Abstract
Homotypic vacuole fusion in yeast requires Sec18p (N-ethylmaleimide–sensitive fusion protein [NSF]), Sec17p (soluble NSF attachment protein [α-SNAP]), and typical vesicle (v) and target membrane (t) SNAP receptors (SNAREs). We now report that vacuolar v- and t-SNAREs are mainly found with Sec17p as v–t-SNARE complexes in vivo and on purified vacuoles rather than only transiently forming such complexes during docking, and disrupting them upon fusion. In the priming reaction, Sec18p and ATP dissociate this v–t-SNARE complex, accompanied by the release of Sec17p. SNARE complex structure governs each functional aspect of priming, as the v-SNARE regulates the rate of Sec17p release and, in turn, Sec17p-dependent SNARE complex disassembly is required for independent function of the two SNAREs. Sec17p physically and functionally interacts largely with the t-SNARE. (a) Antibodies to the t-SNARE, but not the v-SNARE, block Sec17p release. (b) Sec17p is associated with the t-SNARE in the absence of v-SNARE, but is not bound to the v-SNARE without t-SNARE. (c) Vacuoles with t-SNARE but no v-SNARE still require Sec17p/Sec18p priming, whereas their fusion partners with v-SNARE but no t-SNARE do not. Sec18p thus acts, upon ATP hydrolysis, to disassemble the v–t-SNARE complex, prime the t-SNARE, and release the Sec17p to allow SNARE participation in docking and fusion. These studies suggest that the analogous ATP-dependent disassembly of the 20-S complex of NSF, α-SNAP, and v- and t-SNAREs, which has been studied in detergent extracts, corresponds to the priming of SNAREs for docking rather than to the fusion of docked membranes.
The targeting and fusion of vesicles to their respective membrane requires the N-ethylmaleimide–sensitive fusion protein (NSF),1 soluble NSF attachment proteins (SNAPs), and SNAP receptors (SNAREs) (for review see Ferro-Novick and Jahn, 1994; Pfeffer, 1996; Rothman and Wieland, 1996). SNAREs are integral membrane proteins that are found on both the vesicle (v-SNAREs) and on the target membrane (t-SNAREs). The SNARE hypothesis postulates that vesicles are targeted to their respective organelle membrane via the specific heterotypic interaction of v- and t-SNAREs (Rothman, 1994). In contrast, the fusion of organelles or mitotic Golgi fragments with themselves has been termed homotypic. Most fusion reactions in the secretory pathway depend on NSF, but there are also examples where NSF does not seem to be required, e.g., the traffic from the endosome to the vacuole and apical sorting in polarized cells (Graham and Emr, 1992; Ikonen et al., 1995). Homologous proteins can substitute for NSF in certain reactions, like the NSF-related protein p97 in the reassembly of mitotic Golgi fragments (Acharya et al., 1995; Rabouille et al., 1995) or its homologue Cdc48 in ER–ER fusion (Latterich et al., 1995). Both p97 and Cdc48 are ATPases, indicating that ATP hydrolysis by an NSF-like protein might be a conserved requirement in trafficking.
The role of NSF in trafficking reactions is receiving intensive study (for review see Morgan and Burgoyne, 1995; Hay and Scheller, 1997). It has been proposed that NSF and SNAP(s) are recruited into the v–t-SNARE complex after docking of the vesicle with its target membrane and that NSF catalyzes bilayer fusion via the ATP-dependent disassembly of the docked SNARE proteins (Söllner et al., 1993a ,b; Rothman, 1994). In a detergent extract v- and t-SNAREs can assemble with NSF and α-SNAP into a 20 S particle, which is dissociated upon ATP hydrolysis by NSF. This assay has linked the functions of NSF and SNAPs with v- and t-SNAREs and allowed the identification of a number of new SNAREs (Söllner et al., 1993a ; Søgaard et al., 1994; Paek et al., 1997). However, it has not established whether the 20 S complex is present before, during or after fusion. Indeed, several studies of secretion, neuronal transmission and Golgi trafficking have questioned the role of NSF in lipid bilayer fusion (Holz et al., 1989; Wattenberg et al., 1992; Hiebsch and Wattenberg, 1992; Chamberlain et al., 1995; Banerjee et al., 1996). Recently, highly purified synaptic vesicles have been shown to contain both synaptobrevin, a neuronal v-SNARE, as well as syntaxin and SNAP-25, members of the t-SNARE family (Walch-Solimena et al., 1995). Each of these proteins was found in an SDS-resistant complex that was disassembled by NSF and α-SNAP (Otto et al., 1997). These data suggest that NSF and α-SNAP can even act upstream of the docking and fusion reactions in neurons. Studies of homotypic vacuole fusion in Saccharomyces cerevisiae have suggested that NSF/Sec18p primes vacuoles as an essential prerequisite for subsequent docking and fusion. Sec17p/ α-SNAP is released from the vacuole membrane through ATP hydrolysis via Sec18p/NSF well before vesicle docking (Mayer et al., 1996), and this release is in fact a prerequisite for docking (Mayer and Wickner, 1997). Sec17p was also required early in the heterotypic reaction when vacuoles containing only t- or v-SNARE were studied in this in vitro fusion assay (Nichols et al., 1997).
Our lab has developed an in vitro assay that measures the homotypic fusion of vacuoles (Conradt et al., 1992; Haas et al., 1994). During cell division, a stream of membranous tubules and vesicles from the mother vacuole is delivered into the daughter bud and fuses there to form the new vacuole (Weissman and Wickner, 1988; Gomes de Mesquita et al., 1991; Raymond et al., 1992). The priming and docking that lead to this fusion depend on the Rab protein Ypt7p (Haas et al., 1995), LMA1, a heterodimeric complex consisting of thioredoxin and the protease B inhibitor IB 2 (Xu and Wickner, 1996; Slusarewicz et al., 1997; Xu et al., 1997), Sec18p/NSF, Sec17p/α-SNAP (Haas and Wickner, 1996), the t-SNARE Vam3p (Darsow et al., 1997; Götte and Gallwitz, 1997; Nichols et al., 1997; Wada et al., 1997), and the v-SNARE Nyv1p (Nichols et al., 1997). The fusion of docked vacuoles is sensitive to GTPγS and the phosphatase inhibitor microcystein LR (Haas et al., 1994). Our in vitro reaction occurs in distinct steps of priming, docking, and fusion. The priming reaction requires the Sec18p-mediated Sec17p release from the vacuoles. LMA1, which is initially bound to Sec18p, is transferred to the t-SNARE concomitant with Sec17p release (Xu and Wickner, manuscript in preparation). Ypt7p and the vacuolar SNAREs are required for the docking step. We have not yet identified the proteins involved in the fusion reaction per se. We now present studies that link Sec17p release from the vacuole membrane to the dissociation of a complex of the vacuolar SNAREs and to an activation of the t-SNARE for docking. These functional studies complement recent structural studies of NSF and SNAP assembly on a pure SNARE complex (Hanson et al., 1997).
Materials and Methods
Materials
The sources of reagents are as described by Haas (1995), Mayer et al. (1996), and Haas and Wickner (1996). Yeast strains are described in Nichols et al. (1997).
Biochemical Procedures
SDS-PAGE, immunoblotting using enhanced chemiluminescence (Ungermann et al., 1994; Haas et al., 1995), purification of His6-tagged Sec18p (Haas and Wickner, 1996), and assay of Sec17p release were as described (Mayer et al., 1996). LMA1 (Xu and Wickner, 1996) was provided by Dr. Z. Xu. Antibodies to Nyv1p (Nichols et al., 1997) were raised in rabbits against a 12–amino acid peptide (residues 182–195). Sec18p-IgGs were affinity purified and concentrated according to Haas and Wickner (1996). IgGs to Vam3p, Nyv1p, and Ypt7p were purified according to Harlow and Lane (1989), concentrated by ultrafiltration, diluted in PS buffer (10 mM Pipes, pH 6.8, 200 mM sorbitol), and then concentrated to 5 mg/ml (Haas and Wickner, 1996). Aliquots (50 μl) were frozen in liquid nitrogen and stored at −20°C.
Vacuole Fusion
Vacuoles (Haas, 1995) were used immediately after isolation. The standard fusion reaction contained 3 μg of each vacuole type (BJ3505 and DKY6281) in reaction buffer (10 mM Pipes, pH 6.8, 200 mM sorbitol, 150 mM KCl, 1 mM MgCl2, 0.5 mM MnCl2, 0.5 mM ATP, 3 mg/ml cytosol, 3.5 U/ml creatine kinase, 20 mM creatine phosphate, 7.5 μM pefabloc SC, 7.5 ng/ml leupeptin, 3.75 μM ortho-phenanthroline, 37.5 ng/ml pepstatin). To reduce proteolytic effects in the Sec17p release reaction and the coimmunoprecipitation experiments, only the protease A–deficient BJ3505 vacuoles were analyzed. One unit of fusion activity is defined as 1 μmol para-nitrophenol hydrolyzed per min and μg BJ3505.
Coimmunoprecipitation
Antiserum (1 ml) was incubated with 500 μl of protein A–Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) in 0.1 M TrisCl, pH 8.0, for 1 h at room temperature on a nutator. Beads were suspended in 5 ml of 0.2 M sodium borate buffer, pH 9.0, collected by sedimentation (30 s, 16,000 g, 4°C), and then resuspended in 5 ml of 0.2 M sodium borate buffer, pH 9.0. Solid dimethylpimelimidate (Pierce Chemical Co., Rockford, IL) was added to a final concentration of 20 mM. The beads were again incubated for 30 min at room temperature, and then reisolated in a clinical centrifuge, suspended in 0.2 M ethanolamine/Cl, pH 8.0, as above, sedimented again, and then resuspended in 5 ml of ethanolamine/Cl, and incubated for 2 h at room temperature on a nutator. Beads were again isolated by centrifugation, suspended in 5 ml PBS buffer (100 mM Na-Phosphate buffer, pH 7.4, 150 mM NaCl), reisolated, and then resuspended to a final volume of 1 ml in PBS. The protein A–coupled IgGs were stored at 4°C in 0.002% sodium azide.
For coprecipitation, vacuoles (60 μg) from the indicated reactions were reisolated by centrifugation (3 min, 8,000 g), suspended in 200 μl 10 mM Pipes/KOH, 200 mM sorbitol (PS) buffer and again reisolated. Vacuoles were solubilized in 1 ml of lysis buffer (1% digitonin, 100 mM Hepes/ KOH, pH 7.4, 110 mM NaCl, 5 mM EDTA, 1 mM PMSF, 30 μM pefablock SC, 30 ng/ml leupeptin, 15 μM ortho-phenanthroline, 150 ng/ml pepstatin), and then incubated on ice for 10 min. Unsolubilized material was removed by centrifugation (10 min at 16,000 g). Proteins were TCA precipitated from 90 μl of the detergent extract. They served as a control for the efficiency of the coprecipitation. The remaining detergent extract was added to the protein A–coupled IgGs and incubated on a nutator for 1.5 h at 4°C. The beads were reisolated by a 30-s centrifugation at 16,000 g and twice sedimented, resuspended in 1 ml of lysis buffer, and then incubated for 10 min. Proteins were eluted from the beads by addition of SDS–sample buffer and heating to 95°C for 4 min, resolved by SDS-PAGE on 12% polyacrylamide gels, transferred to nitrocellulose, and then immunoblotted as described (Haas et al., 1995).
Results
For our fusion assay, vacuoles are isolated from two yeast strains. One strain (DKY6281) has normal vacuolar proteases but lacks the vacuolar alkaline phosphatase, whereas the other (BJ3505) lacks the maturation proteinase A and has only the catalytically inactive pro-alkaline phosphatase. After fusion, the lumenal contents mix and pro-alkaline phosphatase is processed and activated. The amount of active alkaline phosphatase, assayed spectrophotometrically, is a measure of fusion (Haas et al., 1994; Haas, 1995). Assays of the release of Sec17p (Mayer et al., 1996) and coprecipitation of vacuolar membrane proteins from detergent extracts are also used in the present study.
Vacuolar v–t-SNARE Complexes Are Dissociated by Sec18p and ATP In Vitro
The vacuolar SNAREs Vam3p (t-SNARE) and Nyv1p (v-SNARE) are essential for homotypic vacuole fusion (Nichols et al., 1997). They are in a complex on isolated vacuoles, as shown by their coprecipitation from vacuolar detergent extracts by an antibody to the t-SNARE Vam3p (Fig. 1 A, lane 4). Since the ratio of v- and t-SNARE was similar in anti-Vam3p immunoprecipitates (Fig. 1 A, lane 4) as in the total vacuolar extract (Fig. 1 A, lane 1), most of the Nyv1p must have been in this complex. However, SNARE proteins may assemble spontaneously in detergent solution (Hayashi et al., 1994; Fasshauer et al., 1997). To determine whether the complex we detected was present on the vacuole or had formed in the detergent extract, vacuoles from cells without the v-SNARE (t), or without the t-SNARE (v) were extracted with detergent and the mixed extracts were analyzed by immunoprecipitation with α-Vam3p bound to protein A–Sepharose beads. When detergent extracts from t-SNARE–only vacuoles were mixed with those from v-SNARE–only vacuoles, no Nyv1p was coprecipitated with Vam3p (Fig. 1 A, lane 7). This indicates that the v-SNARE Nyv1p did not form a complex with the t-SNARE Vam3p under our detergent extraction conditions before coprecipitation. The coprecipitation assay therefore reflects associations on the vacuolar membrane and not in the detergent extract.
Figure 1.

The vacuolar v- and t-SNAREs are in a complex that is dissociated by ATP hydrolysis. (A) Vacuolar v–t-SNARE complexes are present on the vacuole. Isolated vacuoles (100 μg) from BJ3505 (v,t) and BJ3505, with deletion in the v-SNARE (t) or the t-SNARE (v) were solubilized in 1 ml of 1.5% Triton X-100, 100 mM NaPi, pH 7.4, 150 mM NaCl, 1 mM PMSF (TX buffer). A portion (5%) of the total detergent extract was TCA precipitated before the immunoprecipitation analysis. Coprecipitation with immobilized antibodies to Vam3p was performed overnight at 4°C. In lane 7, detergent extracts of both deletion vacuoles were mixed before addition of the protein A–Sepharose immobilized α-Vam3p. Proteins were removed from beads by suspension in 200 μl of 100 mM glycine/Cl, pH 2.2, and precipitated with TCA before SDS-PAGE analysis. Proteins were transferred to nitrocellulose and decorated with antibodies to Vam3p and Nyv1p. (B) Vacuolar v–t-SNARE complexes are dissociated by the action of Sec18p and ATP. Vacuoles were isolated from sec18-1 cells and incubated for 10 min in PS buffer on ice or at 37°C, and then mixed with reaction buffer without (lane 2) or with 1 mM Mg-ATP (lane 3), and then incubated for 10 min at 25°C. After reisolation, vacuoles were solubilized in detergent, coprecipitated with α-Vam3p and processed as in A. (C) v–t-SNARE complexes are abundant in vivo. Cells carrying the sec18-1 mutation were converted to spheroplasts at 25°C (Lewis et al., 1997), and incubated at either 25° or 37°C for 30 min before lysis. After the incubation, spheroplasts were lysed in TX buffer, which either contained 1 mM Mg-ATP (lanes 3 and 4) or did not contain ATP (lanes 5 and 6). Coprecipitation analysis with antibodies to Vam3p was as in A. (D) Nyv1p is only found in a complex with Sec17p in the presence of the t-SNARE. Vacuoles from wild-type cells or from cells with only a vacuolar t-SNARE or v-SNARE were lysed and coprecipitated with an immobilized antibody to Sec17p as described in the Materials and Methods section. 10% of the detergent extract was TCA-precipitated before the coprecipitation analysis. Proteins were dissolved in sample buffer, resolved by SDS-PAGE and transferred to nitrocellulose. The membrane was decorated with antibodies to Sec17p, Vam3p, and Nyv1p. The efficiency of coprecipitation was not altered when Triton X-100 was used instead of digitonin (data not shown).
The v–t-SNARE complex on the isolated vacuoles was dissociated by Sec18p (Fig. 1 B). When vacuoles were prepared from sec18-1 cells and incubated with ATP before detergent solubilization and coprecipitation with α-Vam3p antibodies, the v–t-SNARE complex was disassembled (Fig. 1 B, top, lane 3). Dissociation was not seen without ATP (Fig. 1 B, top, lane 2). If the vacuoles were preincubated at 37°C for 10 min to inactivate the Sec18-1p, and then incubated with ATP (Fig. 1 B, bottom, lane 3), the v–t-SNARE complex was preserved. Similarly, v–t-SNARE dissociation was observed when vacuoles prepared from wild-type cells were preincubated with ATP. Antibodies to Sec18p, which do not precipitate the SNARE complex under these conditions, blocked this dissociation (data not shown). Thus both functional Sec18 protein and ATP are necessary to mediate SNARE dissociation.
We then asked whether the amount of vacuolar v–t-SNARE complexes is increased if Sec18p function is compromised in vivo, as reported for other organelles (Søgaard et al., 1994; Lupashin and Waters, 1997). Sec18-1 cells were converted to spheroplasts and incubated for 30 min at permissive (25°C) or nonpermissive (37°C) temperature. The spheroplasts were then detergent solubilized in the presence of ATP, and Vam3p was immunoprecipitated as before (Fig. 1 C). When Sec18p was active, the complex between Vam3p and Nyv1p was dissociated (Fig. 1 C, lane 3), as seen on isolated vacuoles (Fig. 1 B, top, lane 3). However, when Sec18p was inactive, the complex was preserved (Fig. 1 C, lane 4). Strikingly, Sec18p-mediated dissociation of the v–t-SNARE complex required ATP in the lysate and did not occur if ATP was omitted (Fig. 1 C, lanes 5 and 6). Furthermore, the amount of SNARE complex precipitated from detergent extracts of isolated vacuoles from wild-type cells in the absence of ATP (Fig. 1 A, lane 1 vs. lane 4) was similar to that precipitated from extracts of sec18-1 spheroplasts without ATP or at the nonpermissive temperature (Fig. 1 C, lanes 4 and 5). The simplest explanation for these data is that vacuolar v- and t-SNAREs are mainly in a complexed form, both in vivo and in vitro, and that this complex is dissociated by the action of Sec18p and ATP in vitro. The incubation of sec18-1 cells at the nonpermissive temperature prevents the dissociation of the complex that would otherwise occur in the lysate, rather than causing an in vivo accumulation of SNARE complexes. Since Sec17p and Sec18p act exclusively before docking (Mayer et al., 1996; Mayer and Wickner, 1997), the SNARE complex recognized by Sec18p is composed of SNAREs on the same membrane.
Sec17p is also present in the v–t-SNARE complex. A stable complex of Sec17p, Vam3p, and Nyv1p was coprecipitated by an antibody to Sec17p (Fig. 1 D, lane 4). At least 50% of the Vam3p was coprecipitated with Sec17p. The efficiency of coprecipitating Vam3p with immobilized antibodies to Sec17p was only 30% lower with t-SNARE– only vacuoles than with wild-type vacuoles (Fig. 1 D, lane 5). Thus, Sec17p association with the t-SNARE does not depend on the presence of the v-SNARE.
Vacuolar v- and t-SNAREs are thus in a complexed form, both in vivo and in vitro. Sec17p is part of this complex (Fig. 1 D), and is released in a Sec18p-dependent reaction (Mayer et al., 1996), paralleled by the disassembly of the v–t-SNARE complex (Fig. 1 B).
Both SNAREs of the Complex Affect Its Function
To follow the functional changes accompanying the separation of the SNAREs, we asked whether the addition of antibodies to the t-SNARE to just one wild-type vacuole partner would block its fusion with an untreated partner. We have previously demonstrated that v-SNARE–only vacuoles fuse with wild-type and t-SNARE–only vacuoles (Nichols et al., 1997). Thus, one might expect that the v-SNARE should still be able to mediate docking when the t-SNARE on the same membrane is masked by an antibody. However, since v- and t-SNAREs are found in a complex on the vacuolar membrane, the addition of IgGs to one (t) SNARE might obstruct the interaction of the second (v) SNARE with untreated vacuoles. To address these possibilities, vacuoles from the two strains of our assay were primed in separate tubes, and then placed on ice (Fig. 2). Antibodies to Vam3p were added to one portion of the vacuoles and allowed to bind for 10 min on ice. The vacuoles (still separate) were diluted, reisolated, resuspended in reaction buffer, and then combined for a standard 90-min fusion reaction. As expected, addition of α-Vam3p to both vacuole populations strongly inhibited the fusion (Fig. 2 A, lane 4). Fusion was even blocked when the antibody was only added to one of the vacuolar partners that had been kept on ice (Fig. 2 A, lanes 2 and 3). However, after priming the vacuoles with ATP at 27°C (Fig. 2 B), the addition of antibody to only one of the vacuole samples only partially blocked fusion (Fig. 2 B, lanes 2 and 3). These data suggest that the physical separation of the t-SNARE from the v-SNARE during ATP-dependent priming (Fig. 1 B) allows the t-SNARE to be inhibited without affecting the function of the v-SNARE. Thus, the physical separation coincides with the functional separation of the SNAREs upon priming.
Figure 2.
Functional separation of the v- and t-SNAREs during priming. Two double-scale reactions containing either BJ3505 or DKY6281 were incubated on ice without ATP or at 27°C with ATP. All tubes were then placed on ice and α-Vam3p IgGs were added to one tube containing each vacuole type. Reactions were kept on ice for 10 min, allowing the antibody to bind. Ice-cold PS buffer (350 μl) was then added, vacuoles were reisolated (3 min; 8,000 g) and resuspended in the original volume of reaction mixture containing ATP and cytosol. A 15-μl portion of vacuole sample was combined with the corresponding partner such that neither partner, one partner, or both saw the antibody in the second incubation. Vacuoles were incubated for an additional 90 min at 27°C with cytosol and analyzed for fusion as described in the Materials and Methods section. Cytosol, but not LMA1 and Sec18p (not shown), was essential in the last step to insure a normal fusion signal, suggesting that an additional cytosolic factor might be necessary after these preincubations. The lower overall signal in the primed samples is because of the lability of the vacuolar membrane induced by this treatment (Mayer et al., 1997; Xu et al., 1997).
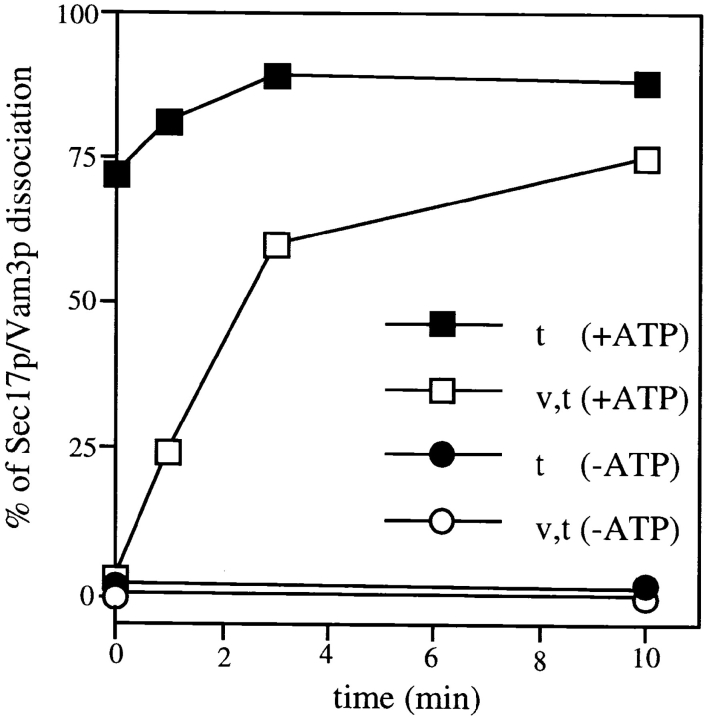
Sec17p is in a complex with the t-SNARE, both on the wild-type and t-SNARE–only vacuoles (Fig. 1 D). As SNARE separation is one of the functions of Sec17p/ Sec18p, we asked whether the rate of the Sec17p release was altered if the v-SNARE was absent from the vacuoles. With wild-type vacuoles, efficient crossprecipitation of Vam3p by an antibody to Sec17p, set as “0% dissociation,” is seen when vacuoles are kept on ice in the presence of ATP (Fig. 3, □; t = 0 min), or when ATP is omitted (○). In the presence of ATP, the complex of Sec17p and Vam3p on wild-type vacuoles is dissociated over a 10-min period, consistent with the rate of Sec17p release from the vacuoles themselves (Fig. 3, □; Mayer et al., 1996). In the absence of ATP, the complex of Sec17p and Vam3p is also maintained on t-SNARE–only vacuoles (Fig. 3, •). However, this complex is immediately dissociated by hydrolyzable ATP even at t = 0 (Fig. 3, ▪). This ATP-dependent dissociation of Sec17p from Vam3p, whether on wild-type vacuoles or t-SNARE–only vacuoles, is blocked by an antibody to Sec18p (not shown), consistent with the block of Sec17p release from the vacuolar membrane by the addition of Sec18p antibodies (Mayer et al., 1996). The slower dissociation of Sec17p from the v–t-SNARE complex may reflect a direct role of Sec17p in SNARE dissociation before its release from the vacuole.
Figure 3.
ATP-dependent dissociation of the Sec17p/ Vam3p complex is enhanced in the absence of the v-SNARE. For each time point, 80 μg of vacuoles (BJ3505 [v,t] or BJ3505 Δnyv1 [t]) in 400-μl fusion reaction mixtures were incubated for 10 min at 27°C without nucleotide or with 1 mM Mg-ATP. PS buffer (400 μl) was then added, vacuoles were reisolated (10 min; 8,000 g) and suspended in 200 μl of PS buffer, and then reisolated again (5 min; 8,000 g). Vacuoles were solubilized in digitonin buffer and processed for coprecipitation with protein A–Sepharose immobilized Sec17p-IgGs as described in Materials and Methods. Proteins were dissociated from the beads by addition of sample buffer, resolved on SDS-PAGE, transferred to nitrocellulose, and then decorated with antibodies to Sec17p and Vam3p. Vam3p bands of the enhanced chemiluminescence–developed film were quantitated by laser densitometry. The stability of the Sec17p/Vam3p complex was unaltered when Triton X-100 was used instead of digitonin (not shown).
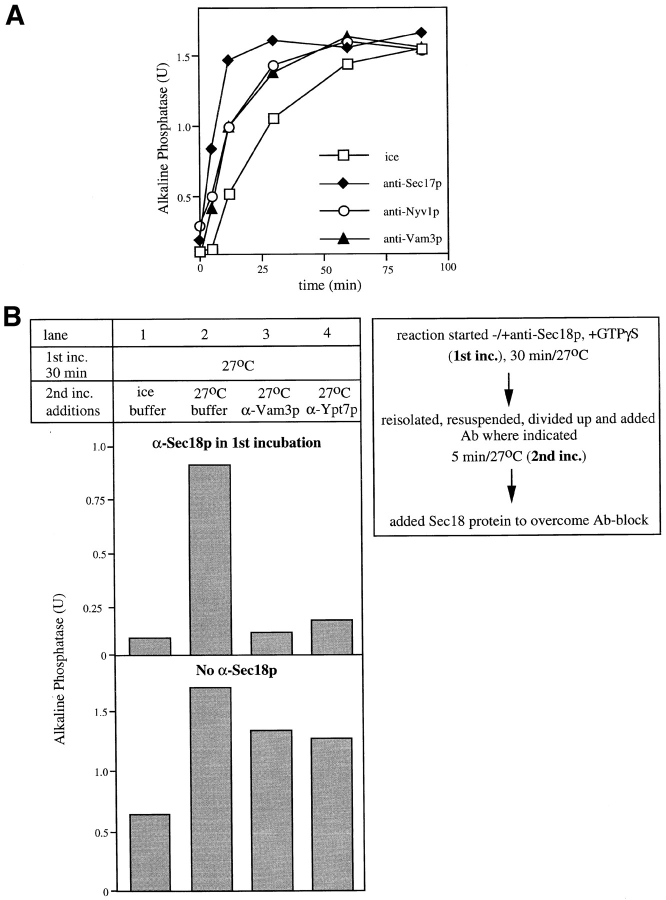
We have previously reported that our in vitro reaction becomes resistant to inhibition by an antibody to Vam3p with kinetics that parallel resistance to anti-Ypt7p, a catalyst of docking (Nichols et al., 1997). These kinetics are also paralleled by the acquisition of resistance to anti-Nyv1p (Fig. 4 A), further indicating that Vam3p and Nyv1p act together in the docking stage of the reaction. It has previously been shown that resistance to anti-Ypt7p can only be achieved after Sec17p/Sec18p have primed the vacuoles (Mayer et al., 1997). To determine whether Sec17p/Sec18p-catalyzed priming is also needed for resistance to anti-Vam3p, we performed a two stage reaction (Fig. 4 B). In a first incubation, vacuoles were incubated at 27°C with ATP in the presence (Fig. 4 B, top) or absence (Fig. 4 B, bottom) of antibodies to Sec18p. GTPγS was added to retard fusion (Mayer and Wickner, 1997) during the first incubation. After 30 min, vacuoles were reisolated, suspended in fresh reaction buffer, and then divided into five portions. One was placed on ice, one incubated without further additions, one received an antibody to Ypt7p and one an antibody to Vam3p. After 5 min, the Sec18p function was restored by addition of purified Sec18 protein, and the reactions were incubated for an additional 90 min at 27°C. Resistance to α-Ypt7p (Mayer et al., 1997) or α-Vam3p was only achieved if the samples had been preincubated with functional Sec18p, indicating that priming is required for a later SNARE-dependent reaction, presumably docking.
Figure 4.
Sensitivity of the stages of the vacuole fusion reaction to antibody to SNAREs. (A) Resistance to anti-Nyv1p and anti-Vam3p is coincident during the fusion reaction. A 30× scale fusion reaction was started in the presence of ATP at 27°C. Aliquots (30 μl) were removed at indicated times, added to antibodies to Sec17p, Vam3p, or Nyv1p, and then incubated at 27°C or placed on ice. Fusion reactions were incubated for a total of 90 min at 27°C before being assayed for alkaline phosphatase activity. (B) Sec18p action must precede that of the t-SNARE in the fusion assay. Two sixfold reactions containing vacuoles from BJ3505 and DKY6801 were started in the presence of cytosol, ATP, and 1.5 mM GTPγS. Half received α-Sec18p. A 30-μl portion of each was removed and placed on ice. The rest was incubated for 30 min at 27°C, and then vacuoles were reisolated, resuspended in the previous volume of reaction buffer containing ATP and cytosol, and divided into 30-μl reactions. Where indicated, IgGs to Vam3p or Ypt7p were added. One reaction was left on ice, the others were returned to 27°C. After 5 min, 400 ng of pure Sec18p was added to each and incubations were continued for 90 min at 27°C and analyzed for fusion as described above. The background activity (0.25 U) was subtracted in all lanes.
Sec17p Function Is Largely t-SNARE Related
Sec17p is released from the SNARE complex early in the reaction. This release is blocked by antibodies to Sec17p and Sec18p that affect the priming reaction, but not by those to Ypt7p that interfere with the docking of primed vacuoles (Mayer et al., 1997). We therefore tested whether antibodies to Vam3p or Nyv1p would influence the Sec17p release from the vacuolar membrane. Only antibodies to the t-SNARE Vam3p blocked Sec17p release, whereas antibodies to the v-SNARE Nyv1p had no effect (Fig. 5). It is possible that this effect is partially due to the specificity of the antibodies; however, IgGs to Nyv1p and Vam3p efficiently inhibit the overall reaction (Fig. 4 A). This asymmetry in the inhibition of Sec17p release by the antibodies to the SNAREs is consistent with the physical interaction of the SNAREs with Sec17p. In contrast to the t-SNARE, which was found in a tight complex with Sec17p (Fig. 1 D, lane 5), the v-SNARE was only found in association with Sec17p when the t-SNARE was also present on the vacuoles (Fig. 1 D, lane 6). Even long exposures of the blot decorated with anti-Nyv1p did not give any signal in Fig. 1 D, lane 6 (not shown). The lysate controls show that the amount of Nyv1p is equal in the detergent extracts of both wild-type and v-SNARE–only vacuoles (Fig. 6 B, bottom row; and Fig. 1 D, lanes 1 and 3). Thus, Sec17p is not found in a complex with the v-SNARE alone, suggesting that Sec17p associates with the SNARE complex directly via the t-SNARE itself or a t-SNARE–specific element of the complex.
Figure 5.
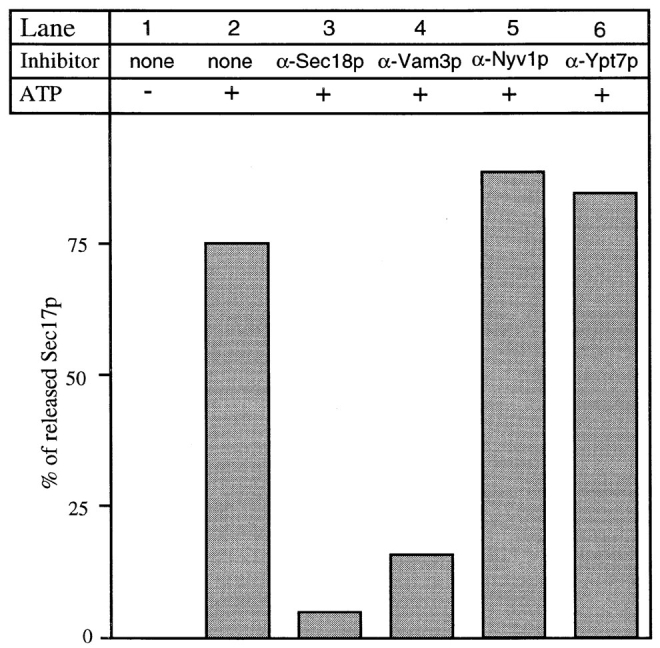
Sec17p release depends on Sec18p and ATP, and is blocked by t-SNARE antibodies. For each condition, a fourfold scale reaction was incubated for 10 min at 27°C. Where indicated, vacuoles were preincubated with IgGs for 3 min on ice at the following concentrations: α-Sec18p (100 μg/ml), α-Vam3p, α-Nyv1p, or α-Ypt7p (each 300 μg/ml). After the incubation, vacuoles were reisolated at 8,000 g and assayed for bound Sec17p as described by Mayer et al. (1996).
Figure 6.
Vacuoles with the t-SNARE, but not those with the v-SNARE, require Sec17p/Sec18p. A double-scale reaction containing cytosol was started with vacuoles from BJ3505 Δvam3 (v) and DKY6281 Δnyv1 (t) in separate tubes. Reactions were either kept on ice in the presence of antibodies to Sec18p or were incubated for 5 min at 27°C in the presence of ATP. Then 15 μl of each reaction was combined with 15 μl of the other partner in the presence of α-Sec18p such that either both, one, or none had been primed. Samples were then incubated for an additional 90 min at 27°C, and analyzed for fusion as before. The background activity (0.2 U) was subtracted in all lanes. Similar results were obtained with DKY6281 Δvam3 and BJ3505 Δnyv1 (data not shown). (B) Binding of Sec17p and Sec18p to the vacuole is independent of the Nyv1p and Vam3p. Vacuoles from BJ3505 (v,t), and the parental strain with deletion in the v-SNARE (t), the t-SNARE (v), or both SNAREs (−) were isolated as described in Materials and Methods. Isolated vacuoles (20 μg) were suspended in SDS–sample buffer, separated by SDS-PAGE, transferred to nitrocellulose, and then decorated with the indicated antibodies.
Sec18p-mediated Sec17p release causes the separation of a preexisting v–t-SNARE complex (Fig. 1). However, fusion of vacuoles with only v-SNAREs with vacuoles bearing only t-SNAREs still requires Sec18p/Sec17p- mediated priming (Nichols et al., 1997), raising the question of whether priming may activate separate v- or t-SNAREs as well as v–t-SNARE pairs. As Sec18p action is needed to separate the SNAREs before docking and fusion, wt-vacuoles would not allow us to ask which SNARE is the primary target of Sec17p/Sec18p action. We therefore addressed this question by pretreating single SNARE vacuoles separately with antibodies to Sec18p. Vacuoles were kept in separate tubes and either primed for 5 min at 27°C in the presence of ATP or left on ice and pretreated with an antibody to Sec18p. After 5 min, they were combined in the presence of α-Sec18p to prevent further priming (Mayer et al., 1996), and allowed to complete the fusion reaction (Fig. 6 A). We find that v-SNARE–only vacuoles showed almost no sensitivity to Sec18p antibodies, whereas the t-SNARE–only vacuoles were strongly inhibited when pretreated with the antibody. However, despite this linkage between the t-SNARE and Sec17p in the priming reaction, the content of Sec17p and Sec18p is not reduced if the t-SNARE is absent from the vacuole (Fig. 6 B). This indicates that there are receptors for Sec17p or Sec18p in addition to the identified vacuolar SNAREs and that the failure of antibody to Sec18p to inhibit the v-SNARE–only vacuoles does not simply reflect an absence of Sec18p from the vacuole. Sec17p/Sec18p therefore acts primarily on the t-SNARE when analyzed in single SNARE vacuoles.
Discussion
Our results link the early function of Sec17p/Sec18p to the disassembly and activation of vacuolar SNAREs, and place the disassembly of a SNARE complex into the functional context of a biochemical fusion assay. As postulated by the SNARE hypothesis (Rothman, 1994), vacuolar homotypic fusion depends on v- and t-SNAREs on opposing vacuoles (Nichols et al., 1997). The ATP-dependent dissociation of a v–t-SNARE complex on the vacuolar membrane by Sec18p/ NSF and Sec17p/α-SNAP is consistent with the disassembly of the 20-S fusion complex in detergent solution or on immobilized, purified SNAREs in vitro (Söllner et al., 1993a ,b; Hayashi et al., 1995). It had been suggested that NSF and α-SNAP mediate membrane fusion through the ATP-dependent disassembly of the docked v- and t-SNAREs (Söllner et al., 1993a ,b; Rothman, 1994). However, we find that Sec17p/Sec18p promote the ATP- dependent disassembly of a v–t-SNARE complex in the priming reaction, an essential prerequisite to the docking step that must itself precede fusion. In addition to resolving the SNARE complex, Sec17p and Sec18p activate the t-SNARE, even when there is no Nyv1p present.
Our findings (Mayer et al., 1996; Nichols et al., 1997; this study) are in agreement with studies of other trafficking reactions where ATP-dependent priming and ATP-independent fusion have been reported. Endosome–endosome fusion and regulated exocytosis also require ATP and NSF in a reaction that precedes docking (Banerjee et al., 1996; Colombo et al., 1996; Martin, 1997). Even in the pseudo-heterotypic fusion between vacuoles with only t-SNARE, and those with only v-SNARE, Sec17p and Sec18p are only required at an early stage of the reaction (Nichols et al., 1997). Jahn and colleagues (Otto et al., 1997) have reported that NSF might be required on both the vesicle and the target membrane in synapses. They found that highly purified synaptic vesicles contain both synaptobrevin (a v-SNARE) and syntaxin and SNAP-25 (two t-SNAREs) (Walch-Solimena et al., 1995). Strikingly, these proteins are exclusively found in an SDS-resistant complex, which is similar to that found on the synaptic plasma membrane (Hayashi et al., 1994, 1995). This v–t-SNARE complex on the vesicle is also dissociated by purified NSF and α-SNAP. Since even synaptic vesicles contain v–t-SNARE complexes, both our data and studies of neuronal synapses suggest that NSF and α-SNAP allow docking by dissociating preexisting v–t-SNARE complexes on both the vesicle and the target organelle membrane. Genetic data have not clarified the site of Sec18p (NSF) action. A ts-allele of SEC18, sec18-1, causes the accumulation of small, ER- derived vesicles in the cell (Rexach and Scheckman, 1991). However, extracts prepared with ATP of thermally inactivated sec18-1 mutant cells showed a large increase in SNARE complexes (Søgaard et al., 1994). These complexes were proposed to be on docked vesicles which had accumulated 20-S complexes. However, their data are also consistent with these complexes resulting from an inefficient in vitro disassembly of v–t-SNARE complexes that had accumulated both before docking and after fusion had occurred. Our data support the latter interpretation, since vacuolar v–t-SNARE complexes disassemble in a Sec18p-dependent reaction, when cells are lysed in the presence of ATP but are preserved when ATP is omitted. When the omission of ATP prevents Sec18p function in vitro, the thermal inactivation of sec18-1 is not required for maximal level of vacuolar v–t-SNARE complexes. This places earlier studies (Søgaard et al., 1994; Sapperstein et al., 1996; Lupashin et al., 1997), in which ATP was present in the detergent extracts, in a new context. Thus, the v–t-SNARE complex may be a more predominant form of SNAREs in the cell than heretofore realized.
In addition to Sec17p, Sec18p, and the SNAREs, LMA1 (a complex of thioredoxin with IB 2) is required for heterotypic and homotypic fusion (Xu and Wickner, 1996; Barlowe, 1997; Xu et al., 1997; Xu, Z., and W. Wickner, manuscript in preparation). Xu and Wickner (in preparation) have shown that LMA1 binds directly to the vacuoles via Sec18p. During the priming reaction of Sec18p-mediated ATP hydrolysis, LMA1 is transferred from Sec18p to the t-SNARE. LMA1 stabilizes vacuoles immediately after priming (Xu et al., 1997), and is released from the vacuole at a late stage of the reaction. These observations suggest that LMA1 binds to and protects the activated t-SNAREs for docking and/or fusion. LMA1 is also necessary for the heterotypic docking and fusion of ER vesicles with Golgi membranes (Barlowe, 1997). Therefore, both vacuole–vacuole fusion and ER–Golgi fusion use many of the same soluble proteins.
Both physical and functional data point to the t-SNARE Vam3p as the main target of the action of Sec17p/Sec18p. Release of Sec17p from Vam3p, and hence from the vacuole, is completely blocked by both IgGs to Sec18p (Mayer et al., 1996) and IgGs to Vam3p. Furthermore, the tight association of Sec17p with the t-SNARE on isolated vacuoles does not depend on the presence of the v-SNARE, as it is seen on t-SNARE–only vacuoles. This is consistent with in vitro binding studies with neuronal SNAREs. There, syntaxin was shown to suffice for α-SNAP binding. A complex of syntaxin and synaptobrevin increased the binding of α-SNAP severalfold; synaptobrevin alone, however, was not sufficient to bind α-SNAP (McMahon and Südhof, 1995; Hanson et al., 1995; Hayashi et al., 1995). This agrees with our finding that 30% less Sec17p is associated with the t-SNARE Vam3p on t-SNARE–only vacuoles, though the absence of the vacuolar v-SNARE has a lower effect on Sec17p association with the t-SNARE than reported with purified neuronal SNAREs (McMahon and Südhof, 1995). This difference may reflect additional components present in the vacuolar SNARE complex. However, the v-SNARE Nyv1p was only recovered in a complex with Sec17p when Vam3p was present as well (Fig. 1 D). Furthermore, in vitro assays showed that syntaxin undergoes a conformational change after NSF and α-SNAP action (Hanson et al., 1994). This is in agreement with our finding that Sec18p/ Sec17p action is required on single SNARE vacuoles for activation of the t-SNARE, but not for the v-SNARE (Fig. 4). Whether this activation includes a conformational change and/or a disassembly of a t-SNARE–specific subcomplex on the t-SNARE–only vacuoles remains to be tested. Thus, the vacuolar priming and docking reaction and those for heterotypic fusion have very similar requirements.
We have focused on three proteins of the v–t-SNARE complex in this study, the Sec17p, the v-SNARE Nyv1p, and the t-SNARE Vam3p. However, the SNARE complex has other important components. Sec18p and LMA1 are also associated with the SNARE complex, but are not retained at the salt concentrations used for coprecipitation with anti-Vam3p or anti-Sec17p (Xu, Z., and W. Wickner, manuscript in preparation; unpublished observation). Preliminary results indicate that Vps33 and Ypt7 are also part of the complex (not shown). Further studies are needed to identify the other components of this complex and to characterize their function.
Our current working model of the homotypic vacuolar fusion reaction is summarized in Fig. 7. Before priming, Sec17p is associated with a v–t-SNARE complex, bound primarily to the t-SNARE. ATP hydrolysis by Sec18p triggers four coupled reactions: Sec17p release, dissociation of the SNARE complex, activation of the t-SNARE, and transfer of LMA1 to the activated SNAREs to stabilize them for docking and fusion (Xu, Z., and W. Wickner, manuscript in preparation). These four biochemical events, which constitute the priming reaction, may be needed for analogous functions in the heterotypic yeast ER to Golgi and neural synaptic transmission reactions as well. Unlike vacuoles, v- and t-SNAREs of the heterotypic trafficking reaction are considered to be largely on separate organelles. The dissociation of v- and t-SNARE complexes may occur after fusion on the distal organelle to allow selective retrograde transport of the v-SNARE and priming of the t-SNARE for interaction with subsequently arriving vesicles. However, retrograde sorting of v-SNAREs from one organelle (e.g., the Golgi) to another organelle (e.g., the ER) might also include the paired t-SNARE, which would then be found on the vesicle in the following anterograde targeting process (e.g., from the ER to the Golgi). Subsequently, even the vesicle would need Sec18p action to dissociate the v–t-SNARE complex, consistent with data from Otto et al. (1997) as discussed above. Despite extensive studies of v- and t-SNAREs, there are surprisingly few reports where SNAREs of a specific trafficking step have been clearly shown to reside exclusively on the donor, vesicle or target membrane. During vacuole docking, which requires the G protein Ypt7p, v- and t-SNAREs from opposing vacuoles are believed to interact and to remain in a v–t-SNARE complex on the vacuole after fusion. The parallel arrangement of the coiled-coil domains of SNAREs could then serve as a driving force for the fusion of docked vacuoles (Hanson et al., 1997). Further studies are needed to directly measure this postulated SNARE reassociation and to determine whether SNARE conformations are altered during the fusion event.
Figure 7.
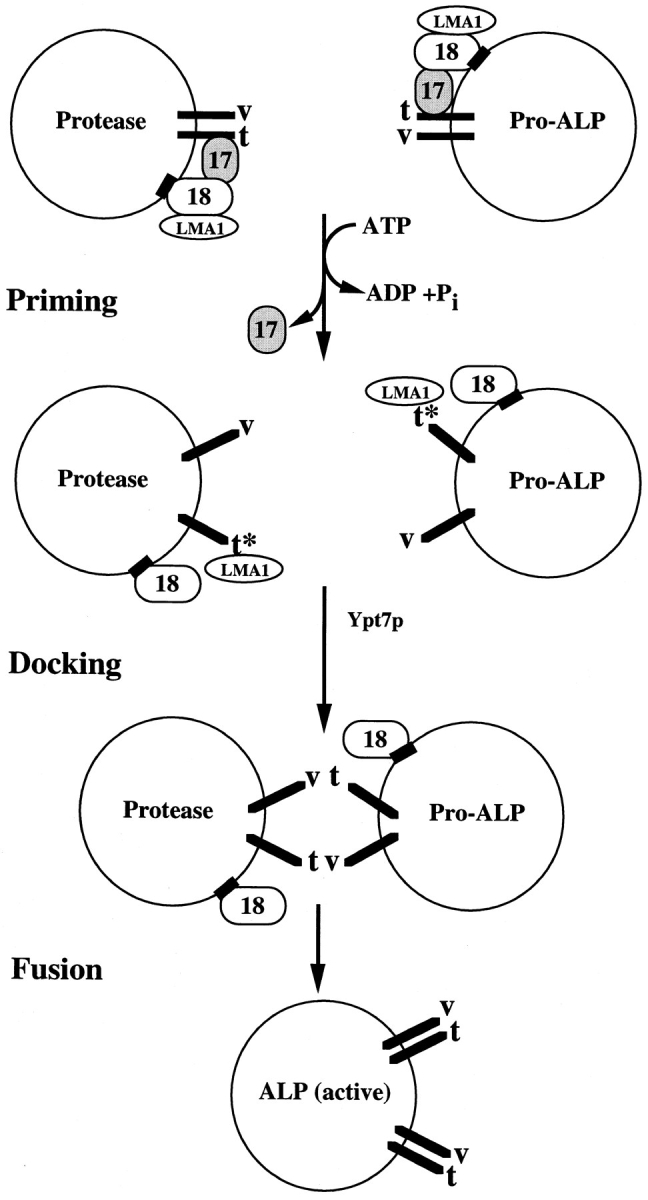
Working model for homotypic vacuolar fusion. The box on Sec18p indicates a hypothetical Sec18p receptor. Abbreviations are as follows: Sec17p (17), Sec18p (18), Nvy1p (v), Vam3p (t), pro-alkaline phosphatase (Pro-ALP), and alkaline phosphatase (ALP). A star on the t-SNARE indicates the activated state of the protein after priming. See text for details.
Acknowledgments
We thank Drs. A. Haas, and Z. Xu for reagents, advice, and discussion; and Drs. C. Barlowe, G. Eitzen, A. Haas, D. Klionsky, and Z. Xu for critical comments on the manuscript.
Abbreviations used in this paper
- NSF
N-ethylmaleimide–sensitive fusion protein
- SNAP
soluble NSF attachment proteins
- SNARE
SNAP receptor
- t
target membrane
- v
vesicle membrane
Footnotes
The work was supported by grants from the Medical Research Council to H. Pelham and B. Nichols; the National Institute of General Medical Sciences to W. Wickner and the Deutsche Forschungsgemeinschaft to C. Ungermann.
Address all correspondence to W. Wickner, Department of Biochemistry, Dartmouth Medical School, 7200 Vail Building, Hanover, NH 03755-3844. Tel.: (603) 650-1701. Fax: (603) 650-1353. E-mail: Wickner.William@Dartmouth.edu
References
- Acharya U, Jacobs R, Peters J-M, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. doi: 10.1016/0092-8674(95)90269-4. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Barry V, DasGupta BR, Martin TF. N-ethylmaleimide sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- Barlowe C. Coupled ER to Golgi transport reconsituted with purified cytosolic proteins. J Cell Biol. 1997;139:1–12. doi: 10.1083/jcb.139.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LH, Roth D, Morgan A, Burgoyne RD. Distinct effects of α-SNAP, 14-3-3 proteins, and calmodulin on priming and triggering of regulated exocytosis. J Cell Biol. 1995;130:1063–1070. doi: 10.1083/jcb.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo MI, Taddese M, Whiteheart S, Stahl PD. A possible predocking attachment site for N-ethylmaleimide-sensitive fusion protein. J Biol Chem. 1996;271:18810–18816. doi: 10.1074/jbc.271.31.18810. [DOI] [PubMed] [Google Scholar]
- Conradt B, Shaw J, Vida T, Emr S, Wickner W. In vitroreactions of vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1992;119:1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SE. A multi-specificity syntaxin- homologue, Vam3p, essential for autophagy and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Fasshauer D, Otto H, Eliason WK, Jahn R, Brünger AT. Structural changes are associated with soluble N-ethylmaleimide sensitive fusion protein attachment protein receptor complex formation. J Biol Chem. 1997;272:28936–28941. doi: 10.1074/jbc.272.44.28036. [DOI] [PubMed] [Google Scholar]
- Götte M, Gallwitz D. High expression of the yeast syntaxin-related Vam3 protein suppresses the protein transport defects of a pep12 null mutant. FEBS (Fed Eur Biochem Soc) Lett. 1997;411:48–52. doi: 10.1016/s0014-5793(97)00575-9. [DOI] [PubMed] [Google Scholar]
- Gomes de Mesquita DS, ten Hoopen R, Woldringh CL. Vacuolar segregation to the bud of Saccharomyces cerevisiae: an analysis of morphology and timing in the cell cycle. J Gen Microbiol. 1991;137:2447–2454. doi: 10.1099/00221287-137-10-2447. [DOI] [PubMed] [Google Scholar]
- Graham TR, Emr SE. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. A quantitative assay to measure homotypic vacuole fusion in vitro. . Methods Cell Sci. 1995;17:283–294. [Google Scholar]
- Haas A, Wickner W. Homotypic vacuolar fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF) EMBO (Eur Mol Biol Organ) J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitroreactions of vacuole inheritance. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO (Eur Mol BiolOrgan) J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Otto H, Barton N, Jahn R. The N-ethylmaleimide-sensitive fusion protein and α-SNAP induce a conformational change in syntaxin. J Biol Chem. 1995;270:16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and O. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory.
- Hay JC, Scheller R. SNAREs and NSF in targeted membrane fusion. Curr Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO (Eur Mol Biol Organ) J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H. Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. . EMBO (Eur Mol Biol Organ) J. 1995;14:2317–2325. doi: 10.1002/j.1460-2075.1995.tb07226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebsch RR, Wattenberg BW. Vesicle fusion in protein transport through the Golgi in vitrodoes not involve long-lived prefusion intermediates. A reassessment of the kinetics of transport as measured by glycosylation. Biochemistry. 1992;31:6111–6118. doi: 10.1021/bi00141a022. [DOI] [PubMed] [Google Scholar]
- Holz RW, Bittner MA, Peppers SC, Senter RA, Eberhard DA. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. J Biol Chem. 1989;264:5412–5419. [PubMed] [Google Scholar]
- Ikonen E, Tagaya M, Ullrich O, Montecucco C, Simons K. Different requirements for NSF-SNAP, and Rab proteins in apical and basolateral transport in MDCK cells. Cell. 1995;81:571–580. doi: 10.1016/0092-8674(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Latterich M, Fröhlich K-U, Schekman R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell. 1995;82:885–894. doi: 10.1016/0092-8674(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Rayner JC, Pelham HRB. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1997;16:3017–3024. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin VV, Waters MG. t-SNARE activation through transient interaction with a rab-like guanosine triphosphatase. Science. 1997;276:1255–1258. doi: 10.1126/science.276.5316.1255. [DOI] [PubMed] [Google Scholar]
- Martin TFJ. Stages of regulated exocytosis. Trends Cell Biol. 1997;7:271–276. doi: 10.1016/S0962-8924(97)01060-X. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) precedes docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Südhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP-25 forms high affinity α-SNAP binding site. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- Morgan A, Burgoyne RD. Is NSF a fusion protein? . Trends Cell Biol. 1995;5:335–339. doi: 10.1016/s0962-8924(00)89059-5. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HRB, Wickner W, Haas A. Homotypic vacuolar fusion mediated by v- and t-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Otto H, Hanson PI, Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin and SNAP-25 in the membrane of synaptic vesicles. Proc Natl Acad Sci USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek I, Orci L, Ravazzola M, Erdjument-Bromage H, Amherdt M, Tempst P, Söllner T, Rothman JE. ERS-24, a mammalian v-SNARE implicated in vesicle traffic between the ER and the Golgi. J Cell Biol. 1997;137:1017–1028. doi: 10.1083/jcb.137.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking: SNARE and associates. Annu Rev Cell Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Levine TP, Peters J-M, Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 1995;82:905–914. doi: 10.1016/0092-8674(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Raymond CK, Roberts CJ, Moore KE, Howald I, Stevens TH. Biogenesis of the vacuole in Saccharomyces cerevisiae. . Intern Rev Cytol. 1992;139:59–120. doi: 10.1016/s0074-7696(08)61410-2. [DOI] [PubMed] [Google Scholar]
- Rexach MF, Schekman RW. Distinct biochemical requirements for the budding, targeting, and fusion of ER-derived transport vesicles. J Cell Biol. 1991;114:219–229. doi: 10.1083/jcb.114.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular membrane fusion. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sapperstein SK, Lupashin VV, Schmitt HD, Waters MG. Assembly of the ER to Golgi SNARE complex requires Uso1p. J Cell Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarewicz P, Xu Z, Seefeld K, Haas A, Wickner W. IB 2is a small cytosolic protein that participates in vacuole fusion. Proc Natl Acad Sci USA. 1997;94:5582–5587. doi: 10.1073/pnas.94.11.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Söllner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993a;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart S, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitrothat may correspond to sequential steps of synaptic vesicle docking, activation and fusion. Cell. 1993b;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Neupert W, Cyr DM. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science. 1994;266:1250–1253. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- Wada Y, Nakamura N, Ohsumi Y, Hirata A. Vam3p, a new member of syntaxin related proteins, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. . J Cell Sci. 1997;110:1299–1306. doi: 10.1242/jcs.110.11.1299. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Blasi J, Edelmann L, Chapman ER, von Mollard GF, Jahn R. The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg BW, Raub TJ, Hiebsch RR, Weidman PJ. The activity of Golgi transport vesicles depends on the presence of the N-ethylmaleimide-sensitive factor (NSF) and a soluble NSF attachment protein (α-SNAP) during vesicle formation. J Cell Biol. 1992;118:1321–1332. doi: 10.1083/jcb.118.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman LS, Wickner WT. Intervacuole exchange in the yeast zygote: a new pathway in organelle communication. Science. 1988;241:289–291. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wickner W. Thioredoxin is required for vacuole inheritance in S. cerevisiae. . J Cell Biol. 1996;132:787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Mayer A, Muller E, Wickner W. A heterodimer of thioredoxin and IB 2cooperates with Sec18p(NSF) to promote yeast vacuole inheritance. J Cell Biol. 1997;136:299–306. doi: 10.1083/jcb.136.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]