Abstract
Abnormal functional brain connectivity is a candidate factor in developmental brain disorders associated with cognitive dysfunction. We analyzed a substantial (ten minute per subject) record of dense array EEG with spectral power and coherence methods in ADHD (n = 42) and Control (n = 21) 10–13 year old children. We found topographically distinct narrow band coherence differences between subject groups: ADHD subjects showed elevated coherence in the lower alpha (8 Hz) band and reduced coherence in the upper alpha (10–11 Hz) band. The 8 Hz ADHD elevation, and a 2–6 Hz Control group coherence elevation, were independent of stimulus presentation. In response to visual stimulation, the ADHD group exhibited reduced evoked potential power and elevated frontal coherence. Only the upper alpha band control group coherence elevation discriminated according to ADHD group medication status. The findings suggest a static state of deficient connectivity in ADHD, and a stimulus induced state of over-connectivity within and between frontal hemispheres.
Keywords: attention deficit/hyperactivity disorder, pediatric EEG, alpha rhythm, synchrony, induced rhythms
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most prevalent disorders of childhood, with an estimated lifetime recognition rate of 7.8% in children aged 4–17 years in the United States (Center for Disease Control, 2005). ADHD is characterized by developmentally inappropriate inattention, motor overactivity and impulsiveness. Differences in distributed cortical and sub-cortical networks that support basic cognitive functions (such as attention, motor control and self-regulation) have been proposed as a neural basis for ADHD (Castellanos, 1997; Solanto, 2002; Swanson et al., 1998). The major substrate of anatomical connectivity among cortical regions is an extensive system of white matter fibers, composing about half of the brain’s overall volume, that continue to mature throughout normal childhood, adolescence and early adulthood (Paus et al., 1999, 2001). Reduced volumes of within-hemisphere (corticocortical) and callosal white matter have been noted in ADHD (Baumgardner et al., 1996; Castellanos et al. (2002); Durston et al., 2004; Giedd et al., 1994; Hynd et al., 1991; Filipek, et al., 1997; Semrud-Clikeman et al., 1994). This suggests the possibility that the symptoms of ADHD may be related to impaired interactions within brain networks, rather than impaired function of specialized cortical regions.
Functional connectivity in brain networks is typically inferred from statistical relationships found between neurophysiological signals measured from spatially separated neuronal regions (Bressler, 1995; Friston, 1994; Lehmann et al., 1987; Nunez, 1995; Varela et al., 2001). In adult populations functional connectivity has been studied with a variety of neuroimaging tools including PET and magnetic resonance. In contrast, electroencephalography (EEG) is a non-invasive neuroimaging technology well suited to pediatric subject groups. Cortical neural populations that exhibit a high degree of oscillatory synchrony over relatively large areas (on the order of at least 100–1000 mm2) can generate electrical potentials that are measurable with electrodes placed on the scalp (Lopes da Silva and Pfurtsceller, 1999; Nunez and Srinivasan, 2006). The levels of synchronization between such neural populations distributed across different parts of the brain can be estimated from EEG recordings via coherence measurements. Coherence is a squared correlation coefficient that provides a measure of the linearity of the relationship between two EEG electrodes at one frequency. High coherence between two EEG signals indicates the contribution of synchronized neuronal oscillations to each electrode, suggesting functional integration between neural populations, while low coherence suggests functional segregation. EEG coherence is primarily a measure of phase correlation and is believed to reflect functional cortical connectivity on a centimeter scale (Nunez and Srinivasan, 2006; Srinivasan et al., 1998a) either directly via corticocortical fiber systems or indirectly through networks that include other cortical or subcortical structures.
In humans, the development of EEG coherence from birth into adulthood has been studied in detail by Thatcher and colleagues (Thatcher et al., 1986; Thatcher et al., 1987; Thatcher, 1994), who demonstrated maturational changes in patterns of coherence that overlap stages of cognitive development. In studies of EEG coherence with pediatric populations, pathology associated with cognitive dysfunction has been associated with increased coherence, suggesting impaired functional segregation of neuronal populations. Increased coherence has been observed within healthy low IQ and low academic achievement subject groups (Thatcher et al., 1983), and coherence elevations among ADHD subjects in the eyes closed resting state have been observed (Barry et al., 2002; Chabot and Serfontein, 1996a; Montagu, 1975).
Three technical issues limit the interpretation of many previously published pediatric studies employing coherence methods:
Reports of coherence values averaged over broad frequency bands, e.g., alpha across 8–12 Hz, provide only coarse frequency resolution and risk that frequency-specific effects within bands may cancel out or otherwise go undetected (Klimesch, 1999; Nunez, 1995). Cognitive studies have illustrated robust functional distinctions between lower (8–10 Hz) and upper (11–12 Hz) alpha bands in a number of tasks, including attention and working memory (Klimesh et al., 2000, Nunez et al., 2001, Ding et al, 2005). In many of these studies, power and coherence in the lower and upper alpha bands are modulated in opposite directions. Human alpha and theta bands appear to include many rhythms with distinct spatial and functional properties that can only be effectively characterized with narrow frequency bands (Nunez et al. 2001; Nunez and Srinvasan, 2006).
Volume conduction (the passive flow of current across the scalp, skull, and cerebrospinal fluid) strongly influences scalp potential EEG coherence (Srinivasan et al., 1998a; Nunez and Srinivasan, 2006). Volume conduction has been shown in electrical models of the head to introduce artificial coherence between electrodes separated by less than 10–12 centimeters (Srinivasan et al., 1998a). Thus, EEG potential coherence measurements are only meaningful for widely spaced electrode pairs.
Any choice of reference electrode placement distributes the signal at the reference site throughout the array of electrodes (Andrew and Pfurtscheller, 1996; Srinivasan et al., 1998a; Srinivasan et al., 1998b). Most previous pediatric studies of EEG coherence use either one ear potential, or the average of two ear potentials, as a reference. Either choice confounds coherence estimates by redistributing the potentials at the reference site (Srinivasan et al., 1998a; Nunez and Srinivasan, 2006). We have demonstrated that using a high density of recording electrodes, EEG coherence estimates obtained using the average reference provide a reasonable estimate of the coherence of scalp potentials without contamination by reference electrode effects (Srinivasan et al., 1998b). Using this information, Srinivasan (1999) differentiated genuine spatial correlations from volume conduction and reference electrode effects, and found cortical areas contributing to the alpha rhythm to be far more weakly correlated with each other in pre-adolescent children than in adults.
In this study, we make use of this approach to investigate functional connectivity of the brains of ADHD and typical children using high-density EEG coherence. We presented ADHD and control children with visual stimulation typical of cognitive experiments, within the context of task demands. We examined coherence only among long-range electrode pairs to characterize genuine spatial correlations free of volume conduction confounds. We characterized the narrow band power and coherence of EEG signals resulting from processes in the eyes open attentive state, when subjects were alert and expecting a stimulus. In the interval following the stimulus, we examined modulation of EEG power and coherence by the visual stimulation, and the extent to which that modulation differed between ADHD and typically developing children.
Methods
Subjects
Twenty-one (17 male, 4 female) typically developing control children and 42 (34 male, 8 female) children with ADHD ranging in age from 10–13 years (mean age of Control children was 134 months, of ADHD, 141 months) participated. Two additional ADHD subjects were not included in the present analysis; one could not remain still enough for EEG recording, the other due to software error. ADHD and control subjects were drawn from a cohort of children at the University of California at Irvine, one of the six sites of the Multimodal Treatment study of ADHD (MTA Cooperative Group, 1999). This study was conducted approximately 3 years after the initiation of the MTA. Control subjects were recruited from classrooms in the same schools and grades of the ADHD children. Written consent of parents and assent of children was obtained to undergo 2 days of neuropsychological assessment and EEG recordings.
Inclusion criteria for clinical subjects included a DSM-IV diagnosis of ADHD-Combined type, including the endorsement of at least six of nine symptoms of inattention and six of nine symptoms of hyperactivity/impulsivity. All subjects were evaluated on entry into the MTA with an assessment battery (Hinshaw et al, 1997; MTA Group, 1999 and 2004), which included the Diagnostic Interview Schedule for Children (DISC) to evaluate DSM-IV criteria for ADHD and other disorders, and Swanson Nolan and Pelham (SNAP) rating scale to measure the severity of ADHD symptoms (Swanson, 1995a). All of the ADHD subjects and none of the Control subjects met DISC criteria for ADHD Combined Type. Subjects were free of other child psychiatric diagnoses as measured by the DISC. Average SNAP ratings per item above 2.0 are considered severe and below 1.0 considered within the normal range. The ADHD group had higher average SNAP symptom ratings than controls for parent ratings of inattention (2.27 vs. 0.43) and hyperactivity/impulsivity (2.07 vs. 0.21). School-teacher ratings also verified symptom severity of inattention (2.32 vs. 0.65), and hyperactivity/impulsivity (2.0 vs. 0.35). Twenty-three children in the ADHD group were being treated with medication during the time in which they participated in this study, which was determined partially by the assigned treatment in the 14-month randomized clinical trial (which included stimulant medication for half the subjects) and partially by decision to stop or start medication during the subsequent naturalistic follow-up phase of the MTA. Of these, 19 were treated with stimulant formulations including methylphenidate (16), mixed amphetamine salts (1), dextroamphetamine (1), and pemoline (1). Four other ADHD participants were treated with other psychotropics (bupropion HCl, buspirone, nortriptyline, and risperidone). The medication status of 2 ADHD participants could not be confirmed. All subjects refrained from medication for at least 24 hours prior to assessment, longer than the serum and behavioral half-lives of stimulant medications (Swanson et. al 1995b). Our Control group was slightly but not significantly younger (134.3 months) than the unmedicated ADHD (140.5 months) group, and significantly younger (p < .01) than the medicated ADHD (143.6 months) group. Details of demographics, ADHD symptoms, and psychometric test scores of both groups have been previously reported (Swanson et al., 2000).
Stimuli
Subjects were visually presented with single words in a task designed to investigate brain processes involved in word processing (Abdullaev and Posner, 1998; Raichle, 1994). This task was originally designed to differentiate brain processes involved in verbal word generation, as compared to a baseline verbal word repetition condition. In this study, we averaged over both trial conditions in order to maximize the number of trials available for analysis. The present dataset consisted of an average of more than 300 stimulus presentations per subject.
The stimuli employed here were nearly identical to those employed by Abdullaev and Posner (1998). Stimuli consisted of 100 common nouns. Trials were initiated with the presentation of a small foveal fixation point, which remained on screen for 1 second before stimulus onset. Stimuli remained on screen for 195 ms, and were replaced by fixation point for 805 ms. A response prompt ("?") then appeared, cueing the subject to make a verbal response to the stimulus presented 1 second before. Thus, two one-second segments were available for analysis: an alert interval in which subjects anticipated stimulus presentation, and a stimulus interval in which subjects viewed stimuli and prepared a response. EEG following the response prompt was contaminated with movement artifact and not used in this analysis. A random interval between 2 and 5 seconds separated trials. Each word was presented once within a block, with all 100 nouns presented twice in two separate blocks. In the first block, subjects were instructed to repeat aloud the words they saw presented on the video screen. In the second block, the subjects saw the same words (in a different random order), and were instructed to say aloud a use for each word. For example, in response to the word "hammer", the subject might say "pound"; in response to "broom", "sweep". Words ranged from 3–7 characters and subtended a horizontal visual angle ranging from 2.4 – 3.0° and a vertical angle of approximately 0.7°. Words were displayed in black lowercase letters presented against a white background.
EEG acquisition
EEG was recorded from 128 electrodes using the Geodesic Sensor Net (Electrical Geodesics, OR). Impedances were < 50 kOhm. EEG was recorded with reference to the vertex (Cz) electrode, amplified, and analog filtered (elliptical) between .1 and 50 Hz. Signals were digitized at 250 samples/second. Electrode application and experimental procedures were well tolerated by all subjects. Recordings were carried out in an electrically shielded, sound attenuated booth. Subjects were instructed to refrain from movement, were monitored for eye and head movements via video camera, and were reminded as necessary to remain still.
EEG data was manually edited to remove segments with artifact due to EOG, eyeblink, or motion, and to reject electrodes with a preponderance of noise resulting from poor electrode contact with the scalp. Twenty electrodes, primarily on the outer ring of the electrode array, were eliminated from the study due to excessive artifact. Additionally, an amplitude thresholding criterion was applied to reject electrodes that exceeded +/− 150 microvolts during a trial, and if any given electrode was rejected in more than 25% of trials, it was eliminated entirely for that subject. Averaged power spectra for each subject were visually inspected at all electrodes for EOG and EMG artifact contamination. The average number of included trials for the Control group was 326 (out of an average 351 trials presented); for the ADHD group, 316 (of 355).
At each time point, the potentials at each electrode were re-referenced to the instantaneous average of all electrodes, yielding average referenced potentials. The average reference is a reasonable estimate of reference independent potentials when the number of electrodes is adequate, and superior and inferior surfaces of the head are sampled (Bertrand et al., 1985).
Power spectrum analysis
The time-series data was segmented into one-second epochs corresponding to the alert and stimulus intervals, and liner trends removed. Epochs were Fourier transformed using the fast (FFT) algorithm (MATLAB). Fourier coefficients Fmq (f ) were calculated using one-second epochs (Δf = 1 Hz) at each electrode m, for each epoch q, and frequency f between 2–50 Hz.
Because the alert interval began with the visual presentation of a fixation point, and the stimulus interval began with the presentation of a word, non-stationarities were present in the EEG time-series. The evoked potential constitutes a non-stationary mean value, which must be removed to obtain meaningful measures of power and coherence (Bendat and Piersol, 2001). The average phase locked, or evoked potential (EP) power spectrum PEP is taken from the complex valued mean over Q epochs:
| (1) |
where
| (2) |
A factor of 2 accounts for negative frequencies. The power spectrum is calculated by subtracting the complex average FEP from each epoch:
| (3) |
Coherence spectrum analysis
The coherence between two signals is a correlation coefficient (squared) that measures the phase consistency of the two signals as a function of frequency. Coherence at a given frequency measures the fraction of variance in EEG record at a given electrode that has amplitude and phase predicted by the other electrode, across recording epochs. To obtain the coherence between two electrodes m and n across Q epochs, the average cross spectrum Cmn at each frequency f is computed from the Fourier coefficients of each EEG record, after removing the non-stationary mean values:
| (4) |
(The * indicates complex conjugation). The cross spectrum is squared and normalized by the average residual power spectrum of the individual electrodes to obtain the coherence , which is highly sensitive to the consistency of the phase difference between the recordings (Bendat and Piersol, 2001):
| (5) |
Note that the form of this equation closely resembles that of a squared correlation coefficient, in which the cross spectrum is analogous to covariance and the power spectrum is analogous to variance. At frequency f, a coherence value of 1 indicates that the two EEG records maintain the same phase difference on every epoch, whereas a coherence value near 0 indicates that the phase difference is random from epoch to epoch.
Because electrode pairs spaced close together are subject to significant volume conduction effects (Srinivasan et al, 1998a), we focused analysis on the 4,107 electrode pairs spaced further than 10 cm apart from one another. The statistical error in coherence estimates depends on both the coherence of the stochastic neural activity and the number of epochs used in the coherence estimate. Our coherence estimates are based on more than 300 epochs, well above the minimum of 40 epochs apparently required to obtain reasonable coherence estimates (Nunez & Srinivasan, 2006).
Contrasts of interest
We tested for group differences in the power and coherence measurements described above, at all frequencies and scalp electrode locations. We evaluated differences in Control (n=21) vs. ADHD (n=42) groups using the t statistic
| (6) |
for all electrodes (power) and electrode pairs (coherence), at each frequency. We also grouped ADHD subjects by medication status (with methylphenidate, or not) and computed t statistics for Control vs. ADHD children being treated with methylphenidate formulations (n=16); for Control vs. ADHD children not being treated with any drug (n=17); and for medicated vs. unmedicated ADHD children.
Statistical Methods
We tested each t statistic against bootstrap estimates of the t statistic distribution, instead of against an assumed normal distribution. This nonparametric bootstrap method is robust in the face of unequal sample sizes and outlier subjects (Efron and Tibshirani, 1993). The bootstrap technique involved resampling the subject data to obtain a distribution of average differences for the null hypothesis that the two subject groups contrasted were drawn from the same distribution. We sampled 2,000 draws of randomly selected subjects (with replacement) to form “groups” and calculated the t statistic for each measure (power or coherence) at each electrode (or electrode pair). These distributions were used to estimate the probability (p value) that our observed t statistics could be obtained by chance, if our two groups were in fact drawn from the same population. In other words, we established a 95% confidence interval for the power and coherence differences observed between randomly selected groups, and tested our observed group differences against this distribution.
Multiple Comparisons
To address the multiple comparison problem imposed by recording from large numbers of electrodes (for power) and electrode pairs (for coherence), we calculated two-tailed tests for group at the p < 0.05 level, and examined whether 5% of the contrasts across frequencies and electrodes (or electrode pairs) are significant. This correction helps guard against the increased likelihood of type 1 error imposed by comparing large electrode arrays. For example, if one frequency is tested, 205 electrode pairs of the 4,107 pairs tested can be expected by chance to achieve significance. If two frequencies are tested, 410 pairs can be expected by chance, etc. At a single frequency, we examined 109 electrodes for the EP, 109 electrodes for power, and 4,107 electrode pairs for coherence. We conducted four tests (Control vs. ADHD, Control vs. ADHD medicated, Control vs. ADHD unmedicated, and ADHD medicated vs. ADHD unmedicated) for each measure (EP, power, coherence) in either the stimulus interval (EP) or both the alert and stimulus intervals (power, coherence), for a total of 8,541 combined tests at a single contrast and frequency. Across the 4 contrasts, 34,164 tests were performed at each frequency. Figure 1 shows the proportion of significant effects found across all of these tests and measures, as a function of frequency. Summing across all tests and measures, the proportion of significant effects meets the 5% criterion over a broad (2–20 Hz) range. Figure 1 also shows the proportion of significant effects we found separately for each measure (power, EP power, and coherence), summed across all tests, as a function of the number of frequencies tested. We find that coherence and EP effects meet the 5% criterion over a broad (2–20 Hz) range, but power effects do not. Thus, our evidence for power differences is weaker, and as a consequence we have emphasized coherence and the EP in this paper.
Figure 1.
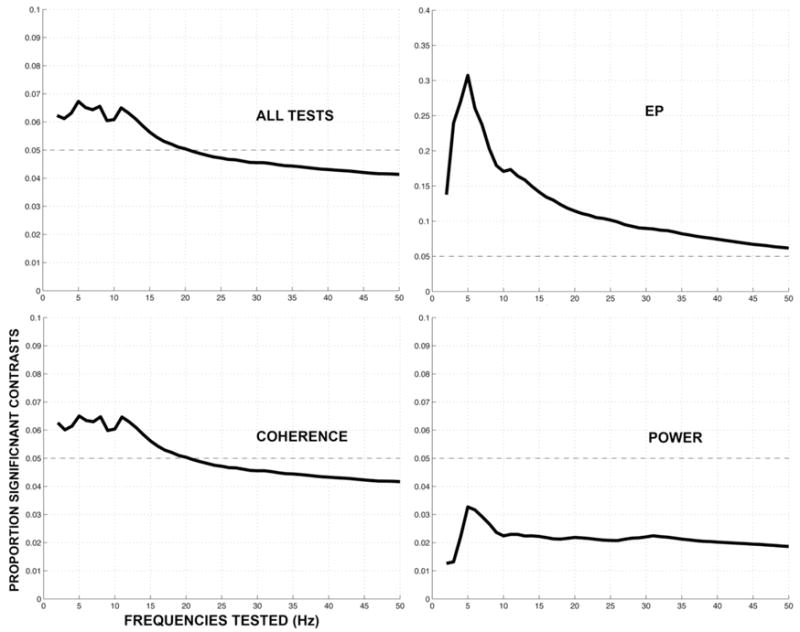
(A) Proportion of all significant findings as a function of the total number of tests performed. At a single frequency, we examined 109 electrodes for the EP, 109 electrodes for power, and 4,107 electrode pairs for coherence. Four tests (Control vs. ADHD, vs. ADHD medicated, vs. ADHD unmedicated, and ADHD medicated vs. ADHD unmedicated) for each measure (EP, power, coherence) were conducted in either the stimulus interval (EP) or both the alert and stimulus intervals (power, coherence), for a total of 34,164 combined tests at a single frequency. When examining two frequencies, there 2*34,164 tests. More than 5% of these contrasts are significant when testing frequencies between 2–20 Hz (19*34,164 tests). (B–D) Proportion of significant effects for each individual measure (coherence, EP and power) combined from all tests as a function of the number of frequencies tested. Coherence and EP effects, but not power, meet the 5% criterion over a broad (2–20 Hz) range.
The group differences we observed were typically distributed over narrow frequency bands that were identifiable as distinct peaks in the power and coherence spectra. Power and coherence differences that met our significance criterion were invariably spatially consistent, with effects spread across adjacent scalp regions. We adopted a criterion of accepting any particular frequency as significant if more than 5% of the electrodes or electrode pairs in the array showed differences that exceeded the 95% confidence interval.
Results
Power spectrum reflects normal features of the EEG
Power and coherence spectra in the 2–50 Hz range in both ADHD and Control subjects reflected normal features of the EEG, including alpha band peaks that desynchronized upon visual stimulation. In the alert interval, 5 Hz power was significantly lower in the ADHD group at 10% of electrode pairs located over predominantly central and temporal scalp regions. In the stimulus interval, 20% of electrodes reached significance at 5 Hz; these included the same sensors as the alert interval and additional sensors extending anteriorly toward frontal and central regions (from Cz to FC3/FCz/FC4).
Elevated Control group EP power
The evoked potential (EP) strongly differentiated groups, with the ADHD group presenting smaller potentials. Although EPs are usually contrasted at specific latencies in the time domain (Figure 2a), our main analyses were based on frequency-domain measures of power and coherence and we therefore examined the spectrum of the EP (Figure 2b). At no frequency did the EP of the ADHD group exceed Controls. Statistical results are presented as estimated p values for the EP power difference between ADHD and Controls (Figure 2c). Topographies of the EP at frequencies that most strongly differentiated subject groups are shown in Figure 2, with significant (p < .05, two tailed) electrodes marked. At 3 Hz, 30% of electrodes reached statistical criterion. These were located bilaterally in frontal and central regions, and in left hemisphere occipital and parietal regions. At 4 and 5 Hz, 39% and 44% of electrodes reached criterion, respectively, located bilaterally over frontal, central, parietal and occipital regions. At 11 Hz, 11% of electrodes reached criterion, located primarily over left hemisphere central and parietal regions.
Figure 2.

Upper left: Time domain event related potential (ERP) at left parietal electrode P7. Upper right: frequency domain evoked potential (EP) of the stimulus interval at P7. Lower: bootstrap p values at all electrodes. Lower: Topography of evoked potential. Black dots indicate sensors statistically differing between Control ADHD groups at p < .025.
EP effects by medication status
Tested against each other, unmedicated ADHD group EP power exceeded medicated ADHD group power at 4 Hz at 12.8% of electrodes, and 7 Hz at 9.2% of electrodes. These differences were located in prefrontal and frontal regions. Medicated ADHD group EP power exceeded unmedicated only at 2 Hz, in 13.7% of electrodes, located over bilateral frontal and left occipital scalp regions.
Coherence spectra
In the alert interval, summation across both tails of the t-statistic differentiated groups (at 2 Hz, 7.4% of electrode pairs; at 3 Hz, 5.1%; 4 Hz, 6.9%; 5 Hz, 8.8%; 6 Hz, 5.9%; 7 Hz, 5.8%; 8 Hz, 12.1%; 9 Hz, 2.7%; 10 Hz, 8.3%; 11 Hz, 9.7%, at and above 12 Hz, < 5%). In the stimulus interval, larger group differences were present than in the alert interval below 8 Hz (at 2 Hz, 8.8% of electrode pairs differentiated groups; at 3 Hz, 11.7%; 4 Hz, 10.8%; 5 Hz, 11.4%; 6 Hz, 7%; 7 Hz, 7.8%; 8 Hz, 7.2%; 9 Hz, 2.6%; 10 Hz, 3.9%, 11 Hz, 5.1% at and above 12 Hz, < 5%.). Supplementary figures 1 and 2 illustrate the topographies of significant coherence differences for each frequency between 2–12 Hz (in 1 Hz steps), for both the alert and stimulus intervals.
We visually inspected significant channel pairs at each frequency for both tails of the t distribution, and identified four distinct spatial patterns that differentiated groups. We describe these patterns below, illustrating each with a topographic plot of a representative frequency showing the spatial distribution of electrode pairs that significantly differentiated groups. Example coherence spectra reflect electrode pairs from scalp regions showing the greatest number of significant effects at that frequency, and represent the dominant coherence differences between groups.
ADHD coherence is elevated in the lower alpha band (8 Hz)
In the alert interval, ADHD coherences exceeded Control coherences in the lower alpha band at 8 Hz (11.4% of electrode pairs), primarily among parietal-frontal and prefrontal-parietal electrode pairs. In the stimulus interval, 8 Hz ADHD coherences were elevated relative to Control coherences with the same topography, but reached significance at fewer electrode pairs (6.8%). Significant channel pairs included interhemispheric and intrahemispheric anterior-posterior pairs, and interhemispheric frontal pairs. In both intervals, the majority of significant electrode pairs were located close to the midline. Average spectra among electrode pairs spanning frontal/prefrontal and parietal regions showed the Control group peaking narrowly at 10 Hz in both the alert and stimulus intervals, while the ADHD group peaked broadly across 8–10 Hz.
The topography of 8 Hz ADHD group coherence elevations in the alert interval is illustrated in Figure 3a. At 8 Hz, 33% of significant ADHD group elevations included frontal electrodes; 15% involved prefrontal; 8% central; 7% temporal; 22% parietal; 5% occipital, and 10% included midline electrodes. 51% of the 8 Hz elevations were observed between hemispheres; 30% were within the right hemisphere; and 19% were within the left hemisphere.
Figure 3.

4A (upper left): Topography of ADHD group coherence elevation at 8 Hz in the alert interval. 4B (upper right): Control group coherence elevations at 10 Hz. Within hemisphere electrode pairs are plotted in blue, between hemisphere pairs in green. 4C & 4D (lower left and right): example spectra for both groups in the alert and stimulus intervals. Spectra are averages of the labeled electrode clusters, each representing averages across 9 electrode pairs. The left frontal cluster of electrodes "A" includes AF7 (Geodesic Sensor Net electrode #27); AF3 (24) and F5 (28). The right frontal cluster "B" includes AF8 (2); AF4 (3) and F6 (123). The right posterior cluster "C" includes P4 (87); P6 (93) and P04 (86). The left anterior temporal cluster "D" includes F9 (39); FT7 (40) and T9 (45). Geodesic Sensor Net 10-10 electrode equivalents from Luu & Feree (2000).
Control coherence is elevated in the upper alpha band (10–11 Hz)
Control group coherences were elevated in the alert interval in the upper alpha band at 10 Hz (8.1 % of electrode pairs) and 11 Hz (8.9%). These elevations were predominantly interhemispheric, spanning prefrontal with contralateral anterior temporal, central, midline and parietal electrode pairs; and among temporal with contralateral central and parietal electrode pairs. Significant intrahemispheric electrode pairs involved prefrontal and frontal electrodes with central, temporal and parietal electrodes in both hemispheres; and with occipital electrodes in the right hemisphere. Channel pairs differentiating groups were situated laterally, away from the midline. Average spectra among electrode pairs spanning anterior temporal and parietal regions showed both the Control and ADHD groups peaking narrowly at 10 Hz in alert and stimulus intervals, with smaller magnitudes evident in the ADHD group. In the stimulus interval, the 10–11 Hz Control group coherence elevation was significant at fewer electrode pairs, and did not meet significance criterion.
The topography of the Control group coherence elevations at 10 Hz in the alert interval is illustrated in Figure 3b. At 10 Hz, 27% of significant Control group elevations included prefrontal electrodes; 15% involved frontal; 14% central, 23% temporal; 11% parietal; 6% occipital, and 4% included midline electrodes. 58% of the 10 Hz elevations were observed between hemispheres; 25% were within the right hemisphere; and 17% were within the left hemisphere.
Control coherence is elevated in delta and theta bands (2–6 Hz)
Control group coherences were elevated between 2–6 Hz in both the alert and stimulus intervals. These elevations were largely interhemispheric, and involved connections spanning right hemisphere prefrontal and temporal electrodes with left hemisphere central, temporal, and parietal electrodes. Figure 4 illustrates the topography of Control group coherence elevations at 5 Hz in the alert interval (8.7% of electrode pairs). At 5 Hz, 28% of significant Control group elevations included prefrontal electrodes (18% in the right hemisphere); 8% involved frontal; 22% central (16% in the right hemisphere), 19% temporal (13% in the right hemisphere); 13% parietal, 3% occipital; and 7% included midline electrodes. 51% of the 5 Hz elevations were observed between hemispheres; 26% were within the right hemisphere; and 23% were within the left hemisphere.
Figure 4.

Control group coherence elevation between 2–6 Hz. Spectra are averages of the labeled electrode clusters, each representing averages across 9 electrode pairs. The right prefrontal cluster "A" is composed of electrodes near Fp1(22, 23, 26). The left prefrontal cluster ""B" is composed of electrodes near Fp2 (8,9,14). The right anterior temporal cluster "C" is composed of electrodes T8 (109); T10 (115) and FT8 (116). The left parietal cluster "D" is composed of electrodes around P1 (61, 67 and 54).
Stimulus-interval ADHD coherence is elevated in delta and lower theta bands (2–4 Hz)
ADHD group coherences were significantly elevated in the stimulus interval among electrode pairs spanning frontal with posterior temporal and occipital regions. These elevations were largely interhemispheric among anterior and contralateral anterior pairs, with more intrahemispheric pairs evident in the left hemisphere. Figure 5 illustrates the topography of ADHD group coherence elevations at 3 Hz in the stimulus interval (5.2% of electrode pairs). At 3 Hz, 15% of significant stimulus interval ADHD group elevations included prefrontal electrodes (10% in the left hemisphere); 30% involved frontal (21% left hemisphere); 3% central, 17% temporal; 11% parietal, 15% occipital; and 9% included midline electrodes. 40% of the 3 Hz elevations were observed between hemispheres; 18% were within the right hemisphere; and 42% were within the left hemisphere.
Figure 5.
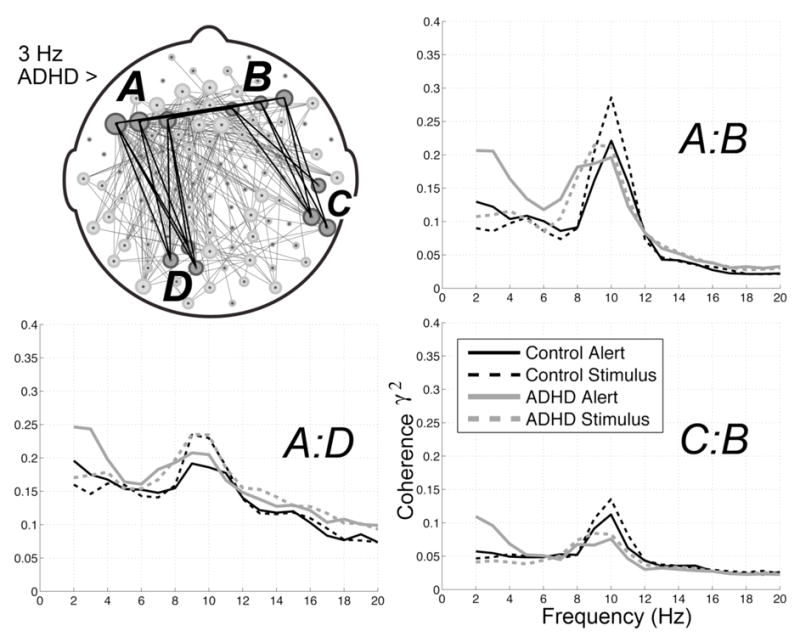
ADHD group coherence elevations between 2–4 Hz. Spectra are averages of the labeled electrode clusters, each representing averages across 9 electrode pairs. ADHD group coherence elevations between 2–4 Hz. The left frontal cluster "A" is composed of electrodes near F3(25), F5(28), and F7(34)The right frontal cluster "B" is composed of electrodes near AF8 (2), AF4(3), and F2 (4). The right temporal cluster "C" is composed of electrodes near T8 (109), TP10(101), and T10 (102). The left cluster "D" is composed of electrodes near 01 (72), P07(66,67)
Upper alpha band (10–12 Hz) coherence depends on medication status
Having found robust coherence differences between Control and ADHD subjects, we tested each frequency for significant group differences according to ADHD group medication status. In the alert interval, unmedicated ADHD coherences exceed the medicated ADHD group at 7 Hz in 5.4% of electrode pairs, at 10 Hz (5.5%), 11 Hz (12.3%), and 12 Hz (5.1%). In the stimulus interval, unmedicated ADHD coherences exceeded the medicated ADHD group at 10 Hz (8.5%) and 11 Hz (12%), and 12 Hz (5.9%). The topography of the unmedicated ADHD group coherence elevations was consistent across the alert and stimulus intervals. In the alert interval at 11 Hz, 15% of significant elevations included prefrontal electrodes; 19% involved frontal; 17% central, 19% temporal; 16% parietal, 7% occipital; and 7% included midline electrodes. 58% of the 3 Hz elevations were observed between hemispheres; 18% were within the right hemisphere; and 24% were within the left hemisphere.
Discussion
We analyzed a substantial record of dense array EEG with spectral power and coherence methods in 10–13 year old combined type ADHD and typically developing control children. Averaged evoked activity, and pre- and post-stimulus EEG were examined in a visual stimulation paradigm typical of cognitive evoked potential studies. We found coherence differences that were independent of stimulus presentation, with the ADHD group coherences reduced in delta, theta and upper alpha bands, and elevated in the lower alpha band. In response to visual stimuli, we observed globally reduced power and elevated frontal coherence in the ADHD group. We found coherence differences in the alpha band between medicated and unmedicated subjects.
EEG Power
Our finding of reduced ADHD group evoked potential power in the frequency domain is consistent with previous event-related potential studies, which have commonly found reduced ERP component amplitudes in ADHD groups (Barry et al., 2003b). Reduced theta band power been a frequent finding in ADHD (Barry et al., 2003a), most typically in the eyes closed resting state. Our subjects were in the eyes open, alert state, and we found no reduction in absolute ADHD group theta band power.
Prior coherence studies in ADHD
Direct comparison of our findings with prior investigations is problematic because of the sparse electrode arrays, reference dependence, and wide frequency bands employed in earlier studies. However, some of our results appear broadly compatible with previous coherence studies in ADHD, which have typically been made with subjects in the eyes closed resting state. Montague (1975) found significantly elevated intrahemispheric and reduced interhemispheric coherences in hyperkinetic children. Chabot and Serfontein (1996a) reported reduced parietal, and increased intrahemispheric coherence in frontal and central regions in children with attention deficit disorder. Elevated ADHD frontal coherence in the delta and theta bands has been a fairly consistent finding (Chabot et al 1996a and 1996b, Barry et al 2002, and Clarke et al., 2005). We found low frequency (2–4 Hz) ADHD frontal-frontal and frontal-temporal coherence elevations in the stimulus (but not alert) interval. Disparity with these studies may be methodological: we found reduced ADHD coherence over interhemispheric central electrode pairs between 2–6 Hz, while Barry et al.’s 2002 report of elevated delta and theta coherences included central, parietal, and occipital interhemispheric electrode pairs averaged together. Also inconsistent with that study is our finding of within hemisphere low alpha (8 Hz) ADHD coherence elevations. Barry et al. (2002) reported reduced long-range ADHD intrahemispheric alpha coherences, however, they examined only frontal-occipital electrode pairs, whereas the majority of ADHD elevations we observed were frontal-parietal. Generally, our finding of contrasting coherence effects in the lower (ADHD elevated) and upper (ADHD reduced) alpha range suggests that averaging across the wide 7.5–12.5 Hz range may obscure narrow band effects.
Methodological considerations
This study makes use of several methodological advances to estimate functional connectivity using EEG coherence (Srinivasan et al., 1998; Nunez and Srinivasan, 2006). Our ability to make reasonable inferences about cortical regions contributing to our results relies upon a large number of spatial measurements to enable the appropriate use of an average reference. Recordings made from sparse electrode arrays with a linked ears reference strategy limit interpretation of prior literature on EEG coherence in developmental psychopathology, including ADHD. Depending on the nature of the (unknown) signals at “recording” and “reference” sites, changes in power or phase at the reference location can easily be reflected as changes in coherence between two other recording electrodes. When recorded with reference to linked ears, the problem is compounded by the contribution of activity at two reference sites to all electrodes. Further, any differences in electrode contact impedance between the two reference sites in a physically linked ears recording will lead to a unknown bias in recordings to one hemisphere or the other (Nunez & Srinivasan, 2006), and this bias will inevitably differ among subjects, leading to a random reference. We have used 128 electrodes and an average reference, which is a reasonable approximation to reference independent potentials in simulation studies of EEG coherence (Srinivasan et al., 1998a).
Coherence between closely spaced EEG electrodes is elevated even when the underlying brain sources are entirely uncorrelated (Srinivasan et al., 1998a). Moreover, increases in the strength of one cortical source region will increase coherence between two electrodes located within 10 cm. of the source region (Nunez and Srinivasan, 2006) confounding source strength with coherence. Changes in coherence among potentials recorded at inter-electrode distances less than 10 cm. can reflect either changes in power of one source (detected by both electrodes), or genuine changes in coherence between two distinct sources. Thus, we only examined all possible (4,107) long-range electrode pairs in our electrode array, excluding electrode pairs that are separated by less than 10 cm. By restricting analysis to long-range electrode pairs, we avoided confounds due to volume conduction.
The present study is limited to combined-type attention deficit-hyperactivity disorder. Our findings therefore cannot be assigned either to the inattentive or hyperactive-impulsive components of the combined subtype. Because this study was conducted approximately 3 years after initial diagnosis of the children, our results are limited to children with a previous diagnosis of ADHD. Although Control group participants were selected from the same classrooms as ADHD participants, Controls were not matched on IQ or socioeconomic status. Because this study examined twice as many ADHD subjects than Controls, it potentially has more power to pick up effects in the ADHD group than in the Control group.
Anatomical considerations
MRI studies suggest overall brain volume reductions of up to 5% in ADHD, including reduced frontal lobe (Castellanos et al., 1996, 2002; Filipek et al., 1997; Sowell et al., 2003) and dorsolateral prefrontal cortical volumes (Durston et al., 2004; Giedd et al., 2001; Sowell et al., 2003). Grey matter reductions in frontal cortex and caudate nucleus have been reported (Overmeyer et al., 2001) as well as grey matter density increases in posterior aspects of the temporal lobes and inferior aspects of the parietal lobes (Sowell et al., 2003). Cerebellar volumes appear reduced in ADHD, as are caudate nucleus and globus pallidus volumes of the basal ganglia (Aylward et al., 1996; Castellanos et al., 1996; Filipek et al., 1997; Hynd et al., 1993). Reduced white matter volumes have been noted in right frontal (Semrud-Clikeman et al., 2000) and bilateral retrocallosal brain regions (Filipek, et al., 1997). The splenium of the corpus callosum, connecting temporal and parietal cortex, appears smaller in ADHD (Hynd et al., 1991, Lyoo et al. 1996; Semrud-Clikeman et al., 1994). The rostrum, the most anterior and inferior portion of the callosum connecting frontal and prefrontal cortical hemispheres, may be also appear smaller in ADHD groups (Baumgardner et al., 1996; Giedd et al., 1994; Hynd et al., 1991).
As many of the coherence differences we found were across hemispheres, our findings may reflect differences in functional callosal connections between the two groups of children. In humans, EEG coherence patterns appear consistent with anatomical cortical connectivity (Nunez, 1995; Tucker et al., 1986). Reduced coherences have been observed in pathological lesions of the corpus callosum and visual cortex (Knyazeva et al., 1999; 2002). These and other studies, reviewed by Knyazeva and Innocenti (2001) imply that corticocortical connections mediate EEG coherence. Impaired callosal connectivity in the ADHD group could account for the observation of elevated Control group coherences across hemispheres in the 2–5 Hz and 10–11Hz. These observations are independent of stimulus presentation, and may thus reflect a relatively static property of cortical connectivity. However, we also noted stimulus induced elevated ADHD coherence in frontal-temporal and frontal-frontal electrode pairs. Neuronal interpretations of elevated coherence in psychopathology may appear paradoxical, especially when disorders appear associated with deficits in white matter. In considering abnormal cortical connectivity in schizophrenia, Innocenti (2003) suggested that a partial loss of callosal axons might serve to enhance cortical connectivity, by inducing remaining axons to increase their number of boutons and/or their extent into contralateral cortex. Thus, callosally projecting neurons in one hemisphere could more powerfully control excitability of neurons in the other hemisphere. Given the intrahemispheric white matter and rostral callosal volume reductions that have been observed in ADHD, this account may also explain the elevated, stimulus induced frontal ADHD coherences we observe at low (2–4 Hz) frequencies.
Maturation
Symptoms of ADHD have been suggested to result from a deviant process of maturation and development. The maturational lag hypothesis (El-Sayed et al., 2003) has been suggested to include delays in brain myelination, which could be reflected in EEG coherence. Our observed interhemispheric 8 Hz ADHD coherence elevation may be consistent with the maturational lag hypothesis, since, like power, peak coherence spectra appears to develop with age, exhibiting a gradual decrease in lower frequencies and increase in higher frequencies (Marosi et al., 1997; Fornara et al., 1997). The 8 Hz coherence elevation was seen mostly at channel pairs located close to the midline, suggesting the possibility of elevated lower alpha range coherence representing a delayed maturation of cingulate cortex. Our control group coherence spectra exhibit a narrow peak alpha coherence at 10 Hz, while the ADHD group is broadly peaked between 8–10 Hz. This may also be consistent with a delay in development, at least among the long-range frontal-parietal regions near the midline shown in our data. Cross-sectional or longitudinal studies would be necessary to confirm delays in maturational (Bresnahan et al., 1999), however, this speculation is apparently consistent with Barry et al’s. 2005 study of coherence development in 8–12 year old ADHD and controls, which found within hemisphere alpha band (7.5–12.5 Hz) coherence values increased with age in healthy (but not ADHD subjects) across long inter-electrode distances.
Medication
Evidence from neuroimaging and psychopharmacological studies suggest that methylphenidate (MPH), a stimulant catecholamine agonist that is the primary drug for treatment of ADHD, principally acts by modulating fronto-striatal brain systems (Mehta et al., 2000), with therapeutic effects thought to rely on its ability to block dopamine and norepinephrine transporters (Solanto, 2002). Castellanos et al. (2002) found that unmedicated ADHD patients had significantly smaller white matter volumes, compared to controls and medicated children with ADHD. Prior reports of MPH effects on EEG coherence measures have been negative. Lubar et al. (1999) found no clear coherence changes within medicated ADHD children following MPH administration, and Clarke et al. (2005) found no coherence changes in ADHD subjects before and after a 6 month MPH trial. We compared ADHD subjects medicated with MPH and unmedicated ADHD subjects, and found narrow band coherences in the upper alpha (10–12 Hz) range were smaller within the medicated group. While we note medication effects, this was not a drug study by design. The present study lacks necessary control conditions such as random assignment to medication groups, control over medication histories, dosage, and administration that would be necessary to infer mediation of coherence by methylphenidate.
Conclusion
Anatomical and psychological evidence suggests that ADHD is characterized by reductions in regions of the corpus callosum, frontal lobes, basal ganglia, and cerebellum. These networks involve input-output processing and attention, including alerting and executive function. Consistent with neuroanatomical networks of attention, deficits in attention, information processing, alerting, orienting and working memory may be mediated primarily in the prefrontal cortex. The present findings suggest a static state of deficient connectivity between hemispheres in ADHD, and a stimulus induced state of over-connectivity within and between frontal hemispheres. They suggest delayed maturation of key cortical (e.g. cingulate) regions may be associated with ADHD. They imply that in addition to the anatomical and neuropsychological impairments associated with frontal cortex dysfunction, altered functional connectivity, particularly among frontal regions, is implicated in ADHD.
Acknowledgments
This research was supported in part by the UC Irvine Department of Cognitive Sciences, and by the National Institute of Mental Health, Grant R01-MH068004. Thanks to Greg Owen for graphical assistance, and to Bruce Berg, Geraldine Dawson and anonymous reviewers for critiques of earlier drafts.
References
- Abdullaev Y, Posner M. Event-related brain potential imaging of semantic encoding during processing of single words. Neuroimage. 1998;7:1–13. doi: 10.1006/nimg.1997.0309. [DOI] [PubMed] [Google Scholar]
- Andrew C, Pfurtscheller G. Dependence of coherence measurements on EEG derivation type. Med Biol Eng Comput. 1996;34:232–238. doi: 10.1007/BF02520079. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Reiss AL, Reader MJ, Singer HS, Brown JE, Denckla MB. Basal ganglia volumes in children with attention-deficit hyperactivity disorder. J Child Neurol. 1996;11:112–115. doi: 10.1177/088307389601100210. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003a;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M. EEG coherence in attention-deficit/hyperactivity disorder: a comparative study of two DSM-IV types. Clin Neurophysiol. 2002;113:579–585. doi: 10.1016/s1388-2457(02)00036-6. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ, Hsu C-I, Bond D, Wallace MJ, Magee CA. Age and gender effects in EEG coherence: II. Boys with attention deficit/hyperactivity disorder. Clinical Neurophysiology. 2005;116:977. doi: 10.1016/j.clinph.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Johnstone SJ, Clarke AR. A review of electrophysiology in attention-deficit/hyperactivity disorder:II. Event-related potentials. Clin Neurophysiol. 2003b;114:184–198. doi: 10.1016/s1388-2457(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Baumgardner T, Singer H, Denckla M, Rubin M, Abrams M, Colli M, Reiss A. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47:477–482. doi: 10.1212/wnl.47.2.477. [DOI] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Random Data: Analysis and Measurement Procedures. New York: John Wiley & Sons; 2001. [Google Scholar]
- Bertrand O, Perrin F, Pernier J. A theoretical justification of the average reference in topographic evoked potential studies. Electroencephalogr Clin Neurophysiol. 1985;62:462–464. doi: 10.1016/0168-5597(85)90058-9. [DOI] [PubMed] [Google Scholar]
- Bresnahan SM, Anderson JW, Barry RJ. Age-related changes in quantitative EEG in attention- deficit/hyperactivity disorder. Biological Psychiatry. 1999;46(12):1690. doi: 10.1016/s0006-3223(99)00042-6. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Large-scale cortical networks and cognition. Brain Research Reviews. 1995;20:288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- Castellanos FX. Neuroimaging of attention-deficit hyperactivity disorder. Child & Adolescent Psychiatric Clinics of North America. 1997;6:383–411. doi: 10.1016/j.chc.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clinical Pediatrics. 1997;36:381–393. doi: 10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh JL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kayen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL. Quantitative brain magnetic resonance imaging inattention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Mental Health in the United States: Prevalence of Diagnosis and Medication Treatment for Attention-Deficit/Hyperactivity Disorder--United States, 2003. JAMA: The Journal of the American Medical Association. 2005;294:2293-a. [Google Scholar]
- Chabot R, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biol Psychiatry. 1996a;40:951–963. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- Chabot RJ, Merkin H, Wood LM, Davenport TL, Serfontein G. Sensitivity and specificity of QEEG in children with attention deficit or specific developmental learning disorders. Clin Electroencephalogr. 1996b;27:26–34. doi: 10.1177/155005949602700105. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Johnstone SJ, Abbott I, Croft RJ, Magee CA, Hsu C-I, Lawrence CA. Effects of methylphenidate on EEG coherence in Attention-Deficit/Hyperactivity Disorder. International Journal of Psychophysiology. 2005;58:4. doi: 10.1016/j.ijpsycho.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Ding J, Sperling G, Srinivasan R. Attentional Modulation of SSVEP Power Depends on the Network Tagged by the Flicker Frequency. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Hulshoff PHE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, Kahn RS, van Engeland H. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2004:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- El-Sayed E, Larsson J-O, Persson H, Santosh P, Rydelius P-A. “Maturational lag” hypothesis of attention deficit hyperactivity disorder: an update. Acta Paediatrica. 2003;92:776–784. [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Fornara C, Medaglini S, Cursi M, Locatelli T, Minicucci F, Leocani L, Weber G, Cerai LP, Chiumello G, Comi G. Coherence EEG modifications in children with congenital hypothyroidism. Electroencephalography and Clinical Neurophysiology. 1997;103:118. [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Human Brain Mapping. 1994;2:56–78. [Google Scholar]
- Giedd J, Castellanos F, Casey B, Kozuch P, King A, Hamburger SJLR. Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. American Journal of Psychiatry. 1994;151:665–669. doi: 10.1176/ajp.151.5.665. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Molloy E, Castellanos FX. Brain Imaging of Attention Deficit/Hyperactivity Disorder. Annals of the New York Academy of Sciences. 2001;931:33–49. doi: 10.1111/j.1749-6632.2001.tb05772.x. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, March JS, Abikoff H, Arnold LE, Cantwell DP, Conners CK, et al. Comprehensive assessment of childhood attention deficit-hyperactivity disorder in the context of a multisite, multimodal clinical trial. Journal of Attention Disorders. 1997;1:217–234. [Google Scholar]
- Hynd G, Semrud-Clikeman M, Lorys A, Novey E, Eliopulos D, Lyytinen H. Corpus callosum morphology in attention deficit-hyperactivity disorder: morphometric analysis of MRI. J Learn Disabil. 1991;24:141–146. doi: 10.1177/002221949102400302. [DOI] [PubMed] [Google Scholar]
- Hynd GW, Hern KL, Novey ES, Eliopulos D, Marshall R, Gonzalez JJ, Voeller KK. Attention deficit-hyperactivity disorder and asymmetry of the caudate nucleus. Journal Of Child Neurology. 1993;8:339–347. doi: 10.1177/088307389300800409. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Molecular Psychiatry. 2003;8:261–274. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- Jemel B, Achenbach C, Muller BW, Ropcke B, Oades RD. Mismatch negativity results from bilateral asymmetric dipole sources in the frontal and temporal lobes. Brain Topography. 2002;15(1):13. doi: 10.1023/a:1019944805499. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Rohm D, Pollhuber D, Stadler W. Simultaneous desynchronization and synchronization of different alpha responses in the human electroencephalograph: a neglected paradox? Neuroscience Letters. 2000;284:97. doi: 10.1016/s0304-3940(00)00985-x. [DOI] [PubMed] [Google Scholar]
- Knyazeva MG, Innocenti GM. EEG coherence studies in the normal brain and after early-onset cortical pathologies. Brain Res Brain Res Rev. 2001;36:119–128. doi: 10.1016/s0165-0173(01)00087-x. [DOI] [PubMed] [Google Scholar]
- Knyazeva MG, Kiper DC, Vildavski VY, Despland PA, Maeder-Ingvar M, Innocenti GM. Visual stimulus-dependent changes in interhemispheric EEG coherence in humans. J Neurophysiol. 1999;82:3095–3107. doi: 10.1152/jn.1999.82.6.3095. [DOI] [PubMed] [Google Scholar]
- Knyazeva MG, Maeder P, Kiper DC, Deonna T, Innocenti GM. Vision after early-onset lesions of the occipital cortex: II. Physiological studies. Neural Plast. 2002;9:27–40. doi: 10.1155/NP.2002.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D, Ozaki H, Pal I. EEG alpha map series: brain micro-states by space-oriented adaptive segmentation. Electroencephalography and Clinical Neurophysiology. 1987;67:271–288. doi: 10.1016/0013-4694(87)90025-3. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F, Pfurtsceller G. Basic concepts on EEG synchronization and desynchronization. In: Pfurtscheller G, Lopes da Silva FH, editors. Event-related desynchronization. 11. Vol. 3. New York: Elsevier; 1999. [DOI] [PubMed] [Google Scholar]
- Lubar JF, WhiteJr JN, Swartwood MO, Swartwood JN. Methylphenidate effects on global and complex measures of EEG. Pediatric Neurology. 1999;21:633. doi: 10.1016/s0887-8994(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Luu P, Ferree T. Determination of the Geodesic Sensor Nets' Average Electrode Positions and Their 10-10 International Equivalents. Eugene, OR: Electrical Geodesics, Inc; 2000. [Google Scholar]
- Lyoo IK, Noam GG, Lee CK, Lee HK, Kennedy BP, Renshaw PF. The corpus callosum and lateral ventricles in children with attention-deficit hyperactivity disorder: A brain magnetic resonance imaging study. Biological Psychiatry. 1996;40:1060. doi: 10.1016/s0006-3223(96)00349-6. [DOI] [PubMed] [Google Scholar]
- Marosi E, Harmony T, Reyes A, Bernal J, Fernandez T, Guerrero V, Rodriguez M, Silva J, Yanez G, Rodriguez H. A follow-up study of EEG coherences in children with different pedagogical evaluations. Int J Psychophysiol. 1997;25:227–235. doi: 10.1016/s0167-8760(96)00745-3. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate Enhances Working Memory by Modulating Discrete Frontal and Parietal Lobe Regions in the Human Brain. J Neurosci. 2000;20:65RC. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagu J. The hyperactive child: a behavioral, electrodermal and EEG investigation. Dev Med Child Neurol. 1975;17:299–305. [PubMed] [Google Scholar]
- MTA Group . A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- MTA Group . Nine Months of Multicomponent Behavioral Treatment for ADHD and Effectiveness of MTA Fading Procedures. Journal of Abnormal Child Psychology. 2004;32:39–51. doi: 10.1023/b:jacp.0000007579.61289.31. [DOI] [PubMed] [Google Scholar]
- Nunez P, Wingeier B, Silberstein R. Spatial-temporal structures of human alpha rhythms: Theory, microcurrent sources, multiscale measurements, and global binding of local networks. Human Brain Mapping. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL. Neocortical dynamics and human EEG rhythms. Oxford University Press; 1995. [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. 2. New York: Oxford University Press; 2006. [Google Scholar]
- Overmeyer S, Bullmore ET, Suckling J, Simmons A, Williams SC, Santosh PJ, Taylor E. Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychological Medicine. 2001;31:1425–1435. doi: 10.1017/s0033291701004706. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural Maturation of Neural Pathways in Children and Adolescents: In Vivo Study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Images of the mind: Studies with modern imaging techniques. Annual Review of Psychology. 1994;45:333–356. doi: 10.1146/annurev.ps.45.020194.002001. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Filipek PA, Biederman J, Steingard R, Kennedy D, Renshaw P, Bekken K. Attention-deficit hyperactivity disorder: Magnetic resonance imaging morphometric analysis of the corpus callosum. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:875. doi: 10.1097/00004583-199407000-00014. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Steingard R, Filipek P, Biederman J, Bekken K, Renshaw P. Using MRI to examine brain-behavior relationships in males with attention deficit disorder with hyperactivity. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:477–484. doi: 10.1097/00004583-200004000-00017. [DOI] [PubMed] [Google Scholar]
- Silberstein R, Farrow M, Levy F, Pipingas A, Hay D, Jarman F. Functional brain electrical activity mapping in boys with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1998;55:1105–1112. doi: 10.1001/archpsyc.55.12.1105. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behavioural Brain Research. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Sowell E, Thompson P, Welcome S, Henkenius A, Toga A, Peterson B. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Srinivasan R. Spatial structure of the human alpha rhythm: global correlation in adults and local correlation in children. Clinical Neurophysiology. 1999;110:1351–1362. doi: 10.1016/s1388-2457(99)00080-2. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Silberstein RB. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Trans Biomed Eng. 1998a;45:814–826. doi: 10.1109/10.686789. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Tucker DM, Murias M. Estimating the spatial Nyquist of the human EEG. Behavior Research Methods, Instruments & Computers. 1998b;30:8–19. [Google Scholar]
- Swanson J, Castellanos FX, Murias M, LaHoste G, Kennedy J. Cognitive neuroscience of attention deficit hyperactivity disorder and hyperkinetic disorder. Current Opinion in Neurobiology. 1998;8 doi: 10.1016/s0959-4388(98)80150-5. [DOI] [PubMed] [Google Scholar]
- Swanson J. SNAP-IV Scale. Irvine: University of California Child Development Center; 1995. [Google Scholar]
- Swanson J, McBurnett K, Christian D, Wigal T. Stimulant medications and the treatment of children with ADHD. Adv Clin Child Psychol. 1995;17:265–232. [Google Scholar]
- Swanson J, Oosterlaan J, Murias M, Schuck S, Flodman P, Spence MA, Wasdell M, Ding Y, Chi HC, Smith M, Mann M, Carlson C, Kennedy JL, Sergeant JA, Leung P, Zhang YP, Sadeh A, Chen C, Whalen CK, Babb KA, Moyzis R, Posner MI. Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proc Natl Acad Sci U S A. 2000;97:4754–4759. doi: 10.1073/pnas.080070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher RW. Cyclic cortical reorganization: origins of human cognitive development. In: Dawson G, Fischer K, editors. Human Behavior and the Developing Brain. New York: Guilford; 1994. pp. 232–266. [Google Scholar]
- Thatcher RW, Krause PJ, Hrybyk M. Cortico-cortical associations and EEG coherence: a two-compartmental model. Electroencephalography and Clinical Neurophysiology. 1986;64:123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, McAlaster R, Lester ML, Horst RL, Cantor DS. Hemispheric EEG asymmetries related to cognitive functioning in children. In: Perecman A, editor. Cognitive processing in the right hemisphere. New York: Academic Press; 1983. pp. 125–146. [Google Scholar]
- Thatcher RW, Walker RA, Guidice S. Human cerebral hemispheres develop at different rates and age. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Roth DL, Bair TB. Functional connections among cortical regions: topography of EEG coherence. Electroencephalography and Clinical Neurophysiology. 1986;63:242–250. doi: 10.1016/0013-4694(86)90092-1. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]


