides evidence that trimers are not inherently poor antigens. Thus, if we can find a way to steer B cells away from irrelevant targets, we may be able to entice responses against native trimers.
MATERIALS AND METHODS
Plasmids and mutagenesis
The plasmid pCAGGS was used to express membrane-bound forms of the primary R5 isolate, JR-FL (Binley et al., 2003; Moore et al., 2006). We expressed full-length gp160 and gp160ΔCT, truncated at amino acid 709, leaving 3 amino acids of the gp41 cytoplasmic tail. The SOS and UNC mutants to stabilize gp120-gp41 association have been described previously (Binley et al., 2000). Gp160-expressing plasmids of isolates SF162, BaL, HXB2, ADA, and 1196 were obtained from Drs. Leo Stamatatos, David Montefiori, and the NIH AIDS Repository. A mutant of SF162 Env in which the threonine residue at 308 of the V3 loop was exchanged for histidine (SF162 308T/H) was created by Quikchange mutagenesis. Truncated gp160ΔCT versions of 1196, SF162, SF162 308T/H, and SIVmac316 Envs were cloned into pCAGGS in a similar manner to that described for JR-FL (Binley et al., 2003). The plasmid pVSV-G was obtained from Dr. Nathaniel Landau. Sub-genomic plasmids pNL4-3.Luc.R-E- and pSG3ΔEnv have been described previously (Binley et al., 2002; Li et al., 2005a).
MAbs, soluble CD4, recombinant gp120 and HIV+ donor plasmas
Anti-gp120 monoclonal antibodies (mAbs) included b12 and 15e, directed to epitopes that overlap the CD4 binding site (CD4bs) (Burton et al., 1994); 2G12, directed to a unique glycan-dependent epitope on gp120 (Sanders et al., 2002a; Scanlan et al., 2002); X5 directed to a CD4-inducible (CD4i) epitope (Darbha et al., 2004; Labrijn et al., 2003); LE311, 39F and PA1 directed to the V3 loop (Crooks et al., 2005; Schulke et al., 2002); and B12, directed to an epitope in the C2 domain of gp120 that is preferentially exposed on denatured forms of the molecule (Moore et al., 2006). Anti-gp41 mAb 7B2 is directed to the cluster I region (Binley et al., 2000).
MAb 2G12 was provided by Dr. H. Katinger (Polymun Scientific Inc., Vienna, Austria). MAbs 39F, LE311, and 7B2 were provided by J. Robinson (Tulane University). MAb B12 was provided by Dr. George Lewis (Institute of Human Virology, Baltimore, MD). MAb PA1, four-domain soluble CD4 (sCD4) and JR-FL gp120 were provided by Progenics Pharmaceuticals Inc (Tarrytown, NY). MAbs b12 and 2G12 are broadly neutralizing (Binley et al., 2004); mAb X5 neutralizes primary isolates in the presence of soluble CD4 (Crooks et al., 2005; Moulard et al., 2002); the V3 loop-specific Abs neutralize a subset of primary isolates (Binley et al., 2004); mAb 7B2 is nonneutralizing. The HIV-1-infected donor plasmas A62, L503 and N308 were described previously (Crooks et al., 2005). The anti-p24 serum was obtained from the NIH AIDS Reagent Program.
Peptides
Eleven residue-overlapping 15-mer peptides of the V3 loop of JR-FL were obtained from the NIH AIDS Reagent Program. Five residue-overlapping 15-mer peptides of the V1/V2 loop of JR-FL were synthesized at The Torrey Pines Institute for Molecular Studies and purified to >90%. A peptide of the gp41 immunodominant region, sequence RVLAVERYLKDQQLLGIWGCSGKLIC, termed the “AVERY” peptide was purchased from AnaSpec (San Jose, CA).
Production of VLPs
VLPs were produced by transient transfection of 293T cells with plasmids pNL4-3.Luc.R-E- and a pCAGGS-based Env-expressing plasmid by calcium phosphate precipitation. Two days later, supernatants were collected, precleared by low speed centrifugation, filtered through a 0.45 μM filter and pelleted at 50,000 xg in a Sorvall SS34 rotor. To remove residual medium, pellets were diluted with 1ml of PBS, then recentrifuged in a microfuge at 15,000 rpm. VLPs were then resuspended in PBS at 1000 x the original concentration. VLPs were referred to as SOS-VLPs, UNC-VLPs, WT-VLPs, depending on the form of Env on their surfaces, or as “naked-VLPs” bearing no Env (produced by transfecting pNL4-3.Luc.R-E- alone). Particles were inactivated using aldrithiol (AT-2) (Rossio et al., 1998), after which they were re-centrifuged and washed with PBS.
Immunoprecipitation
Supernatants of 293T cells were metabolically labeled and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), as previously described (Binley et al., 2000). Reduced and nonreduced samples were prepared by boiling for 5 min in Laemmli sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue) in the presence or absence of 20mM dithiothreitol (DTT).
SDS-PAGE, Native PAGE and Western blot
VLP preparations were separated by SDS-PAGE, and Western blots were probed with mAbs PA1 and B12 diluted to 1 μg/ml in PBS containing 2% nonfat milk, detected with a goat anti-mouse alkaline phosphatase conjugate (Jackson) and developed using BCIP/NBT colorimetric reagents (Sigma-Aldrich). VLP-derived Env was also analyzed by Blue Native PAGE (BN-PAGE), as described previously (Moore et al., 2006).
Electron Microscopy
Transfected 293T cells producing VLPs were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3) for 2 h. Samples were then washed in 0.1 M sodium cacodylate, fixed in 1% osmium tetroxide and incubated in 0.5% tannic acid in 0.05 M cacodylate for 30 mins. Following a 10 min wash with 1% sodium sulfate/0.1 M cacodylate buffer and further rinsing in 0.1 M cacodylate, the cells were dehydrated in a graded ethanol series, cleared in propylene oxide and embedded in EMbed-812/Araldite (Electron Microscopy Sciences, Fort Washington PA). Ultra thin sections were cut and mounted on parlodion-coated copper grids, stained with uranyl acetate and lead citrate. The sections were then examined on a Philips CM-100 electron microscope (FEI, Hillsborough, OR) and documented on Kodak SO-163 photographic film.
In high magnification negative stain electron microscopy, aliquots (25 μl) of virus particle preparations were washed in 140 μl of PBS and pelleted by an Airfuge centrifuge (Beckman Coulter) at 100,000 xg for 15 min. The virus particles were then resuspended and fixed for 30 min in 20 μl of 2.5% glutaraldehyde/PBS at 4°C. The fixed virus particles were washed in PBS and pelleted in Airfuge centrifuge again, and finally resuspended in 15 μl of saline. 4.5 μl of resuspended sample was placed on a carbon-covered grid for 30 sec. Excess virus particles and buffer were removed by filter paper blotting, and 5 μl of 1% uranyl formate stain was immediately added and incubated for 1 min. Excess stain was then removed, and the samples were allowed to air dry. Sample grids were screened at x 30,000, and electron micrographs were recorded at a nominal magnification of x 100,000 at 100 kV on a JEOL JEM 1200EX electron microscope.
Immunogenicity of VLPs in guinea pigs
Dunkin Hartley guinea pigs were immunized with SOS-VLPs, UNC-VLPs, “naked” VLPs or gp120, at the Scripps animal facility or Pocono Rabbit Farm (denoted by the animal identifier prefixes “S” and “P”). Five microgram Env equivalents of VLPs were used for each dose, as determined by Western blot, comparing to the known monomeric JR-FL gp120 standard. Naked VLPs were normalized for equivalent p24 to the other VLP preparations in Western blot. Recombinant monomeric gp120 was initially used at 5 μg dose (immunizations 1 and 2) consistent with the dose of Env in Env-VLP vaccinations. However, due to weak responses, the third and final dose was increased to 50 μg. Most immunogens were formulated in an equal mixture with the CpG-based adjuvant ImmunEASY (Qiagen) optimized for rabbits and mice. In some immunizations, the saponin-based QS21 or Ribi Ras3c adjuvants were used. All immunizations were administered by a combination intradermal and intramuscular routes in two locations each, at days 0, 43 and 97. Sera were collected 1 or 2 weeks after each immunization.
Neutralization assays
Sera were heat-inactivated at 56°C for 30 min, then analyzed for neutralization. Each assay was performed in duplicate and repeated at least 2 times. Representative data are shown. HIV+ plasmas A62 and N308 were used as reference controls. Neutralization assays were performed using either Env-pseudotyped viruses produced by transfection of 293T cells, or PBMC-grown viruses.
i) Neutralization assays using CF2 cell targets
Neutralization assays using CF2 cells were described previously (Crooks et al., 2005; Kolchinsky, Kiprilov, and Sodroski, 2001). Briefly, virus was incubated with graded dilutions of Ab for 1 h at 37°C. The mixture was then added to CF2 cells, spinoculated, then incubated for 2 h at 37°C, after which the medium was changed. The cells were cultured a further 3 days and then luciferase activity was measured. Post-CD4 binding neutralization was assayed by treating VLPs with sCD4 and graded dilutions of sera and using CF2 cells bearing only CCR5 as targets (Crooks et al., 2005). In “peptide interference” neutralization experiments, fixed concentrations of V3 peptides were added to virus-Ab mixture to adsorb V3-specific nAbs.
ii) Neutralization assays using TZM-BL cell targets
A neutralization assay using TZM-BL cells has been described previously (Li et al., 2005b), in which trypsinized TZM-BL cells were added to virus-serum mixtures and left for 3 days without a change of medium or 2) a “wash out” format in which virus-Ab mixtures were added to wells that had been pre-seeded with TZM-BL cells, and the medium changed after a 2 h incubation, followed by 2 days of culture.
iii) Neutralization assays using PBMC targets
Live virus (JR-FL or SIVmac251) was cultured in activated human PBMC. Virus was mixed with graded concentrations of Ab for 1 h at 37°C. Mixtures were then added to fresh activated PBMC and cultured for 3 days. The structural proteins p24 or p27 were detected by ELISA, as described previously (Hammonds et al., 2005).
iv) Trimer binding in native PAGE
We measured the ability of antibodies to bind VLP-derived Env trimers, based on the premise that trimer-Ab complexes migrate more slowly than their unliganded counterparts in BN-PAGE (Crooks et al., 2005; Moore et al., 2006). Specifically, JR-FL 140T SOS-VLPs were incubated with HIV+ plasmas, VLP sera and/or mAb b12 Fab for 15 minutes, and then trimers were resolved by BN-PAGE and Western blot. Since the migration properties of trimer-Ab complexes can vary, binding was measured by depletion of the unliganded trimer. Using NIH Image software, a box was drawn around the unliganded trimer band and the density was measured in arbitrary units. The density of a control bands with no ligand added was used as a reference for measuring the % residual unliganded trimer in lanes in which ligands were added.
Adsorption of sera against 293T cells
Sera were adsorbed against 293T cells to remove reactivity against membrane-associated proteins (Giannecchini et al., 2001; Langlois et al., 1992). Briefly, pellets containing 108 293T cells were mixed with 200 μl of serum and mixed overnight at 4 °C. Cells were then pelleted and the serum retrieved. The process was repeated 2 more times. The supernatant serum was completely cleared of cells, and then assayed by ELISA to detect anti-gp120 Abs compared to unabsorbed sera, to determine whether anti-gp120 titers were affected by adsorption.
Flow cytometry
Guinea pig serum binding to 293T, CF2 and HOS cells was assessed. Cells were incubated with sera at graded dilutions for 1 h at RT. A FITC conjugate (Jackson, West Grove, PA) was used to detect guinea pig IgG binding by FACS.
Analysis of serum binding titer and specificity by ELISA
We measured anti-gp120 titers of guinea pig sera by ELISA, as described previously (Binley et al., 1997). A HIV+ donor plasma, A62, was included as a control (Crooks et al., 2005) A goat anti-guinea pig or human alkaline phosphatase conjugate (Accurate) was used to detect bound Ab using the AMPAK system (Dako). Reactivity against epitopes exposed on denatured gp120 was measured against captured gp120 that had been treated with 1% SDS and 50mM DTT and boiled for 2 min and then diluted in PBS before capture on D7324-coated plates. Peptide ELISAs were performed in a similar manner.
Serum mapping by inhibition of mAb-virus capture
To determine the specificity of guinea pig sera, we examined their ability to inhibit JR-FL WT-VLP capture by a panel of mAbs, as described previously (Derby et al., 2006). VSV-G was co-expressed on particle surfaces to enhance detection of captured virus and also to provide a read-out of capture that is essentially unaffected by possible serum neutralization (Moore et al., 2006). Briefly, mAbs were coated on ELISA microwells overnight at 5 μg/ml. Wells were then washed and blocked with 3% bovine serum albumin (BSA) in PBS. Graded dilutions of sera were incubated with JR-FL WT-VLPs bearing VSV-G for 30 min. For X5 mAb capture, a fixed concentration of 5μg/ml sCD4 was added throughout. Mixtures were then added to mAb-coated ELISA wells for 3 h followed by washing with PBS (Poignard et al., 2003). CF2.CD4.CCR5 cells (Kolchinsky, Kiprilov, and Sodroski, 2001) were overlaid and two days later, infection was measured by assaying luciferase.
Acknowledgments
This work was supported by NIH grants AI49566, AI58763, Bill and Melinda Gates Collaboration for AIDS Vaccine Discovery Vaccine Immune Monitoring Consortium grant #38619 and the AIDS and Infectious Disease Science Center of the Torrey Pines Institute for Molecular Studies (JMB), AI55461 (KHR), and AI32292 (DRB). PLM was supported through the Columbia University-Southern Africa Fogarty AIDS International Training and Research Program (grant# D43 TW00231), funded by the Fogarty International Center. Progenics Pharmaceuticals, Inc., provided additional support. We thank Ralph Pantophlet, Michael Zwick, Pascal Poignard, and Jon Cohen for advice and Ann Hessell for reagents, Peter Kwong for providing the trimer model, Scott Conklin for assistance with animal immunizations, Malcolm Wood for help with electron microscopy, Ned Landau for providing the VSV-G plasmid, Joe Sodroski for providing CF2 cells, Doug Richman for providing HIV+ donor plasmas and Devin Shrestha for technical assistance. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: gp160-expressing plasmids, mouse anti-p24 serum, and the overlapping peptide set encompassing the V3 loop of JR-FL Env.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle JH, Aberle SW, Allison SL, Stiasny K, Ecker M, Mandl CW, Berger R, Heinz FX. A DNA immunization model study with constructs expressing the tick- borne encephalitis virus envelope protein E in different physical forms. J Immunol. 1999;163(12):6756–61. [PubMed] [Google Scholar]
- Abrahamyan LG, Markosyan RM, Moore JP, Cohen FS, Melikyan GB. Human immunodeficiency virus type 1 Env with an intersubunit disulfide bond engages coreceptors but requires bond reduction after engagement to induce fusion. J Virol. 2003;77(10):5829–36. doi: 10.1128/JVI.77.10.5829-5836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamyan LG, Mkrtchyan SR, Binley J, Lu M, Melikyan GB, Cohen FS. The Cytoplasmic Tail Slows the Folding of Human Immunodeficiency Virus Type 1 Env from a Late Prebundle Configuration into the Six-Helix Bundle. J Virol. 2005;79:106–115. doi: 10.1128/JVI.79.1.106-115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur LO, Bess JW, Jr, Sowder RC, 2nd, Benveniste RE, Mann DL, Chermann JC, Henderson LE. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258(5090):1935–8. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- Beddows S, Franti M, Dey AK, Kirschner M, Iyer SP, Fisch DC, Ketas T, Yuste E, Desrosiers RC, Klasse PJ, Maddon PJ, Olson WC, Moore JP. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology. 2006 doi: 10.1016/j.virol.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Beddows S, Schulke N, Kirschner M, Barnes K, Franti M, Michael E, Ketas T, Sanders RW, Maddon PJ, Olson WC, Moore JP. Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2005;79(14):8812–27. doi: 10.1128/JVI.79.14.8812-8827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Cayanan CS, Wiley C, Schulke N, Olson WC, Burton DR. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J Virol. 2003;77(10):5678–84. doi: 10.1128/JVI.77.10.5678-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Klasse PJ, Cao Y, Jones I, Markowitz M, Ho DD, Moore JP. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71(4):2799–809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, Moore JP. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion- associated structure. J Virol. 2000;74(2):627–43. doi: 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Sanders RW, Master A, Cayanan CS, Wiley CL, Schiffner L, Travis B, Kuhmann S, Burton DR, Hu SL, Olson WC, Moore JP. Enhancing the proteolytic maturation of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 2002;76(6):2606–16. doi: 10.1128/JVI.76.6.2606-2616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78(23):13232–52. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou-Habib DC, Roderiquez G, Oravecz T, Berman PW, Lusso P, Norcross MA. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68(9):6006–13. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Visciano ML, Tornesello ML, Tagliamonte M, Biryahwaho B, Buonaguro FM. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 clade A virus-like particles administered by different routes of inoculation. J Virol. 2005;79(11):7059–67. doi: 10.1128/JVI.79.11.7059-7067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrer R, Haessig-Einius S, Aubertin AM, Moog C. Neutralizing as well as non-neutralizing polyclonal immunoglobulin (Ig)G from infected patients capture HIV-1 via antibodies directed against the principal immunodominant domain of gp41. Virology. 2005;333(1):102–13. doi: 10.1016/j.virol.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Burton DR, Parren PW. Vaccines and the induction of functional antibodies: time to look beyond the molecules of natural infection? Nat Med. 2000;6(2):123–5. doi: 10.1038/72200. [DOI] [PubMed] [Google Scholar]
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266(5187):1024–7. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- Chakrabarti BK, Kong WP, Wu BY, Yang ZY, Friborg J, Ling X, King SR, Montefiori DC, Nabel GJ. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J Virol. 2002;76(11):5357–68. doi: 10.1128/JVI.76.11.5357-5368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WL, Rodgers A, Hancock RD, Taffs F, Kitchin P, Farrar G, Liew FY. Protection in simian immunodeficiency virus-vaccinated monkeys correlates with anti-HLA class I antibody response. J Exp Med. 1992;176(4):1203–7. doi: 10.1084/jem.176.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertova E, Bess JW, Jr, Crise BJ, Sowder RC, 2nd, Schaden TM, Hilburn JM, Hoxie JA, Benveniste RE, Lifson J, Henderson LE, Arthur LO. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 2002;76(11):5315–5325. doi: 10.1128/JVI.76.11.5315-5325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland SM, Buratti E, Jones TD, North P, Baralle F, McLain L, McInerney T, Durrani Z, Dimmock NJ. Immunogenic and antigenic dominance of a nonneutralizing epitope over a highly conserved neutralizing epitope in the gp41 envelope glycoprotein of human immunodeficiency virus type 1: its deletion leads to a strong neutralizing response. Virology. 2000;266(1):66–78. doi: 10.1006/viro.1999.0041. [DOI] [PubMed] [Google Scholar]
- Cole KS, Steckbeck JD, Rowles JL, Desrosiers RC, Montelaro RC. Removal of N-linked glycosylation sites in the V1 region of simian immunodeficiency virus gp120 results in redirection of B-cell responses to V3. J Virol. 2004;78(3):1525–39. doi: 10.1128/JVI.78.3.1525-1539.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner ME, Zarley CD, Hu B, Parsons S, Drabinski D, Greiner S, Smith R, Jiang B, Corsaro B, Barniak V, Madore HP, Crawford S, Estes MK. Virus-like particles as a rotavirus subunit vaccine. J Infect Dis. 1996;174(Suppl 1):S88–92. doi: 10.1093/infdis/174.supplement_1.s88. [DOI] [PubMed] [Google Scholar]
- Crooks ET, Moore PL, Richman D, Robinson J, Crooks JA, Franti M, Schulke N, Binley JM. Characterizing anti-HIV monoclonal antibodies and immune sera by defining the mechanism of neutralization. Hum Antibodies. 2005;14(3–4):101–13. [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Mazzara GP, Simon MA, Sehgal PK, Kodama T, Panicali DL, Desrosiers RC. High-titer immune responses elicited by recombinant vaccinia virus priming and particle boosting are ineffective in preventing virulent SIV infection. AIDS Res Hum Retroviruses. 1994;10(7):839–51. doi: 10.1089/aid.1994.10.839. [DOI] [PubMed] [Google Scholar]
- Darbha R, Phogat S, Labrijn AF, Shu Y, Gu Y, Andrykovitch M, Zhang MY, Pantophlet R, Martin L, Vita C, Burton DR, Dimitrov DS, Ji X. Crystal structure of the broadly cross-reactive HIV-1-neutralizing Fab X5 and fine mapping of its epitope. Biochemistry. 2004;43(6):1410–7. doi: 10.1021/bi035323x. [DOI] [PubMed] [Google Scholar]
- Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med. 2005;201(9):1407–19. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Schweighardt B, Wrin T, Galovich J, Hoh R, Sinclair E, Hunt P, McCune JM, Martin JN, Petropoulos CJ, Hecht FM. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J Virol. 2006;80(12):6155–64. doi: 10.1128/JVI.00093-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby NR, Kraft Z, Kan E, Barnett SW, Srivastava IK, Binley JMLS. Comparative analysis of antibody responses elicited in macaques immunized with HIV-1 SF162-derived gp140 envelope immunogens with those elicited during homologous SHIVSF162 and heterologous HIV-1 infection. J Virol. 2006;80:8745–8762. doi: 10.1128/JVI.00956-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey AK, David KB, Klasse PJ, Moore JP. Specific amino acids in the N-terminus of the gp41 ectodomain contribute to the stabilization of a soluble, cleaved gp140 envelope glycoprotein from human immunodeficiency virus type 1. Virology. 2006 doi: 10.1016/j.virol.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Doms RW, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1990;87(2):648–52. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Sugiura W, Montefiori DC, Broder CC, Lee SA, Wild C, Lifson J, Moss B. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J Virol. 2001;75(2):645–53. doi: 10.1128/JVI.75.2.645-653.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Ahuja M, Sung T, Baxter KC, Haggarty B, Doms RW, Hoxie JA. Characterization and epitope mapping of neutralizing monoclonal antibodies produced by immunization with oligomeric simian immunodeficiency virus envelope protein. J Virol. 2000;74(17):7922–35. doi: 10.1128/jvi.74.17.7922-7935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TG, Wyss S, Reeves JD, Zolla-Pazner S, Hoxie JA, Doms RW, Baribaud F. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J Virol. 2002;76(6):2683–91. doi: 10.1128/JVI.76.6.2683-2691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini EA, Koff WC. AIDS/HIV. Developing an AIDS vaccine: need, uncertainty, hope. Science. 2004;304(5679):1913–4. doi: 10.1126/science.1100368. [DOI] [PubMed] [Google Scholar]
- Evans DT, Bricker JE, Sanford HB, Lang S, Carville A, Richardson BA, Piatak M, Jr, Lifson JD, Mansfield KG, Desrosiers RC. Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239. J Virol. 2005;79(12):7707–20. doi: 10.1128/JVI.79.12.7707-7720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TG, Bonnez W, Rose RC, Koenig S, Demeter L, Suzich JA, O’Brien D, Campbell M, White WI, Balsley J, Reichman RC. A Phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J Infect Dis. 2001;183(10):1485–93. doi: 10.1086/320190. [DOI] [PubMed] [Google Scholar]
- Farzan M, Choe H, Desjardins E, Sun Y, Kuhn J, Cao J, Archambault D, Kolchinsky P, Koch M, Wyatt R, Sodroski J. Stabilization of human immunodeficiency virus type 1 envelope glycoprotein trimers by disulfide bonds introduced into the gp41 glycoprotein ectodomain. J Virol. 1998;72(9):7620–5. doi: 10.1128/jvi.72.9.7620-7625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda DH, Lever A, Terwilliger E, Sodroski J. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1992;66(6):3306–15. doi: 10.1128/jvi.66.6.3306-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber DA, Silvestri G, Feinberg MB. Prospects for an AIDS vaccine: three big questions, no easy answers. Lancet Infect Dis. 2004;4(7):397–413. doi: 10.1016/S1473-3099(04)01056-4. [DOI] [PubMed] [Google Scholar]
- Garrity RR, Rimmelzwaan G, Minassian A, Tsai WP, Lin G, de Jong JJ, Goudsmit J, Nara PL. Refocusing neutralizing antibody response by targeted dampening of an immunodominant epitope. J Immunol. 1997;159(1):279–89. [PubMed] [Google Scholar]
- Giannecchini S, Del Mauro D, Matteucci D, Bendinelli M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: reevaluation of neutralizing antibody levels elicited by a protective and a nonprotective vaccine after removal of antisubstrate cell antibodies. J Virol. 2001;75(9):4424–9. doi: 10.1128/JVI.75.9.4424-4429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grovit-Ferbas K, Hsu JF, Ferbas J, Gudeman V, Chen IS. Enhanced binding of antibodies to neutralization epitopes following thermal and chemical inactivation of human immunodeficiency virus type 1. J Virol. 2000;74(13):5802–9. doi: 10.1128/jvi.74.13.5802-5809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundner C, Mirzabekov T, Sodroski J, Wyatt R. Solid-phase proteoliposomes containing human immunodeficiency virus envelope glycoproteins. J Virol. 2002;76(7):3511–21. doi: 10.1128/JVI.76.7.3511-3521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar OK, Smithgall MD, Moran PA, Travis BM, Zarling JM, Hu SL. HIV-specific humoral and cellular immunity in rabbits vaccinated with recombinant human immunodeficiency virus-like gag-env particles. Virology. 1991;183(2):487–95. doi: 10.1016/0042-6822(91)90978-k. [DOI] [PubMed] [Google Scholar]
- Hammonds J, Chen X, Ding L, Fouts T, De Vico A, zur Megede J, Barnett S, Spearman P. Gp120 stability on HIV-1 virions and Gag-Env pseudovirions is enhanced by an uncleaved Gag core. Virology. 2003;314(2):636–49. doi: 10.1016/s0042-6822(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Hammonds J, Chen X, Fouts T, DeVico A, Montefiori D, Spearman P. Induction of neutralizing antibodies against human immunodeficiency virus type 1 primary isolates by Gag-Env pseudovirion immunization. J Virol. 2005;79(23):14804–14. doi: 10.1128/JVI.79.23.14804-14814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington PR, Yount B, Johnston RE, Davis N, Moe C, Baric RS. Systemic, mucosal, and heterotypic immune induction in mice inoculated with Venezuelan equine encephalitis replicons expressing Norwalk virus- like particles. J Virol. 2002;76(2):730–42. doi: 10.1128/JVI.76.2.730-742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Honnen WJ, Krachmarov CP, Burkhart M, Kayman SC, Corvalan J, Pinter A. Efficient isolation of novel human monoclonal antibodies with neutralizing activity against HIV-1 from transgenic mice expressing human Ig loci. J Immunol. 2002;169(1):595–605. doi: 10.4049/jimmunol.169.1.595. [DOI] [PubMed] [Google Scholar]
- Issel CJ, Horohov DW, Lea DF, Adams WV, Jr, Hagius SD, McManus JM, Allison AC, Montelaro RC. Efficacy of inactivated whole-virus and subunit vaccines in preventing infection and disease caused by equine infectious anemia virus. J Virol. 1992;66(6):3398–408. doi: 10.1128/jvi.66.6.3398-3408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelonek MT, Maskrey JL, Steimer KS, Potts BJ, Higgins KW, Keller MA. Maternal monoclonal antibody to the V3 loop alters specificity of the response to a human immunodeficiency virus vaccine. J Infect Dis. 1996;174(4):866–9. doi: 10.1093/infdis/174.4.866. [DOI] [PubMed] [Google Scholar]
- Katz E, Moss B. Immunogenicity of recombinant vaccinia viruses that display the HIV type 1 envelope glycoprotein on the surface of infectious virions. AIDS Res Hum Retroviruses. 1997;13(17):1497–500. doi: 10.1089/aid.1997.13.1497. [DOI] [PubMed] [Google Scholar]
- Keller MA, Arora Y. Inhibition of the anti-V3 loop response to a recombinant gp120SF2 vaccine by preexisting monoclonal antibody. AIDS Res Hum Retroviruses. 1999;15(9):855–60. doi: 10.1089/088922299310764. [DOI] [PubMed] [Google Scholar]
- Koff W, Kahn P, Gust ID. AIDS Vaccine Development: Challenges and Opportunities. Horizon Press 2007 [Google Scholar]
- Kolchinsky P, Kiprilov E, Sodroski J. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J Virol. 2001;75(5):2041–50. doi: 10.1128/JVI.75.5.2041-2050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347(21):1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Sattentau QJ, Sodroski J, Hendrickson WA. Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus. J Virol. 2000;74(4):1961–72. doi: 10.1128/jvi.74.4.1961-1972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, Venturi M, Petropoulos CJ, Wrin T, Dimitrov DS, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton DR. Access of Antibody Molecules to the Conserved Coreceptor Binding Site on Glycoprotein gp120 Is Sterically Restricted on Primary Human Immunodeficiency Virus Type 1. J Virol. 2003;77(19):10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCasse RA, Follis KE, Trahey M, Scarborough JD, Littman DR, Nunberg JH. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283(5400):357–62. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- Langlois AJ, Weinhold KJ, Matthews TJ, Greenberg ML, Bolognesi DP. The ability of certain SIV vaccines to provoke reactions against normal cells. Science. 1992;255(5042):292–3. doi: 10.1126/science.1549775. [DOI] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola J, Stamatatos L, Polonis VR, Koutsoukos M, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn B, Montefiori D. Human Immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005a doi: 10.1128/JVI.79.16.10108-10125.2005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005b;79(16):10108–25. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol. 2006;80(3):1414–26. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson JD, Rossio JL, Piatak M, Jr, Bess J, Jr, Chertova E, Schneider DK, Coalter VJ, Poore B, Kiser RF, Imming RJ, Scarzello AJ, Henderson LE, Alvord WG, Hirsch VM, Benveniste RE, Arthur LO. Evaluation of the safety, immunogenicity, and protective efficacy of whole inactivated simian immunodeficiency virus (SIV) vaccines with conformationally and functionally intact envelope glycoproteins. AIDS Res Hum Retroviruses. 2004;20(7):772–87. doi: 10.1089/0889222041524661. [DOI] [PubMed] [Google Scholar]
- Liu J, Ewald BA, Lynch DM, Nanda A, Sumida SM, Barouch DH. Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies. J Virol. 2006;80(24):11991–7. doi: 10.1128/JVI.01348-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorin C, Mollet L, Delebecque F, Combredet C, Hurtrel B, Charneau P, Brahic M, Tangy F. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J Virol. 2004;78(1):146–57. doi: 10.1128/JVI.78.1.146-157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammano F, Salvatori F, Indraccolo S, De Rossi A, Chieco-Bianchi L, Gottlinger HG. Truncation of the human immunodeficiency virus type 1 envelope glycoprotein allows efficient pseudotyping of Moloney murine leukemia virus particles and gene transfer into CD4+ cells. J Virol. 1997;71(4):3341–5. doi: 10.1128/jvi.71.4.3341-3345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney SP, Young KR, Ross TM. Membrane embedded HIV-1 envelope on the surface of a virus-like particle elicits broader immune responses than soluble envelopes. Virology. 2007;358(2):334–46. doi: 10.1016/j.virol.2006.08.032. [DOI] [PubMed] [Google Scholar]
- McKeating JA, McKnight A, Moore JP. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991;65(2):852–60. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna PM, Pomerantz RJ, Dietzschold B, McGettigan JP, Schnell MJ. Covalently linked human immunodeficiency virus type 1 gp120/gp41 is stably anchored in rhabdovirus particles and exposes critical neutralizing epitopes. J Virol. 2003;77(23):12782–94. doi: 10.1128/JVI.77.23.12782-12794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC, Safrit JT, Lydy SL, Barry AP, Bilska M, Vo HT, Klein M, Tartaglia J, Robinson HL, Rovinski B. Induction of neutralizing antibodies and gag-specific cellular immune responses to an R5 primary isolate of human immunodeficiency virus type 1 in rhesus macaques. J Virol. 2001;75(13):5879–90. doi: 10.1128/JVI.75.13.5879-5890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Crooks ET, Porter L, Zhu P, Cayanan CS, Grise H, Corcoran P, Zwick MB, Franti M, Morris L, Roux KH, Burton DR, Binley JM. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol. 2006;80(5):2515–28. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulard M, Phogat SK, Shu Y, Labrijn AF, Xiao X, Binley JM, Zhang MY, Sidorov IA, Broder CC, Robinson J, Parren PW, Burton DR, Dimitrov DS. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc Natl Acad Sci U S A. 2002;99(10):6913–8. doi: 10.1073/pnas.102562599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambi PN, Gorny MK, Bastiani L, van der Groen G, Williams C, Zolla-Pazner S. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J Virol. 1998;72(11):9384–91. doi: 10.1128/jvi.72.11.9384-9391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson RH, Cao SX, Cates G, Yao FL, Klein MH, Rovinski B. Modifications of HIV-1 retrovirus-like particles to enhance safety and immunogenicity. Biologicals. 1998;26(4):255–65. doi: 10.1006/biol.1998.0142. [DOI] [PubMed] [Google Scholar]
- Poignard P, Moulard M, Golez E, Vivona V, Franti M, Venturini S, Wang M, Parren PW, Burton DR. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J Virol. 2003;77(1):353–65. doi: 10.1128/JVI.77.1.353-365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacino PS, Stallard V, Klaniecki JE, Pennathur S, Montefiori DC, Langlois AJ, Richardson BA, Morton WR, Benveniste RE, Hu SL. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in Macaques. J Virol. 1999;73(10):8201–15. doi: 10.1128/jvi.73.10.8201-8215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon B, Hsu JF, Gudeman V, Chen IS, Grovit-Ferbas K. Formaldehyde-treated, heat-inactivated virions with increased human immunodeficiency virus type 1 env can be used to induce high-titer neutralizing antibody responses. J Virol. 2005a;79(16):10210–7. doi: 10.1128/JVI.79.16.10210-10217.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon B, Safrit JT, McClure H, Kitchen C, Hsu JF, Gudeman V, Petropoulos C, Wrin T, Chen IS, Grovit-Ferbas K. Induction of humoral immune responses following vaccination with envelope-containing, formaldehyde-treated, thermally inactivated human immunodeficiency virus type 1. J Virol. 2005b;79(8):4927–35. doi: 10.1128/JVI.79.8.4927-4935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Frezza P, Stephens DM, Davis D, Polyanskaya N, Cranage M, Oxford JS. An experimental chemically inactivated HIV-1 vaccine induces antibodies that neutralize homologous and heterologous viruses. Vaccine. 1995;13(1):54–60. doi: 10.1016/0264-410x(95)80011-2. [DOI] [PubMed] [Google Scholar]
- Richmond JF, Lu S, Santoro JC, Weng J, Hu SL, Montefiori DC, Robinson HL. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol. 1998;72(11):9092–100. doi: 10.1128/jvi.72.11.9092-9100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, Arthur LO, Henderson LE, Lifson JD. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72(10):7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk J. Polio vaccines and polioviruses. Br Med J. 1977;2(6089):765. doi: 10.1136/bmj.2.6089.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, Kwong PD, Moore JP. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002a;76(14):7293–305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Vesanen M, Schuelke N, Master A, Schiffner L, Kalyanaraman R, Paluch M, Berkhout B, Maddon PJ, Olson WC, Lu M, Moore JP. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2002b;76(17):8875–89. doi: 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1-->2 mannose residues on the outer face of gp120. J Virol. 2002;76(14):7306–21. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulke N, Vesanen MS, Sanders RW, Zhu P, Lu M, Anselma DJ, Villa AR, Parren PW, Binley JM, Roux KH, Maddon PJ, Moore JP, Olson WC. Oligomeric and conformational properties of a proteolytically mature, disulfide-stabilized human immunodeficiency virus type 1 gp140 envelope glycoprotein. J Virol. 2002;76(15):7760–76. doi: 10.1128/JVI.76.15.7760-7776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajah S, Puffer B, Pantophlet R, Law M, Doms RW, Burton DR. Comparing antigenicity and immunogenicity of engineered gp120. J Virol. 2005;79(19):12148–63. doi: 10.1128/JVI.79.19.12148-12163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava IK, Stamatatos L, Legg H, Kan E, Fong A, Coates SR, Leung L, Wininger M, Donnelly JJ, Ulmer JB, Barnett SW. Purification and characterization of oligomeric envelope glycoprotein from a primary r5 subtype B human immunodeficiency virus. J Virol. 2002;76(6):2835–47. doi: 10.1128/JVI.76.6.2835-2847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava IK, VanDorsten K, Vojtech L, Barnett SW, Stamatatos L. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J Virol. 2003;77(4):2310–20. doi: 10.1128/JVI.77.4.2310-2320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staropoli I, Chanel C, Girard M, Altmeyer R. Processing, stability, and receptor binding properties of oligomeric envelope glycoprotein from a primary HIV-1 isolate. J Biol Chem. 2000;275(45):35137–45. doi: 10.1074/jbc.M003868200. [DOI] [PubMed] [Google Scholar]
- Tramont EC, Johnston MI. Progress in the development of an HIV vaccine. Expert Opin Emerg Drugs. 2003;8(1):37–45. doi: 10.1517/14728214.8.1.37. [DOI] [PubMed] [Google Scholar]
- Usonis V, Meriste S, Bakasenas V, Lutsar I, Collard F, Stoffel M, Tornieporth N. Immunogenicity and safety of a combined hepatitis A and B vaccine administered concomitantly with either a measles-mumps-rubella or a diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis vaccine mixed with a Haemophilus influenzae type b conjugate vaccine in infants aged 12–18 months. Vaccine. 2005;23(20):2602–6. doi: 10.1016/j.vaccine.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Valenzuela P, Medina A, Rutter WJ, Ammerer G, Hall BD. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298(5872):347–50. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- VanCott TC, Mascola JR, Kaminski RW, Kalyanaraman V, Hallberg PL, Burnett PR, Ulrich JT, Rechtman DJ, Birx DL. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71(6):4319–30. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier F, Moog C, Barre-Sinoussi F, Van der Ryst E, Spenlehauer C, Girard M. Macaque immunization with virions purified from a primary isolate of the human immunodeficiency virus type 1 induced enhancement antibodies. Bull Acad Natl Med. 2000;184(1):67–84. [PubMed] [Google Scholar]
- Vzorov AN, Lea-Fox D, Compans RW. Immunogenicity of full length and truncated SIV envelope proteins. Viral Immunol. 1999;12(3):205–15. doi: 10.1089/vim.1999.12.205. [DOI] [PubMed] [Google Scholar]
- Wagner R, Deml L, Notka F, Wolf H, Schirmbeck R, Reimann J, Teeuwsen V, Heeney J. Safety and immunogenicity of recombinant human immunodeficiency virus- like particles in rodents and rhesus macaques. Intervirology. 1996;39(1–2):93–103. doi: 10.1159/000150480. [DOI] [PubMed] [Google Scholar]
- Wagner R, Teeuwsen VJ, Deml L, Notka F, Haaksma AG, Jhagjhoorsingh SS, Niphuis H, Wolf H, Heeney JL. Cytotoxic T cells and neutralizing antibodies induced in rhesus monkeys by virus-like particle HIV vaccines in the absence of protection from SHIV infection. Virology. 1998;245(1):65–74. doi: 10.1006/viro.1998.9104. [DOI] [PubMed] [Google Scholar]
- Yamshchikov GV, Ritter GD, Vey M, Compans RW. Assembly of SIV virus-like particles containing envelope proteins using a baculovirus expression system. Virology. 1995;214(1):50–8. doi: 10.1006/viro.1995.9955. [DOI] [PubMed] [Google Scholar]
- Yang X, Florin L, Farzan M, Kolchinsky P, Kwong PD, Sodroski J, Wyatt R. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J Virol. 2000;74(10):4746–54. doi: 10.1128/jvi.74.10.4746-4754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Kuhlmann FM, Eller R, Compans RW, Chen C. Production and characterization of simian--human immunodeficiency virus- like particles. AIDS Res Hum Retroviruses. 2000;16(3):227–36. doi: 10.1089/088922200309322. [DOI] [PubMed] [Google Scholar]
- Zanetti G, Briggs JA, Grunewald K, Sattentau QJ, Fuller SD. Cryo-Electron Tomographic Structure of an Immunodeficiency Virus Envelope Complex In Situ. PLoS Pathog. 2006;2(8) doi: 10.1371/journal.ppat.0020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CW, Chishti Y, Hussey RE, Reinherz EL. Expression, purification, and characterization of recombinant HIV gp140. The gp41 ectodomain of HIV or simian immunodeficiency virus is sufficient to maintain the retroviral envelope glycoprotein as a trimer. J Biol Chem. 2001;276(43):39577–85. doi: 10.1074/jbc.M107147200. [DOI] [PubMed] [Google Scholar]
- Zhu P, Chertova E, Bess J, Jr, Lifson JD, Arthur LO, Liu J, Taylor KA, Roux KH. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc Natl Acad Sci U S A. 2003;100(26):15812–7. doi: 10.1073/pnas.2634931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Liu J, Bess J, Jr, Chertova E, Lifson JD, Grise H, Ofek GA, Taylor KA, Roux KH. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441(7095):847–52. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]