Abstract
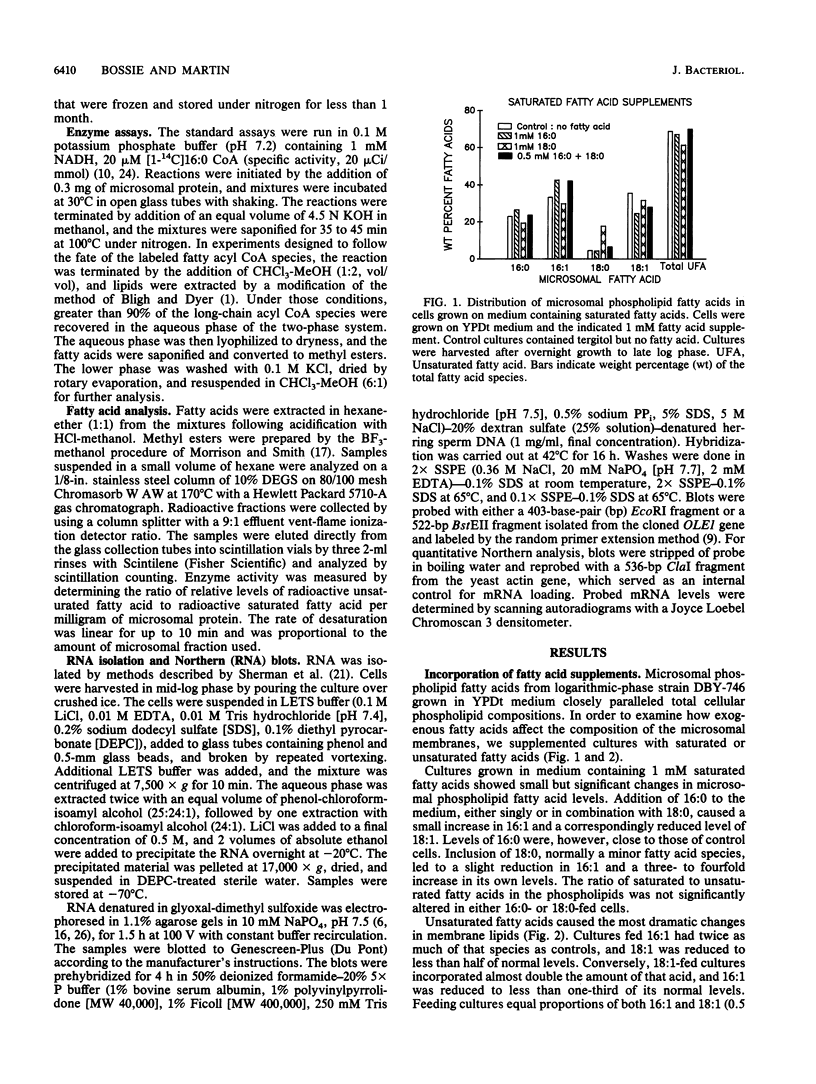
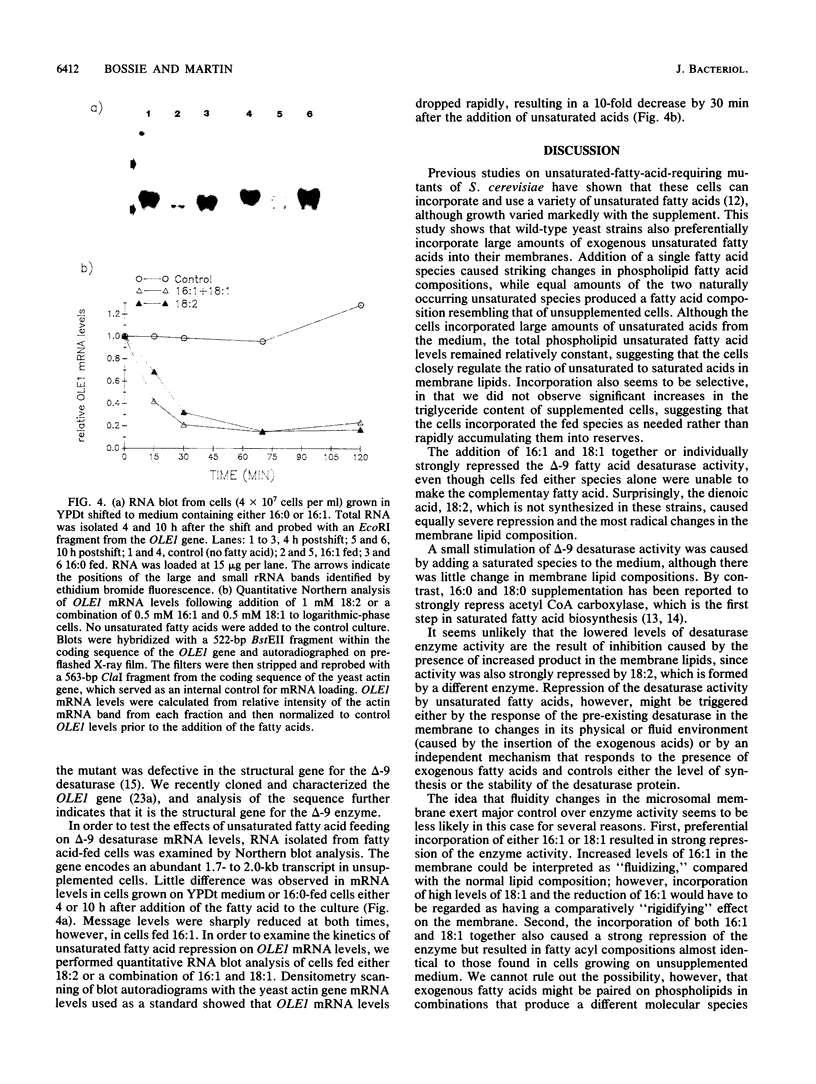
The addition of unsaturated fatty acids to cultures of Saccharomyces cerevisiae significantly altered the microsomal lipid composition. Supplementation with either of the naturally occurring palmitoleic (16:1) or oleic (18:1) acids caused increased levels in membrane phospholipids and reduced levels of the complementary acid. Growth in the presence of equimolar quantities of 16:1 and 18:1 acids, however, produced a fatty acid composition similar to that found in unsupplemented cell membranes. Linoleic acid (18:2) was not found in S. cerevisiae grown under normal conditions. It was preferentially internalized and incorporated into microsomes, however, at levels exceeding 50% of the total fatty acid species. This resulted in an almost total loss of 16:1 and a reduction of 18:1 to 25% of its normal level. The delta-9 fatty acid desaturase, a microsomal enzyme that forms 16:1 and 18:1 from saturated acyl coenzyme A precursors, was affected by the presence of exogenous fatty acids. Enzyme activity toward the 16:0 coenzyme A substrate was elevated in microsomes from saturated-fatty-acid-supplemented cultures and sharply repressed following the addition of unsaturated fatty acids, including 18:2. Northern (RNA blot) and slot-blot analyses of mRNA encoded by the OLE1 gene, which appears to be the structural gene for the delta-9 desaturase, indicated that it was sharply reduced in unsaturated-fatty-acid-fed cells. These data suggest that a significant part of the regulation involves modulation of available transcripts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson L. R., Johnston A. M., Martin C. E. The effects of temperature acclimation on membrane sterols and phospholipids of Neurospora crassa. Biochim Biophys Acta. 1982 Nov 12;713(2):456–462. doi: 10.1016/0005-2760(82)90265-x. [DOI] [PubMed] [Google Scholar]
- Atkinson K., Fogel S., Henry S. A. Yeast mutant defective in phosphatidylserine synthesis. J Biol Chem. 1980 Jul 25;255(14):6653–6661. [PubMed] [Google Scholar]
- BLOOMFIELD D. K., BLOCH K. The formation of delta 9-unsaturated fatty acids. J Biol Chem. 1960 Feb;235:337–345. [PubMed] [Google Scholar]
- Baker N., Lynen F. Factors involved in fatty acyl CoA desaturation by fungal microsomes. The relative roles of acyl CoA and phospholipids as substrates. Eur J Biochem. 1971 Mar 11;19(2):200–210. doi: 10.1111/j.1432-1033.1971.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Esfahani M., Kucirka E. M., Timmons F. X., Tyagi S., Lord A. E., Jr, Henry S. A. Effect of exogenous fatty acids on growth, membrane fluidity, and phospholipid fatty acid composition in yeast. J Supramol Struct Cell Biochem. 1981;15(2):119–128. doi: 10.1002/jsscb.1981.380150203. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gabrielides C., Hamill A. L., Scott W. A. Requirements of delta 9 and delta 12 fatty acid desaturation in Neurospora. Biochim Biophys Acta. 1982 Sep 14;712(3):505–514. doi: 10.1016/0005-2760(82)90278-8. [DOI] [PubMed] [Google Scholar]
- Gordon P. A., Lowdon M. J., Stewart P. R. Effect of unsaturated fatty acids on the development of respiration and on protein synthesis in an unsaturated fatty acid mutant of Saccharomyces cerevisiae. J Bacteriol. 1972 May;110(2):511–515. doi: 10.1128/jb.110.2.511-515.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Numa S. Reduction of the acetyl coenzyme A carboxylase content of Saccharomyces cerevisiae by exogenous fatty acids. FEBS Lett. 1973 Dec 15;38(1):29–32. doi: 10.1016/0014-5793(73)80505-8. [DOI] [PubMed] [Google Scholar]
- Kamiryo T., Parthasarathy S., Numa S. Evidence that acyl coenzyme A synthetase activity is required for repression of yeast acetyl coenzyme A carboxylase by exogenous fatty acids. Proc Natl Acad Sci U S A. 1976 Feb;73(2):386–390. doi: 10.1073/pnas.73.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A. D., Resnick M. R., Haley A. B. Fatty acid desaturase mutants of Saccharomyces cerevisiae. J Bacteriol. 1969 May;98(2):415–420. doi: 10.1128/jb.98.2.415-420.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Sato R. The dietary control of the microsomal stearyl CoA desaturation enzyme system in rat liver. Arch Biochem Biophys. 1972 Apr;149(2):369–377. doi: 10.1016/0003-9861(72)90335-9. [DOI] [PubMed] [Google Scholar]
- Prasad M. R., Joshi V. C. Purification and properties of hen liver microsomal terminal enzyme involved in stearoyl coenzyme A desaturation and its quantitation in neonatal chicks. J Biol Chem. 1979 Jul 25;254(14):6362–6368. [PubMed] [Google Scholar]
- Resnick M. A., Mortimer R. K. Unsaturated fatty acid mutants of Saccharomyces cerevisiae. J Bacteriol. 1966 Sep;92(3):597–600. doi: 10.1128/jb.92.3.597-600.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter P., Enoch H. G. Purification of stearyl-CoA desaturase from liver. Methods Enzymol. 1978;52:188–193. doi: 10.1016/s0076-6879(78)52020-x. [DOI] [PubMed] [Google Scholar]
- Strittmatter P., Spatz L., Corcoran D., Rogers M. J., Setlow B., Redline R. Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4565–4569. doi: 10.1073/pnas.71.11.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukey J. E., McDonough V. M., Martin C. E. Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J Biol Chem. 1989 Oct 5;264(28):16537–16544. [PubMed] [Google Scholar]
- Tamura Y., Yoshida Y., Sato R., Kumaoka H. Fatty acid desaturase system of yeast microsomes. Involvement of cytochrome b5-containing electron-transport chain. Arch Biochem Biophys. 1976 Jul;175(1):284–294. doi: 10.1016/0003-9861(76)90510-5. [DOI] [PubMed] [Google Scholar]
- Thiede M. A., Strittmatter P. The induction and characterization of rat liver stearyl-CoA desaturase mRNA. J Biol Chem. 1985 Nov 25;260(27):14459–14463. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnieski B. J., Keith A. D., Resnick M. R. Double-bond requirement in a fatty acid desaturase mutant of Saccharomyces cerevisiae. J Bacteriol. 1970 Jan;101(1):160–165. doi: 10.1128/jb.101.1.160-165.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnieski B. J., Kiyomoto R. K. Fatty acid desaturase mutants of yeast: growth requirements and electron spin resonance spin-label distribution. J Bacteriol. 1972 Jan;109(1):186–195. doi: 10.1128/jb.109.1.186-195.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



