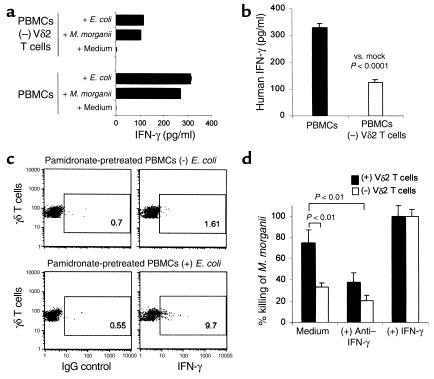
Figure 3.
Vδ2 T cells and IFN-γ play a crucial role in monocyte-mediated killing of extracellular bacteria. IBA-pretreated PBMCs exposed in vitro to dead bacteria for 17 hours induced γδ T cell–dependent IFN-γ secretion (a). At 17 hours after intraperitoneal infection with E. coli (5 × 106 CFUs), SCID mice (n = 5 in each group) reconstituted intraperitoneally with IBA-pretreated, mock-depleted PBMCs had higher levels of serum human IFN-γ than did mice reconstituted with IBA-pretreated PBMCs depleted of Vδ2 T cells (b). Intracellular IFN-γ was produced by 9.7% of γδ T cells harvested from the peritoneal lavage of SCID mice that were reconstituted intraperitoneally with pamidronate-pretreated PBMCs (or IBA-pretreated PBMCs, data not shown) and infected intraperitoneally with E. coli (5 × 106 CFUs) (c, bottom), while only 1.6% of γδ T cells harvested from reconstituted and mock-infected SCID mice produced IFN-γ (c, top). Monocytes from PBMC cultures that were depleted of Vδ2 T cells killed less than half as many bacteria than did monocytes from mock-depleted cultures. Inclusion of neutralizing mAbs to IFN-γ in the culture abrogated the γδ T cell–dependent monocyte-mediated killing of M. morganii. Addition of IFN-γ to the Vδ2 T cell–depleted culture completely reconstituted the antibacterial effect lost by depletion of Vδ2 T cells (d). Use of pamidronate-pretreated PBMCs resulted in data similar to that obtained using IBA-pretreated PBMCs (data not shown). Data were representative of two to four experiments.