Abstract
Here we describe the identification and characterization of a physiological marker that is associated with the chloroquine-resistant (CQR) phenotype in the human malarial parasite Plasmodium falciparum. Single cell in vivo pH measurements revealed that CQR parasites consistently have an elevated cytoplasmic pH compared to that of chloroquine-sensitive (CQS) parasites because of a constitutively activated Na+/H+ exchanger (NHE). Together, biochemical and physiological data suggest that chloroquine activates the plasmodial NHE of CQS parasites, resulting in a transitory phase of rapid sodium/hydrogen ion exchange during which chloroquine is taken up by this protein. The constitutively stimulated NHE of CQR parasites are capable of little or no further activation by chloroquine. We propose that the inability of chloroquine to stimulate its own uptake through the constitutively activated NHE of resistant parasites constitutes a minimal and necessary event in the generation of the chloroquine-resistant phenotype.
Chloroquine was the first choice antimalarial drug for more than three decades until the emergence and spread of chloroquine-resistant Plasmodium falciparum strains rendered its application ineffective in many parts of the world. As other available antimalarial drugs are not as effective, safe, or affordable as chloroquine, the incidence of malaria has soared to ∼500 million clinical cases per year (World Health Organization, 1996). In understanding the molecular mechanism of chloroquine resistance, we may gain valuable insights into the parasite's biology, which, in turn, may inspire rational programs for the development of novel antimalarial drugs with improved pharmacological properties.
Chloroquine targets the intraerythrocytic stages of P. falciparum (Yayon et al., 1983), which feed on the erythrocyte's hemoglobin. The toxic heme moiety released in the process is polymerized in the parasite's acidic vacuole into insoluble and inert hemozoin (Slater et al., 1991). Chloroquine, accumulating to high concentrations in the vacuole, exerts its specific antimalarial effect in the inhibition of heme polymerization (Slater and Cerami, 1992; Dorn et al., 1995; Sullivan et al., 1996). Chloroquine-resistant (CQR)1 parasites accumulate less chloroquine in their vacuoles than do chloroquine-sensitive (CQS) parasites (Fitch, 1970, 1973), suggesting that a reduction in the vacuolar chloroquine concentration, below levels necessary to inhibit heme polymerization, is responsible for chloroquine resistance.
Two different models have been proposed to explain the differences in chloroquine accumulation associated with the resistant phenotype. The first model invokes the acquisition of a rapid chloroquine efflux mechanism by CQR parasite isolates (Krogstad et al., 1987; Martin et al., 1987). The second model proposes that CQR parasites have an elevated pH in their acidic lysosomes that would reduce acidotropic accumulation of the diprotic weak base chloroquine (Ginsburg and Stein, 1991).
We have recently presented compelling evidence in favor of a third model (Sanchez et al., 1997). We found that a carrier-mediated import mechanism is responsible for chloroquine uptake and accumulation in P. falciparum, in contrast to uninfected erythrocytes where chloroquine is solely taken up by nonionic diffusion of the free base (Ferrari and Cutler, 1990; Sanchez et al., 1997). The observation that carrier-mediated chloroquine uptake is competitively inhibited by 5-(N-ethyl-N-isopropyl) amiloride (EIPA), a specific and reversible inhibitor of eukaryotic Na+/H+ exchangers (Vigne et al., 1983; Kleyman and Cragoe, 1990), identified the plasmodial Na+/H+ exchanger (NHE) as a primary candidate for the chloroquine importer (Sanchez et al., 1997). The P. falciparum NHE resides in the parasite's plasma membrane, where it plays an essential role in the maintenance of the parasite's cytoplasmic pH, expelling excess protons generated during metabolism in exchange for sodium ions (Bosia et al., 1993).
The genetic linkage between the CQR phenotype and a reduction in carrier-mediated chloroquine uptake suggests that the P. falciparum NHE is altered in CQR parasites (Sanchez et al., 1997). To verify this hypothesis, we have studied the pH-regulating function of the NHE as well as its putative role in chloroquine transport, in both CQS and CQR parasites. We found that a change in NHE activity, resulting in an elevated cytoplasmic pH, is genetically linked with the CQR phenotype. We further provide evidence for the model that the activity status of the NHE determines the ability of this protein to import chloroquine.
Materials and Methods
P. falciparum Culture
The P. falciparum isolates investigated were cultured in vitro as described (Trager and Jensen, 1976) and then synchronized using the sorbitol method (Lambros and Vanderberg, 1979).
Fluorimetric Assay of Intracellular pH
Fluorimetric in vivo cytoplasmic pH measurements were performed using the fluorochrome 2′,7′-bis-(2-carboxyethyl)-5,6-carboxyfluorescein-acetoxymethylester (BCECF-AM; Molecular Probes, Inc., Eugene, OR) as described (Weiner and Hamm, 1989; Wünsch et al., 1995). Briefly, intraerythrocytic P. falciparum cultures were collected and washed twice in Ringer solution (122.5 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 0.8 mM MgCl2, 5.5 mM d-glucose, 1.0 mM Na2HPO4, 10 mM Hepes, pH 7.4, at 37°C). The cells were incubated for 3 min in Ringer solution containing 3 μM of BCECF-AM. The erythrocytes were seeded onto poly-l-lysine (Sigma Chemical Co.)-coated coverslips and then rinsed with Ringer solution to remove excess BCECF-AM. The coverslip was mounted in a superfusion chamber on the stage of an inverted microscope (model 100 TV Axiovert; Carl Zeiss, Inc., Thornwood, NY), and continuously superfused with Ringer solution prewarmed to 37°C to maintain physiological conditions. Fluorescence emissions at 520 nm were monitored from alternate excitation at wavelengths of 488 and 460 nm, using an automatic filter changing device (Carl Zeiss, Inc.). Using the ratio of the fluorescence signals at the two excitation wavelengths, a pH reading was obtained independently of changes in either cell volume or fluorochrome concentration during the measurement (Weiner and Hamm, 1989; Negulescu et al., 1990). Data acquisition were controlled using Attofluor software (Carl Zeiss, Inc.). This software allows us to define arbitrary areas of interest within a cell where the pH is measured. For each area of interest, a separate pH calibration is performed that compensates for quenching effects and variations in the fluorescence signal intensities emitted from different areas. Calibration of intracellular pH was performed by the nigericin/high potassium method using at least two different buffers of known pH (Thomas et al., 1979). The pH was exclusively determined in the cytoplasm of late trophozoite stage parasites (24–30 h after invasion) positioned horizontally on the slide, as determined by light microscopy. The area where the pH is measured was always defined completely within the parasite's cytoplasm. Where indicated, the Ringer solution was replaced by a bicarbonated buffer (106 mM NaCl, 24 mM NaHCO3, 5.4 mM KCl, 1.2 mM CaCl2, 0.8 mM Na2HPO4, 0.2 mM NaH2PO4, 0.8 mM MgCl2, 5.5 mM d-glucose, pH 7.4, at 37°C, gassed with CO2). Chloroquine exhibits no chromatic activity at the wavelengths used to measure the cytoplasmic pH or sodium ion concentration (data not shown).
Fluorimetric pH Measurements of Isolated P. falciparum Parasites
P. falciparum–infected erythrocytes were loaded with BCECF as described above. The host erythrocyte was lysed using the dipeptide glycyl- l-serine at a concentration of 5% (Elford, 1993), and then solubilized in a modified Hepes Ringer buffer (115 mM NaCl, 10 mM KCl, 1.2 mM CaCl2, 0.8 mM MgCl2, 5.5 mM d-glucose, 1.0 mM Na2HPO4, 10 mM Hepes, pH 7.1, at 37°C). The modified buffer resembles the cytoplasmic ionic environment of the host erythrocyte (Lee et al., 1988). Isolated parasites were then seeded on coverslips and analyzed as described above.
Determination of NHE Activity
NHE activity was determined using the ammonium chloride prepulse technique as described (Boyarsky et al., 1990). Briefly, the parasite's cytoplasmic pH was monitored while the infected erythrocyte was superfused with Ringer solution containing 40 mM of NH4Cl for 2 min. The superfusion buffer was then changed to Ringer solution alone and then the time course of the cytoplasmic pH recovery was monitored. To determine the component of proton flux that is independent of NHE activity, 50 μM of EIPA was added at different time points during the pH recovery phase as described previously (Boyarsky et al., 1990). The EIPA-independent proton flux was subsequently subtracted from the net proton flux. NHE activity was calculated by multiplying the EIPA-sensitive proton flux with the corresponding pH-dependent intracellular buffer capacity βi. The pH-dependent βi was determined as described (Boyarsky et al., 1988). Briefly, cells were superfused with Ringer solution containing 40, 20, 10, 5, and 0 mM of NH4Cl while the cytoplasmic pH of the parasite was monitored. The βi was calculated for each of the different NH4Cl concentrations by dividing the change in the intracellular NH4 + concentration ([NH4 +]i) by the corresponding change in the cytoplasmic pH (ΔpHi). [NH4 +]i was calculated using the Henderson–Hasselbalch equation, assuming that free NH3 is in equilibrium across the plasma membrane.
Fluorimetric Assay of the Intracellular Sodium Ion Concentration
Noninvasive fluorimetric measurements of the cytoplasmic sodium ion concentration were made using the fluorochrome SBFI (sodium-binding benzofuran isophthalate-acetoxymethylester; Molecular Probes, Inc.) as a sodium ion indicator as described (Minta and Tsien, 1989; Negulescu and Machen, 1990). Briefly, intraerythrocytic P. falciparum cultures were maintained for 2 to 3 h in medium containing 10 μM SBFI-AM, dissolved in Pluronic F127 (10% wt/vol DMSO; Molecular Probes, Inc.). Cytoplasmic sodium ion concentrations were then measured using the ratio imaging system described above, with the exception that the fluorescence emissions at 520 nm were monitored at alternate excitation wavelengths of 334 and 380 nm. For internal calibration, cells were permeabilized by the addition of 10 μM of gramicidin followed by superfusion with at least four different buffers of known sodium ion concentration. The buffers for calibration were made by mixing different amounts of the sodium-free buffer A (130 mM K-gluconate, 30 mM KCl, 1.2 mM CaCl2, 0.8 mM MgCl2, 5.5 mM d-glucose, 10 mM Hepes, pH 7.1, at 37°C) with the potassium-free buffer B (130 mM Na-gluconate, 30 mM NaCl, 1.2 mM CaCl2, 0.8 mM MgCl2, 5.5 mM d-glucose, 10 mM Hepes, pH 7.1, at 37°C).
Determination of the IC50 Values for Chloroquine
The susceptibility of the P. falciparum clones investigated to chloroquine was determined according to the WHO guidelines (Wernsdorfer and Payn, 1988). Briefly, synchronized cultures containing ring stage parasites at a parasitemia of 1% were aliquoted into microtiter plates and serial dilutions of chloroquine were added. After incubation for 24 h, the medium was changed and replaced by medium containing the same chloroquine concentration supplemented with [3H]hypoxanthine (2 μCi/ml; Amersham Corp., Arlington Heights, IL). Cells were harvested after a further 24 h of incubation. The incorporation of [3H]hypoxanthine into the parasite's DNA, which occurs during the trophozoite and early schizont stages, was then measured as a function of the chloroquine concentration present in the medium, and then a 50% inhibitory concentration of chloroquine (IC50) was determined. The IC50 value is the chloroquine concentration that inhibits 50% of the parasites in their development from rings to schizonts within a 48-h period. A parasite clone is considered chloroquine resistant if the IC50 value exceeds 160 nM (Wernsdorfer and Payn, 1988).
[3H]Chloroquine Uptake Kinetics
The kinetics of chloroquine uptake in the presence of various NHE inhibitors was determined as previously described (Sanchez et al., 1997). The NHE inhibitors examined were: amiloride (Sigma Chemical Co.); DMA (5-[N,N-dimethyl]amiloride; Sigma Chemical Co.); EIPA (5-[N-ethyl- N-isopropyl]amiloride; Hoechst A.G. Frankfurt, Germany); HMA (5-[N,N-hexamethylene]amiloride; Molecular Probes, Inc.); Hoe 370 (5-chloro- 2-indoloyl guanidine; Hoechst); IBMA (5-[N-isobutyl-N-methyl]amiloride; Molecular Probes Inc.).
Statistical Analysis
Data were evaluated for statistical significance using the Student's paired t test or the ANOVA test as appropriate. Significance was assumed if P < 0.05. Values are given as the mean of (n) independent measurements ± SEM.
Results
Chloroquine Uptake Is Mediated by an NHE in P. falciparum
Based on our finding that chloroquine uptake by P. falciparum is a carrier-mediated process that is competitively inhibited by EIPA, we have recently formulated the hypothesis that the P. falciparum NHE mediates chloroquine uptake (Sanchez et al., 1997). To confirm this hypothesis we determined the effect of other NHE inhibitors on chloroquine uptake. Five NHE inhibitors were chosen: amiloride, DMA, IBMA, HMA, and Hoe 370. Like EIPA, DMA, IBMA, and HMA are amiloride derivatives, whereas Hoe 370 belongs to a structurally unrelated group of NHE inhibitors that are derived from indoloyl-guanidine. The initial velocities of [3H]chloroquine uptake by the CQS parasite clone HB3 were monitored over a range of chloroquine concentrations in the presence of each of these NHE inhibitors. For each NHE inhibitor, three different concentrations were examined and then the data obtained were analyzed using Lineweaver–Burk plots (Fig. 1). It was found that all the NHE inhibitors examined inhibit chloroquine uptake in a strictly competitive manner. This finding indicates that the chloroquine and NHE inhibitors examined compete for binding to the same site. For each inhibitor, the apparent constant of inhibition (K i) was determined by plotting the slopes of the lines versus the corresponding inhibitor concentrations. The apparent K i values obtained range from 450 μM for amiloride, to 8 μM for IBMA, and to 3 μM for HMA (Table I). This establishes a potency scale with HMA being the most potent inhibitor of chloroquine uptake and amiloride being the least (Table I). The ability of these substances to inhibit eukaryotic NHEs is ranked in the same order (Kleyman and Cragoe, 1988, 1990), providing compelling evidence in support of our proposal that the P. falciparum NHE mediates chloroquine uptake.
Figure 1.

Inhibition of initial [3H]chloroquine uptake by NHE inhibitors. The initial rates of [3H]chloroquine uptake by HB3 were measured over a range of chloroquine concentrations in the presence of the NHE inhibitors indicated. The data were analyzed using Lineweaver–Burk plots. The concentrations of the NHE inhibitors used are: amiloride: (□) 0 μM, (▵) 50 μM, (▿) 500 μM, (○) 2 mM, DMA: (□) 0 μM, (▵) 50 μM, (▿) 150 μM, (○) 250 μM; IBMA, HMA, and Hoe 370: (□) 0 μM, (▵) 5 μM, (▿) 10 μM, (○) 50 μM. The mean of three independent experiments is shown. The apparent constants of inhibition determined are listed in Table I. DMA, 5-(N,N-dimethyl)amiloride; EIPA, 5-(N-ethyl-N-isopropyl)amiloride; HMA, 5-(N,N-hexamethylene) amiloride; Hoe 370, 5-chloro-2-indoloyl guanidine; IBMA, 5-(N-isobutyl-N-methyl)amiloride.
Table I.
Apparent Constants of Inhibition of Chloroquine Uptake for Six Different NHE Inhibitors
| Inhibitor | K i | Potency | pKa | |||
|---|---|---|---|---|---|---|
| μM | ||||||
| Amiloride | 450 | 1 | 8.6* | |||
| DMA | 81 | 6 | 8.7* | |||
| Hoe 370 | 12 | 38 | — | |||
| IBMA | 8 | 56 | 8.1‡ | |||
| EIPA | 6 | 75 | 8.6* | |||
| HMA | 3 | 150 | 8.5‡ | |||
The apparent constants of inhibition (Ki) were determined from the data presented in Fig. 1 by plotting the slopes of the lines versus the corresponding NHE inhibitor concentration. The K i of EIPA was taken from Sanchez et al. (1997). The potency of the NHE inhibitor to inhibit chloroquine uptake is given in comparison to amiloride. Abbreviations: DMA, 5-(N,N-dimethyl)amiloride; EIPA, 5-(N-ethyl-N-isopropyl)amiloride; HMA, 5-(N,N-methyl)amiloride; Hoe 370, 5-chloro-2-indoloyl guanidine; IBMA, 5-(N-isobutyl-N-methyl)amiloride. The pKa values were taken from:
Asher et al. (1987); and
Fluorimetric Measurement of Cytoplasmic pH in P. falciparum
As the CQR phenotype is genetically linked with changes in the chloroquine uptake kinetics (Sanchez et al., 1997), this finding suggests that the NHE is altered in response to chloroquine selection. These alterations may also affect the role of the NHE in pH regulation, implying that cytoplasmic pH regulation may differ between CQS and CQR parasite clones. Current protocols to measure cytoplasmic pH rely on single-cell noninvasive fluorimetric techniques that use the pH-sensitive fluorochrome BCECF and a ratio-imaging system to detect changes in the spectral properties of BCECF (Weiner and Hamm, 1989; Wünsch et al., 1995). We initially determined if such a fluorimetric technique can be applied in measuring the cytoplasmic pH (pHi) of P. falciparum while still residing within its host erythrocyte. After loading with BCECF, a strong and readily detectable fluorescence signal was observed in infected erythrocytes in the area of the parasite, as independently identified by light microscopy (Fig. 2 A). The intensity of the fluorescence signal from the parasite was found to be four times higher per squared micrometer than that emitted by the host erythrocyte cytoplasm (Fig. 2 B). More than 90% of the signal remained associated with the parasite even after lysis of its host erythrocyte, using the dipeptide glycyl-l-serine (Elford, 1993; Fig. 2 B). These data indicate that in the area where the parasite lies, most, if not all, of the fluorescence signal is emitted by the parasite itself. The contribution of the host erythrocyte cytoplasm is <0.03 pH units, as calculated from the thickness of the erythrocyte cytoplasm above the parasite of ∼0–0.125 μm (Rosenthal et al., 1988; Sam-Yellowe et al., 1988), and the fluorescence signals emitted from the parasite both within and without the host erythrocyte.
Figure 2.
Noninvasive fluorimetric pH measurements of P. falciparum. (A) Fluorescence signals emitted from infected and noninfected erythrocytes after loading with the pH-sensitive fluorochrome BCECF. Regions of interest where the pH is determined are indicated by squares. RBC, uninfected erythrocyte; iRBC, infected host erythrocyte cytoplasm. (B) The fluorescence signals emitted from the parasite cytoplasm and the host erythrocyte cytoplasm were quantified and expressed in arbitrary units per squared micrometer. The fluorescence signal that remained associated with the parasite after lysis of its host erythrocyte is indicated (hatched column). (C) Fluorimetric determination of the cytoplasmic pH (pHi) of a single erythrocyte (RBC). The arrows indicate the start points of a three-point pH calibration using standardized buffers of pH 7.8, 7.1, and 6.8. (D) Simultaneous cytoplasmic pH measurement within the cytoplasm of the P. falciparum clone FCR3-A2 and the infected host erythrocyte (iRBC). The arrows indicate the start points of a two-point pH calibration using standardized buffers of pH 7.8 and 6.8. Bar, 10 μm.
A pHi of 7.34 ± 0.02 (n = 10) was determined for the CQR parasite clone FCR3-A2 (Fig. 2 D), which is identical to the pHi value obtained for FCR3-A2 after permeabilization of the host erythrocyte (Bosia et al., 1993). The pHi simultaneously determined in the cytoplasm of the host erythrocyte is 7.12 ± 0.02 (n = 5). This pHi value is slightly higher than that of uninfected erythrocytes (7.05 ± 0.02 (n = 121); Fig. 2, C and D), which is not entirely unexpected since the parasite extensively modifies its host cell including band 3 (Cl−/HCO3 − exchanger), which plays a major role in erythrocyte pH maintenance (Crandall and Sherman, 1991). These data indicate that the noninvasive fluorimetric method used in this study allows us to reliably determine the cytoplasmic pH of single, living P. falciparum parasites still within their host erythrocyte.
An Elevated Cytoplasmic pH Is Genetically Linked with the CQR Phenotype
The cytoplasmic pH of two P. falciparum clones were determined: HB3, a fully chloroquine susceptible clone from Honduras (IC50 = 81 nM); and Dd2, a CQR clone from Indochina (IC50 = 733 nM). All pHi measurements were made on late trophozoite-infected erythrocytes. A clear difference in the pHi was found between these two clones. The CQS clone HB3 has a pHi of 7.18 ± 0.02 (n = 39), whereas the CQR clone Dd2 has a significantly higher pHi of 7.32 ± 0.02 (n = 47; P < 0.05; Table II). The differences in cytoplasmic pH observed between HB3 and Dd2 are independent of the superfusion buffers used during the measurement, as the same phenomenon was observed using a CO2-pressurized bicarbonated buffer instead of a Hepes buffer (Table II). However, the pHi values obtained are slightly higher using the CO2-pressurized bicarbonated superfusion buffer, as has also been observed in other systems (Saarikoski et al., 1997; Boyarsky et al., 1988).
Table II.
Fluorimetric Determination of the Cytoplasmic pH of the CQS Parasite Clone HB3 and the CQR Parasite Clone Dd2
| Strain | IC50 | Noninvasive | Isolated | |||||
|---|---|---|---|---|---|---|---|---|
| Hepes | HCO3 − | Hepes* | ||||||
| nM | pHi | |||||||
| HB3 | 81 | 7.18 ± 0.02 | 7.24 ± 0.03 | 7.22 ± 0.02 | ||||
| (n = 39) | (n = 10) | (n = 3) | ||||||
| Dd2 | 733 | 7.32 ± 0.02 | 7.42 ± 0.02 | 7.37 ± 0.02 | ||||
| (n = 19) | (n = 14) | (n = 5) | ||||||
Using the noninvasive protocol described here, the cytoplasmic pH (pHi) was determined in both a Hepes- and a CO2-pressurized bicarbonated superfusion buffer. As a comparison, pHi was determined using isolated parasites in a modified Hepes
buffer that resembles the ionic conditions of the host erythrocyte (Lee et al., 1988). pHi values are expressed as mean ± SEM for n independent determinations.
To exclude the possibility that the differences in the cytoplasmic pH values observed between HB3 and Dd2 result from variations in the ionic or proteinatious environment of their respective host erythrocytes, we isolated these parasites from their host cells using the dipeptide glycyl-l-serine (Elford, 1993). pH measurements were then performed on single isolated parasites as described above. A pHi of 7.22 ± 0.02 was obtained for HB3, and a pHi of 7.37 ± 0.02 for Dd2 (Table II). These data confirm the observation that the CQR parasite clone Dd2 has a significantly higher cytoplasmic pH than does the CQS parasite clone HB3. Furthermore, as the cytoplasmic pH values determined for isolated parasites are comparable to those obtained for live parasites still within their host erythrocytes, this experiment further validates the accuracy of the noninvasive fluorimetric technique used in this study.
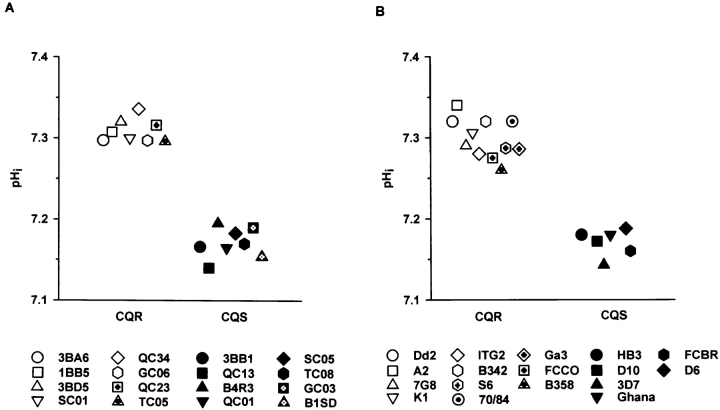
To investigate the possibility of a genetic linkage between the CQR phenotype and a change in the cytoplasmic pH, we examined a genetic cross made with Dd2 and HB3 as parental clones (Wellems et al., 1990, 1991). From this cross 16 independent progeny clones were investigated; 8 are CQS, 8 CQR. The cytoplasmic pH was determined for each of the progeny clones and two groups were clearly identified (Fig. 3 A). All 8 CQS progeny clones share the same cytoplasmic pH as the CQS parental clone HB3. Likewise, the cytoplasmic pH of the 8 CQR progeny clones was the same as that of the CQR parental clone Dd2. Thus, differences in the cytoplasmic pH are genetically linked with the CQR phenotype in the genetic cross between HB3 and Dd2.
Figure 3.
Chloroquine resistance and the cytoplasmic pH. (A) The cytoplasmic pH was determined for each of the 16 progeny clones from a genetic cross between the CQS parental clone HB3 and the CQR parental clone Dd2 (Wellems et al., 1990, 1991). Closed symbols refer to CQS progeny clones, whereas open symbols refer to CQR progeny clones. The names of the progeny clones are indicated below the graph. The average of at least five independent pH measurements are shown for each progeny clone. (B) The cytoplasmic pH was determined for 17 different, geographically dispersed, CQS and CQR parasite clones. Closed symbols refer to CQS clones, whereas open symbols refer to CQR clones. The names of the clones are indicated below the graph. The geographic origin of these clones and their IC50 values for chloroquine are shown in Table III.
Epidemiological studies have suggested that chloroquine resistance emerged simultaneously in two foci— Latin America and Southeast Asia (Payne, 1987). This finding begs the question whether CQR parasite clones from geographic origins other than Southeast Asia also have an elevated cytoplasmic pH. To address this question we measured the cytoplasmic pH of 17 geographically dispersed CQS and CQR P. falciparum clones (Table III). It was found that the 11 CQR parasite clones investigated have a significantly higher cytoplasmic pH than the 6 CQS parasite clones investigated (P < 0.05), independent of their geographic origin (Fig. 3 B).
Table III.
Correlation between Cytoplasmic pH Changes and the CQR Status
| Strain | Origin | IC50 | Status | pHi | pHi (CQ) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| nM | ||||||||||
| D10 | PNG | 74 ± 12 | S | 7.17 ± 0.02 (30) | 7.37 ± 0.01 (6) | |||||
| 3D7 | — | 83 ± 05 | S | 7.14 ± 0.02 (6) | 7.38 ± 0.03 (3) | |||||
| HB3 | Latin-America | 81 ± 06 | S | 7.18 ± 0.02 (39) | 7.40 ± 0.03 (19) | |||||
| FCBR | Latin America | 128 ± 05 | S | 7.19 ± 0.01 (5) | —— | |||||
| Ghana | Africa | 98 ± 07 | S | 7.18 ± 0.03 (4) | —— | |||||
| D6 | Africa | 61 ± 05 | S | 7.16 ± 0.03 (4) | —— | |||||
| Dd2 | S.E. Asia | 733 ± 119 | R | 7.32 ± 0.02 (47) | 7.38 ± 0.03 (19) | |||||
| K1 | S.E. Asia | 1141 ± 84 | R | 7.31 ± 0.03 (4) | 7.37 ± 0.03 (3) | |||||
| S6 | Africa | 687 ± 87 | R | 7.29 ± 0.02 (4) | 7.33 ± 0.03 (4) | |||||
| Ga3 | Africa | 448 ± 40 | R | 7.29 ± 0.02 (11) | 7.31 ± 0.02 (8) | |||||
| FCR3-A2 | Africa | 515 ± 44 | R | 7.34 ± 0.02 (10) | 7.41 ± 0.03 (10) | |||||
| ITG2 | Latin-America | 538 ± 29 | R | 7.28 ± 0.01 (5) | — | |||||
| 7G8 | Latin-America | 685 ± 28 | R | 7.29 ± 0.02 (4) | — | |||||
| 70/84 | Latin-America | 362 ± 50 | R | 7.32 ± 0.02 (4) | — | |||||
| FCCO | Latin-America | 576 ± 65 | R | 7.28 ± 0.02 (4) | — | |||||
| B-358 | Latin-America | 773 ± 70 | R | 7.26 ± 0.01 (3) | — | |||||
| B-342 | Latin-America | 694 ± 21 | R | 7.32 ± 0.01 (3) | — | |||||
| RBC | — | — | — | 7.05 ± 0.02 (121) | 7.07 ± 0.01 (46) | |||||
| iRBC | — | — | — | 7.11 ± 0.02 (6) | 6.98 ± 0.03 (6) | |||||
Cytoplasmic pH of various, geographically dispersed, chloroquine-sensitive and -resistant P. falciparum clones in the absence (pHi) and presence of 50 nM of chloroquine (pHi[CQ]). pHi(CQ) values were determined 30 min after chloroquine addition. The geographic origin of the different P. falciparum clones and the IC50 of chloroquine are given. The IC50 value is that chloroquine concentration that inhibits 50% of the parasites in their development from rings to schizonts within a 24-h period. A parasite clone is considered chloroquine resistant (R) if the IC50 value exceeds 160 nM (Wernsdorfer and Payn, 1988), or it is otherwise designated sensitive (S). pHi values are expressed as mean ± SEM for n independent determinations. PNG, Papua New Guinea; RBC, infected erythrocyte; iRBC, infected host erythrocyte cytoplasm.
Characteristics of NHE Differ between CQS and CQR Parasites
The elevated cytoplasmic pH of CQR parasites is consistent with our proposal that the NHE has been altered in response to chloroquine selection. To verify this hypothesis, we investigated the kinetic and physiological properties of the NHEs in both the CQS parasite clone HB3 and the CQR parasite clone Dd2, using the ammonium chloride prepulse technique (Boyarsky et al., 1990; Fig. 4 A). Although the infected erythrocyte is a two-compartment system, no differences in the temporal pH changes were observed between the host erythrocyte cytoplasm and the parasite cytoplasm during the ammonium chloride pulse. This is most likely a consequence of the short distance of 0–0.125 μm between both compartments (Rosenthal et al., 1988; Sam-Yellowe et al., 1988) and the speed of 0.21 cm/s at which NH3 migrates through the erythrocyte (Labotka et al., 1995). The contribution of proton fluxes, other than those resulting from NHE activity, were determined in the presence of 50 μM of EIPA, which was added during the pHi recovery phase at various time points (Fig. 4 B). The identity of the cytoplasmic acid-loading process unmasked by EIPA is not yet known. It could result from metabolic generation of protons, influx of protons, and/or chloride– base exchange.
Figure 4.
Determination of proton fluxes in CQS and CQR P. falciparum clones. (A) Cytoplasmic pH (pHi) changes during and after an ammonium chloride prepulse. A representative single cell measurement is shown for the CQS parasite clone HB3. Arrows indicate the time points at which the superfusion solutions were changed. Ringer solutions were supplemented with either 40 mM of NH4Cl or 50 μM of EIPA. The rate of pHi recovery was determined from time point 0, at which time the ammonium chloride prepulse is completed. (B) EIPA-insensitive proton flux as a function of the cytoplasmic pH. Data are expressed as means for 7–12 independent experiments. Symbols are: •, CQS parasite clone HB3; and ○, the CQR parasite clone Dd2. (C) Cytoplasmic pH (pHi) changes in response to the ammonium chloride concentration in the superfusion buffer. A representative single cell measurement is shown for the CQS parasite clone HB3. (D) The intracellular buffer capacity (βi) as a function of the cytoplasmic pH. The pH-dependent intracellular buffer capacity was obtained by dividing the change in the intracellular NH4 + concentration by the corresponding change in the cytoplasmic pH. Data are expressed as mean ± SEM from 8 to 15 independent experiments. •, HB3; ○, Dd2.
The NHE activities of both HB3 and Dd2 were quantified by multiplying the EIPA-sensitive component of the proton flux for each parasite clone with the appropriate value of the intracellular buffer capacity of the cytoplasm βi (Boyarsky et al., 1990). βi was determined for both HB3 and Dd2 using stepped ammonium chloride gradients (Boyarsky et al., 1988; Fig. 4 C). No differences in βi were observed between the CQS parasite clone HB3 and the CQR parasite clone Dd2 (Fig. 4 D). The activity of the NHE is given in terms of protons extruded per liter of parasites per minute at a defined cytoplasmic pH (Fig. 5). A sigmoid relationship, in both HB3 and Dd2, was found between the NHE activity and the cytoplasmic pH , as also seen in other eukaryotes (Noel and Pouyssegur, 1995; Fig. 5). However, the pH-dependent activity of the NHEs differ between HB3 and Dd2. At any given cytoplasmic pH, the CQR parasite clone Dd2 has a more active NHE than does the CQS parasite clone HB3. Furthermore, the NHE of Dd2 has an extended pH working range as it continues to be active at higher cytoplasmic pH values than that of HB3. This finding indicates that the set point, the pH value at which NHE ceases activity (Kaila and Vaughan-Jones, 1987), is shifted towards an alkaline pH in Dd2. Thus, the differences in cytoplasmic pH between CQS and CQR parasite clones can be attributed to NHEs with different set points (Noel and Pouyssegur, 1995).
Figure 5.
pH-dependent activities of the NHE from the CQS parasite clone HB3 (•) and the CQR parasite clone Dd2 (○). The NHE activity was determined from the EIPA-sensitive proton flux and multiplied with the appropriate value of the intracellular buffer capacity. NHE activity is given in mmol of proton efflux/liter of parasite volume/minute. Each data point represents a mean of 8 to 10 independent experiments.
Stimulation of the NHE by Chloroquine
We next examined what role the altered properties of the NHE play in the ability of this protein to mediate chloroquine uptake. On the addition of 50 nM of chloroquine to the CQS parasite clone HB3, the pHi increased from 7.18 ± 0.02 to a new steady-state cytoplasmic pH of 7.40 ± 0.03 (n = 19, P < 0.05; Fig. 6 A). In comparison, only a small increase in pHi from 7.32 ± 0.02 to 7.38 ± 0.03 (n = 19, P < 0.05) occurred in the CQR parasite clone Dd2 (Fig. 6 A). Experiments with other CQS and CQR parasite clones confirmed these data, as all CQS parasite clones investigated respond to the addition of chloroquine with a strong alkalization, whereas all the CQR parasite clones showed only a small increase in cytoplasmic pH (Table III).
Figure 6.

Effect of chloroquine on the cytoplasmic pH in P. falciparum. (A) The changes in cytoplasmic pH of the CQS parasite clone HB3 (thin line) and the CQR parasite clone Dd2 (thick line) were measured upon the addition of 50 nM of chloroquine. Uninfected erythrocytes (RBC) were included. The time point of chloroquine addition (0 min) is marked by an arrow. Representations of paired single-cell pHi measurements are shown. (B) Time course of [3H]chloroquine incorporation in P. falciparum. Cell suspensions of either the CQS parasite clone HB3 (•) or the CQR parasite clone Dd2 (○) were incubated in 50 nM of [3H]chloroquine at 37°C and then amounts of internalized chloroquine determined at the time points indicated. Results represent the mean of three independent experiments.
Interestingly, the alkalization induced by chloroquine in the cytoplasm of CQS parasite clones is accompanied by a transient acidification of the host cell cytoplasm (iRBC) from 7.11 ± 0.02 (n = 6) to a peak value of 6.98 ± 0.03 (n = 6, P < 0.05) 5 min after chloroquine addition (Table III). In comparison, no significant change in the cytoplasmic pH of uninfected erythrocytes (RBC) was observed upon the addition of 50 nM of chloroquine (Fig. 6).
The chloroquine-induced cytoplasmic alkalization observed in CQS parasite clones is fully inhibited by EIPA. A slight acidification is observed in the cytoplasm of HB3 when both chloroquine and EIPA were added simultaneously (Fig. 7 A), a finding consistent with the inhibition of NHE activity (Noel and Pouyssegur, 1995). Together, these data suggest that the chloroquine-induced cytoplasmic pH changes, i.e., alkalization in the parasite and acidification of the host erythrocyte, result from the activity of the parasite's NHE, rather than the consumption of protons by chloroquine-free base, which may have crossed the parasite membrane by nonionic diffusion.
Figure 7.

Correlation between proton efflux, sodium ion influx, and chloroquine uptake. Effect of chloroquine on both the cytoplasmic pH (A) and sodium ion concentration (B) were measured in the CQS P. falciparum clone HB3. At time point 0, the superfusion solution was changed to either a buffer containing 50 nM of chloroquine (upper trace), or 50 nM of chloroquine and 50 μM of EIPA (lower trace). A representative trace from six independent paired single-cell measurements is shown. (C) Time course of chloroquine uptake. Cell suspensions of the CQS P. falciparum clone HB3 were incubated in 50 nM of [3H]chloroquine at 37°C in the presence (▴) and absence (•) of 50 μM of EIPA and the amounts of internalized [3H]chloroquine determined at the time points indicated.
As the NHE transports protons in exchange for sodium ions, changes in NHE activity may not only affect the cytoplasmic pH but also the cytoplasmic sodium ion concentration (Nai), as shown in other systems (Borin and Siffert, 1990; Ye et al., 1996). To explore this hypothesis, we determined the cytoplasmic sodium ion concentration of HB3 in the presence and absence of chloroquine, using the fluorochrome SBFI as a sodium ion indicator and the ratio imaging system to detect changes in the spectral properties of SBFI (Minta and Tsien, 1989; Negulescu and Machen, 1990). It was found that upon the addition of 50 nM of chloroquine the cytoplasmic sodium ion concentration increased in HB3 from 21 ± 1 mM to 65 ± 4 mM (n = 10; Fig. 7 B). EIPA completely inhibits the chloroquine-induced increase in Nai (Fig. 7 B). In comparison, no change in the cytoplasmic sodium ion concentration was observed for the CQR parasite clone Dd2 upon the addition of chloroquine (data not shown).
To establish the temporal relationship between chloroquine uptake and the chloroquine-induced changes in pH and sodium ion concentration, we determined the time course of [3H] chloroquine uptake in both HB3 and Dd2 (Figs. 6 B and 7 C). A comparative analysis clearly reveals a coincidence of these events. As chloroquine enters the parasite, protons are extruded from the parasite's cytoplasm into the host erythrocyte's cytoplasm, while sodium is taken up. As all three events are completely inhibited in the presence of EIPA (Fig. 7), this suggests that the plasmodial NHE mediates chloroquine uptake together with sodium and in exchange for protons.
However, the amount of chloroquine taken up by HB3, ∼40 μM, could not readily account for the degree of alkalization and hypernaturesis observed in the cytoplasm of HB3 should we assume a simple exchange reaction. Therefore, we explored the possibility that the NHE is activated by chloroquine, giving rise to the extent of the cytoplasmic alkalization and hypernaturesis observed. To verify this hypothesis, we determined the NHE activities of both HB3 and Dd2 in the presence of 50 nM of chloroquine. It was found that chloroquine activates the NHE of HB3, causing an increase in its pH-dependent activity and pH working range (Fig. 8). Thus, in the presence of chloroquine, the NHE of the CQS parasite clone HB3 acquires kinetic and physiological properties similar to those of the CQR parasite clone Dd2. As a result of this activation, large quantities of protons are exchanged for sodium ions, giving rise to the cytoplasmic alkalization and hypernaturesis observed. Conversely, no significant stimulatory effect was exerted by chloroquine on the NHE of the CQR parasite clone Dd2 (data not shown). These findings establish a link between the extent of chloroquine uptake and the ability of chloroquine to activate the P. falciparum NHE.
Figure 8.
Effect of chloroquine on NHE activity. The pH-dependent NHE activity was determined for the CQS parasite clone HB3 in the presence of 50 nM of chloroquine (•). For comparison, the pH-dependent activities of the NHE in the absence of chloroquine are indicated for HB3 (broken-dotted line) and the CQR parasite clone Dd2 (broken line). Each data point represents a mean of eight independent experiments.
Discussion
Chloroquine exerts its specific antimalarial activity after accumulation within the human malarial parasite P. falciparum. Although chloroquine can permeate membranes by nonionic diffusion, and subsequently accumulates in acidic subcellular compartments because of its diprotic weak base properties (Yayon et al., 1984), the extent of chloroquine uptake by P. falciparum cannot be adequately explained by a diffusion-controlled process (Ferrari and Cutler, 1991). P. falciparum accumulates several orders of magnitude more chloroquine than any other eukaryotic cell, including those similar to P. falciparum that contain large acidic vacuoles (Krogstad et al., 1992; MacIntyre and Cutler, 1993). Furthermore, the kinetics of chloroquine uptake by P. falciparum is inconsistent with a diffusion-controlled mechanism as it is temperature-sensitive, saturable, and inhibitable (Sanchez et al., 1997). These findings suggest that a mechanism other than nonionic diffusion, driven by the acidotropic properties of chloroquine, is responsible for chloroquine uptake and accumulation in P. falciparum. We have recently demonstrated that chloroquine uptake is carrier-mediated (Sanchez et al., 1997). Here we present several lines of evidence that suggest the chloroquine importer is the P. falciparum NHE, a plasma membrane protein involved in cytoplasmic pH and cell volume regulation.
Firstly, chloroquine uptake is competitively inhibited by a broad range of NHE inhibitors, including the amiloride derivatives, DMA, EIPA, IBMA, and HMA, as well as the structurally unrelated indoloyl guanidine derivative Hoe 370 (Fig. 1). The apparent constants of inhibition observed vary amongst the NHE inhibitors examined, defining a potency scale with HMA being the most potent inhibitor of chloroquine uptake and amiloride the least (Table I). This potency scale directly correlates with the ability of these NHE inhibitors to block NHE activity (Kleyman and Cragoe, 1988, 1990). We can exclude the possibility that the reduction of chloroquine accumulation by amilorides results from their properties as weak bases, as there is no correlation between their constants of inhibition and their pKa values (Table I). Instead, the clear structure–function relationship observed provides strong evidence that these compounds prevent chloroquine uptake through the specific inhibition of the P. falciparum NHE.
Secondly, chloroquine uptake coincides with changes in the cytoplasmic pH and sodium ion concentration, both of which are indicative of NHE activity. Upon the addition of chloroquine, protons move from the parasite cytoplasm into the host erythrocyte, resulting in an alkalization in the parasite and an acidification of the host erythrocyte. At the same time, the parasite's cytoplasmic sodium ion concentration rises, an effect that has also been noted previously by Lee et al. (1988). The increase in osmotic pressure caused by the influx of sodium ions explains why the parasite starts to swell immediately after the addition of chloroquine (Macomber and Sprinz, 1967; Warhurst and Hockley, 1967). As chloroquine uptake, proton efflux, and sodium ion influx all take place at the same time and, significantly, are all inhibited by EIPA, this would suggest a common basis for these events, i.e., NHE activity. On the basis of these data we propose that the P. falciparum NHE takes up chloroquine during a sodium/proton exchange reaction, although the mechanistic details remain to be determined. The data presented are inconsistent with a diffusion-controlled model of chloroquine uptake, as this model can explain neither the acidification of the host erythrocyte cytoplasm nor the sodium influx into the parasite on the addition of chloroquine.
A quantification of the chloroquine uptake reaction revealed that ∼40 mM of sodium ions (from Fig. 7) and 20 mM of protons were exchanged (from Fig. 4 D), whereas chloroquine was taken up to a concentration of 40 μM (from Fig. 6) by the CQS parasite clone HB3 in reaching a new steady-state equilibrium in the presence of chloroquine. Thus, the stoichiometry of the chloroquine-induced exchange reaction appears to be two sodium ions for one proton. The P. falciparum chloroquine importer is reminiscent, therefore, of electrogenic NHEs found in invertebrate epithelial cells (Ahearn et al., 1994; Ahearn, 1996). This protein is analogous to the vertebrate amiloride-sensitive electroneutral NHE, except that it performs an extensive array of transport functions because of its electrogenic nature, transporting a wide range of both monovalent and divalent cations (Ahearn, 1996). Interestingly, a drug transporting capability has also been demonstrated for an NHE, where bacterial NHEs export the divalent cation tetracycline in exchange for protons (Yamaguchi et al., 1990; Cheng et al., 1996; Yamaguchi, 1997). On the basis of these data, we postulate that the P. falciparum NHE transports the diprotonated form of chloroquine, which, given its pKa values of 8.4 and 10.8, respectively, dominates at a physiological pH of 7.3.
The apparent disparity in the amount of chloroquine taken up in exchange for the huge excess of protons, would suggest that chloroquine uptake by the P. falciparum NHE is not a simple exchange reaction. We estimate that for each chloroquine molecule taken up, ∼500 protons are extruded. It was this disparity that led us to investigate the effect of chloroquine on P. falciparum NHE activity, thereby providing the third line of evidence linking chloroquine uptake with NHE activity. It was found that chloroquine activates the NHE of the CQS parasite clone HB3, resulting in an increase in the pH-dependent activity and working range (Fig. 8). The activation of an NHE is facilitated by the sodium ion gradient across the plasma membrane, as shown in other systems (Noel and Pouyssegur, 1995). Thus, activation of the P. falciparum NHE appears to be required for chloroquine uptake and accumulation, suggesting that it provides the energy, stored in the sodium ion gradient across the parasite plasma membrane, to concentrate chloroquine against its gradient into the parasite (Fig. 9). Once the NHE has reached its now activated steady state in the presence of chloroquine, the surge in ion exchange abates and no more chloroquine is taken up (Figs. 6 and 7). Therefore, chloroquine uptake appears to be a secondary active transport mechanism in P. falciparum. This proposal is supported by the observation that CQS parasite clones are rendered insensitive to chloroquine in the presence of substances, such as monensin, which dissipate the sodium ion gradient (Yayon et al., 1984). How chloroquine stimulates the P. falciparum NHE remains, at present, unknown.
Figure 9.
Model of chloroquine uptake and accumulation by the P. falciparum NHE. The NHE resides in the parasite plasma membrane and is responsible for pH homeostasis, extruding metabolically generated protons for sodium ions. The NHE is in a silent steady state in CQS parasite clones before addition of chloroquine. Chloroquine, which permeates the erythrocyte membrane by nonionic diffusion, activates the NHE, resulting in a rapid transient exchange of protons for sodium ions. During this initial activation phase, chloroquine is taken up by the NHE via an unknown mechanism and concentrated in the parasite. Once the NHE has reached its activated steady state, no more chloroquine is taken up. Both activation of the NHE as well as chloroquine uptake are specifically inhibited by EIPA and other NHE inhibitors. The CQR phenotype is characterized by an NHE that can be considered to be constitutively in the activated steady state, and as such is incapable of concentrating chloroquine within the parasite. EM, erythrocyte plasma membrane; PM, parasite plasma membrane; CQ, chloroquine.
CQR parasite clones appear to have preempted most of the stimulatory effect caused by chloroquine, as chloroquine has no significant effect on the NHE activity, as demonstrated for Dd2 (Fig. 8). Its NHE already has an increased pH-dependent activity and working range in the absence of chloroquine, which suggests that the NHEs of CQR parasite clones are constitutively activated (Figs. 5 and 8). As there is no further activation of the NHE of CQR parasites by chloroquine, there is no release in energy; no transient surge of sodium–hydrogen ion exchange occurs and, hence, no chloroquine is concentrated into the parasite (Fig. 9). Based on these data, we propose that the inability of chloroquine to effectively stimulate its own uptake through the constitutively activated NHEs of CQR parasites constitutes a minimal and necessary event in the generation of the CQR phenotype.
Consistent with our proposal that NHE of CQR parasites are altered in response to chloroquine selection, we have genetically linked both biochemical and physiological properties of the NHE with the CQR phenotype. Biochemical data have demonstrated that a change in the chloroquine uptake kinetics, reducing the affinity and maximal transport rate, is genetically linked with the CQR phenotype in the cross between HB3 and Dd2 (Sanchez et al., 1997). Also, the CQR phenotype is genetically linked with an elevated cytoplasmic pH, a phenotypical marker that is further associated with all CQR parasites, independent of their geographic origin. The latter data were derived from single-cell fluorimetric pHi measurements made on the parasite still within its host erythrocyte. As such, the cytoplasm of the host erythrocyte would contribute to the total fluorimetric signal measured. Given that the parasite's cytoplasmic pH is higher than that of its host erythrocyte, this contribution would result in an underestimation of the parasite's pH by ∼0.03 pH units, as estimated from the thickness of the host erythrocyte cytoplasm above the parasite (which is between 0 and 0.125 μm according to electronmicroscopic examinations [Rosenthal et al., 1988; Sam-Yellowe et al., 1988]), and the fluorescence signals emitted from the parasite both within and without the host erythrocyte. This estimation is confirmed by cytoplasmic pH determinations made on isolated parasites, which are slightly higher than those made on intact cells, yet maintain the pH differences between CQS and CQR parasite clones (Table II). Thus, any differences between CQS and CQR parasites can be solely attributed to differences in the parasites themselves, rather than their host cell environment. Although we know very little about the ionic and proteinatious environment of the host erythrocyte cytoplasm, we observed no differences in either the pH or sodium ion concentration in the absence of chloroquine, regardless of the parasite's CQR phenotype (data not shown).
A constitutively activated NHE could result from mutations within either the NHE itself or factors modulating NHE activity, such as kinases, accessory binding proteins, or Ca2+-calmodulin (Wakabayashi et al., 1994; Noel and Pouyssegur, 1995). Therefore, we would propose that either a P. falciparum NHE or a factor regulating NHE activity resides within the chloroquine resistance locus defined by the genetic cross between HB3 and Dd2 (Wellems et al., 1991). Interestingly, the primary candidate for the CQR phenotype mediator, CG2 (Su et al., 1997), has features we would predict for an NHE. As is typical for electrogenic NHEs, CG2 is a putative membrane protein located at both the plasma membrane and the vacuole (Kimura et al., 1994). Furthermore, CG2 also contains a consensus amiloride binding motif, putative Ca2+ calmodulin binding sites, and the R/K-X-G-R/K-R/K motif found in many of the metabolite proton or sodium ion symporters and the bacterial tetracycline–proton exchangers (Yamaguchi et al., 1990; Yoshida et al., 1990; Noel and Pouyssegur, 1995).
Acknowledgments
We are grateful to H.J. Lang of Hoechst AG (Frankfurt, Germany) for providing us with EIPA and Hoe 370, A. Scherf (Institute Pasteur, Paris, France), D. Walliker (University of Edinburgh, Edinburgh, UK), M.-O. Rojas (Instituto Nacional de Salud, Bogota, Colombia), and A. Cowman (The Walter and Elisa Hall Institute of Medical Research, Melbourne, Victoria, Australia) for P. falciparum clones, and T. Wellems (National Institutes of Health, Bethesda, MD) for allowing us to use the progeny clones from the genetic cross. We thank P. Horrocks for critically reading the manuscript. We thank the Institute of Physiology at the University of Würzburg for allowing us to use the Attofluor ratio imaging system for cytoplasmic pH measurements. Technical assistance was provided by E. Wilken and secretarial support by C. Borde.
This work was supported by grants from the Bundesministerium Für Bildung, Technologie und Forschung, the Deutsche Forschungsgemeinschaft, and the Sander Stiftung.
Abbreviations used in this paper
- βi
intracellular buffer capacity
- BCECF-AM
fluorochromo 2′7′-bis-(2-carboxyethyl)-5,6-carboxyfluorescein acetomethyl ester
- CQR
chloroquine resistant
- EIPA
5-(N-ethyl- N-isopropyl)amiloride
- CQS
chloroquine sensitive
- HMA
5-N(N-hexamethylene)amiloride
- IC50
50% inhibitory concentration
- pHi
intracellular pH
- NHE
Na+/H+ exchanger
- SBFI
sodium-binding benzofuran isophthalate-acetoxymethylester
Footnotes
Address all correspondence to Michael Lanzer, Zentrum für Infektionsforschung, Universität Würzburg, Röntgenring 11, D-97070 Würzburg, Germany. Tel.: (49) 93-13-12-151. Fax: (49) 93-13-12-578.
References
- Ahearn GA. The invertebrate electrogenic 2Na+/1H+exchanger: polyfunctional epithelial workstation. News Physiol Sci. 1996;11:31–35. [Google Scholar]
- Ahearn GA, Zhuang Z, Duerr J, Pennington V. Role of the invertebrate electrogenic 2Na+/1H+antiporter in monovalent and divalent cation transport. J Exp Biol. 1994;196:319–335. doi: 10.1242/jeb.196.1.319. [DOI] [PubMed] [Google Scholar]
- Asher C, Cragoe EJ, Garty H. Effects of amiloride analogues on Na+transport in toad bladder membrane vesicles. J Biol Chem. 1987;262:8566–8573. [PubMed] [Google Scholar]
- Borin M, Siffert W. Stimulation of thrombin increases the cytosolic free Na+concentration in human platelets. J Biol Chem. 1990;265:19543–19550. [PubMed] [Google Scholar]
- Bosia A, Ghigo D, Turrini E, Nissani E, Pescarmona GP, Ginsburg H. Kinetic characterization of Na+H+ antiport of Plasmodium falciparummembrane. J Cell Physiol. 1993;254:527–534. doi: 10.1002/jcp.1041540311. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3 . Am J Physiol. 1988;255:C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ganz MB, Cragoe EJ, Boron WF. Intracellular-pH dependence of Na-H exchange and acid loading in quiescent and arginine vasopressin-activated mesangial cells. Proc Natl Acad Sci USA. 1990;87:5921–5924. doi: 10.1073/pnas.87.15.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Hicks DB, Krulwich TA. The purified Bacillus subtilis tetracycline efflux protein TetA(L) reconstitutes both tetracycline-cobalt/ H+ and Na+(K+)/H+exchange. Proc Natl Acad Sci USA. 1996;93:14446–14451. doi: 10.1073/pnas.93.25.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall I, Sherman IW. Plasmodium falciparum(human malaria)-induced modifications in human erythrocyte band 3 protein. Parasitol. 1991;3:335–340. doi: 10.1017/s0031182000064271. [DOI] [PubMed] [Google Scholar]
- Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG. Malarial haemozoin/β-haematin supports haem polymerization in the absence of protein. Nature. 1995;374:269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- Elford, B.C. 1993. Generating viable extra-erythroytic forms of P. falciparum. TDR News (WHO Bulletin) 41:11.
- Ferrari V, Cutler DJ. Uptake of chloroquine by human erythrocytes. Biochem Pharmacol. 1990;39:753–762. doi: 10.1016/0006-2952(90)90155-e. [DOI] [PubMed] [Google Scholar]
- Ferrari, V., and D.J. Cutler. 1991. Simulation of kinetic data on the influx and efflux of chloroquine by erythrocytes infected with Plasmodium falciparum. Biochem. Pharmacol. 42 p. 167–179. [DOI] [PubMed]
- Fitch CD. Plasmodium falciparumin owl monkeys: drug resistance and chloroquine binding. Science. 1970;169:289–290. doi: 10.1126/science.169.3942.289. [DOI] [PubMed] [Google Scholar]
- Fitch CD. Chloroquine-resistant Plasmodium falciparum: differences in the handling of [14C]-amodiaquin and [14C]-chloroquine. Antimicrob Agents Chemother. 1973;3:545–548. doi: 10.1128/aac.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H, Stein WD. Kinetic modeling of chloroquine uptake by malaria-infected erythrocytes. Biochem Pharmacol. 1991;41:1463–1470. doi: 10.1016/0006-2952(91)90562-j. [DOI] [PubMed] [Google Scholar]
- Kaila K, Vaughan-Jones RD. Influence of sodium-hydrogen exchange on intracellular pH, sodium and tension in sheep cardiac Purkinje fibers. J Physiol (Lond) 1987;390:93–118. doi: 10.1113/jphysiol.1987.sp016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura C, Ahearn G, Busquetes-Turner L, Haley S, Nagao C, Couet H. Immunolocalization of an antigen associated with the invertebrate electrogenic 2Na+/1H+antiporter. J Exp Biol. 1994;189:85–104. doi: 10.1242/jeb.189.1.85. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transporters. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Cation transport probes: The amiloride series. Methods Enzymol. 1990;191:739–752. doi: 10.1016/0076-6879(90)91045-8. [DOI] [PubMed] [Google Scholar]
- Krogstad DJ, Gluzman IY, Kyle DE, Oduola AMJ, Martin SK, Milhous WK, Schlesinger PH. Efflux of chloroquine from Plasmodium falciparum: Mechanism of chloroquine resistance. Science. 1987;238:1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- Krogstad DJ, Gluzman IY, Herwaldt BL, Schlesinger PH, Wellems TE. Energy dependence of chloroquine accumulation and chloroquine efflux in Plasmodium falciparum. . Biochem Pharmacol. 1992;43:57–62. doi: 10.1016/0006-2952(92)90661-2. [DOI] [PubMed] [Google Scholar]
- Labotka RJ, Lundberg P, Kuchel PW. Ammonia permeability of erythrocyte membrane studied by 14N and 15N saturation transfer NMR spectroscopy. Am J Physiol. 1995;263:C686–C699. doi: 10.1152/ajpcell.1995.268.3.C686. [DOI] [PubMed] [Google Scholar]
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparumerythrocyte stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Lee P, Ye Z, Van Dyke K, Kirk G. X-ray microanalysis of Plasmodium falciparumand infected red blood cells: effects of quinghaosu and chloroquine on potassium, sodium, and phosphorus composition. Am Trop Med Hyg. 1988;39:157–165. doi: 10.4269/ajtmh.1988.39.157. [DOI] [PubMed] [Google Scholar]
- Martin SK, Oduola AM, Milhouse WK. Reversal of chloroquine resistance in Plasmodium falciparumby verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- MacIntyre A, Cutler DJ. Kinetics of chloroquine uptake into isolated rate hepatocytes. J Pharm Sci. 1993;82:592–600. doi: 10.1002/jps.2600820610. [DOI] [PubMed] [Google Scholar]
- Macomber PB, Sprinz H. Morphological effects of chloroquine on Plasmodium bergheiin mice. Nature. 1967;214:937–939. doi: 10.1038/214937a0. [DOI] [PubMed] [Google Scholar]
- Minta A, Tsien RY. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989;185:19449–19457. [PubMed] [Google Scholar]
- Noel J, Pouyssegur J. Hormonal regulation, pharmacology and membrane sorting of vertebrate Na+/H+exchanger isoforms. Am J Physiol. 1995;268:C283–C296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- Negulescu PA, Machen TE. Intracellular ion activities and membrane transport in parietal cells measured with fluorescent dyes. Methods Enzymol. 1990;192:38–81. doi: 10.1016/0076-6879(90)92062-i. [DOI] [PubMed] [Google Scholar]
- Payne D. Spread of chloroquine resistance in Plasmodium falciparum. . Parasitol Today. 1987;3:241–246. doi: 10.1016/0169-4758(87)90147-5. [DOI] [PubMed] [Google Scholar]
- Rosenthal PJ, McKerrow JH, Aikawa M, Nagasawa H, Leech JH. A malarial cysteine proteinase is necessary for hemoglobin degradation by Plasmodium falciparum. . J Clin Invest. 1988;82:1590–1566. doi: 10.1172/JCI113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarikoski J, Ruusuvuori E, Koskelainen A, Donner K. Regulation of intracellular pH in salamander retinal rods. J Physiol. 1997;498:61–72. doi: 10.1113/jphysiol.1997.sp021841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam-Yellowe TY, Shio H, Perkins ME. Secretion of Plasmodium falciparumrhoptry protein into the plasma membrane of host erythrocytes. J Cell Biol. 1988;106:1507–1513. doi: 10.1083/jcb.106.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CP, Wünsch S, Lanzer M. Identification of a chloroquine importer in Plasmodium falciparum: differences in import kinetics are genetically linked with the chloroquine resistant phenotype. J Biol Chem. 1997;272:2652–2658. doi: 10.1074/jbc.272.5.2652. [DOI] [PubMed] [Google Scholar]
- Slater AFG, Swiggard WJ, Orton BR, Flitter WD, Goldberg DE, Cerami A. An iron-carboxylate bond links the heme units of malaria pigments. Proc Natl Acad Sci USA. 1991;88:325–329. doi: 10.1073/pnas.88.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater AFG, Cerami A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992;355:167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- Su X-Z, Kirkman LA, Fujioka H, Wellems TE. Complex polymorphisms in an ∼330 kDa protein are linked to chloroquine-resistance P. falciparumin Southeast Asia and Africa. Cell. 1997;91:593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- Sullivan DJ, Jr, Gluzman IY, Russell DG, Goldberg DE. On the molecular mechanism of chloroquine's antimalarial action. Proc Natl Acad Sci USA. 1996;93:11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. . Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vigne P, Frelin C, Cragoe EJ, Lazdunski M. Ethylisopropyl-amiloride: A new and highly potent derivative of amiloride for inhibition of the Na+/H+exchange system in various cell types. Biochem Biophys Res Commun. 1983;116:86–90. doi: 10.1016/0006-291x(83)90384-4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S, Bertrand B, Ikeda T, Pouyssegur J, Shigekawa M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H+-sensitive and Ca2+regulation-defective. J Biol Chem. 1994;269:13710–13715. [PubMed] [Google Scholar]
- Warhurst DC, Hockley DJ. Mode of action of chloroquine on Plasmodium berghei and P. cynomolgi. . Nature. 1967;214:935–936. doi: 10.1038/214935a0. [DOI] [PubMed] [Google Scholar]
- Weiner ID, Hamm LL. Use of fluorescent dye BCECF to measure intracellular pH in cortical collecting tubule. Am J Physiol. 1989;256:F957–F964. doi: 10.1152/ajprenal.1989.256.5.F957. [DOI] [PubMed] [Google Scholar]
- Wellems, T.E., L.J. Panton, I.Y. Gluzman, V.E. do Rosario, R.W. Gwadz, A. Walker-Jonah, and D.J. Krogstad. 1990. Chloroquine resistance is not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 345:253–255. [DOI] [PubMed]
- Wellems TE, Walker-Jonath A, Panton LJ. Genetic mapping of the chloroquine resistance locus on Plasmodium falciparumchromosome 7. Proc Natl Acad Sci USA. 1991;88:3382–3386. doi: 10.1073/pnas.88.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernsdorfer, W.H., and D. Payn. 1988. Drug sensitivity test in malaria parasites. In Malaria. Principles and Practice of Malariology. W.H. Wernsdorfer and Sir L. McGregor, editors. Churchill Livingstone, Edinburgh, UK. 1765– 1800.
- World Health Organization. World malaria situation in 1993. Weekly Epidemiological Record. 1996;71:17–22. [Google Scholar]
- Wünsch S, Gekle M, Kersting U, Schuricht B, Oberleithner H. Phenotypicallly and karyotypically distinct Madin-Darby canine kidney cell clones respond differently to alkaline stress. J Cell Physiol. 1995;164:164–171. doi: 10.1002/jcp.1041640121. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A. Bacterial resistance mechanisms for tetracycline. Nippon Rinsho. 1997;55:1245–1251. [PubMed] [Google Scholar]
- Yamaguchi A, Ono N, Akasaka T, Noumi T, Sawai T. Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J Biol Chem. 1990;265:4809–4813. [PubMed] [Google Scholar]
- Yayon A, Van de Waa JA, Yayon M, Geary TG, Jensen JB. Stage-dependent effects of chloroquine on Plasmodium falciparum in vitro. . J Protozool. 1983;30:642–647. doi: 10.1111/j.1550-7408.1983.tb05336.x. [DOI] [PubMed] [Google Scholar]
- Yayon A, Cabantchik ZI, Ginsburg H. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO (Eur Mol Biol Organ) J. 1984;3:2695–2700. doi: 10.1002/j.1460-2075.1984.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Flores G, Batlle D. Angiotensin II and angotensin-(1-7) effects on free cytosolic sodium, intracellular pH, and the Na+/H+antiporter in vascular smooth muscle. Hypertension. 1996;27:72–78. doi: 10.1161/01.hyp.27.1.72. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureusnorA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]