Abstract
Withdrawal symptoms are a major deterrent when people try to quit smoking. The α7 subunit of the neuronal nicotinic acetylcholine receptor (nAChR) is highly expressed in the brain, and has been suspected to play a major role in nicotine addiction. We studied the influence of α7-containing nAChRs on nicotine withdrawal and tolerance, in wild type mice and mice null for the α 7 nAChR subunit (α7 −/−). For withdrawal experiments, animals were implanted with osmotic minipumps delivering nicotine for 13 days. A single intraperitoneal injection of the nAChR antagonists mecamylamine (MEC) or methyllycaconitine (MLA) was used to precipitate withdrawal. In wild type mice, both MEC and MLA precipitated somatic signs of withdrawal such as increased grooming, scratching and shaking. In α7 −/− mice, the somatic effects of MEC-precipitated nicotine withdrawal were significantly reduced. Interestingly, the presumed α7-specific antagonist MLA also precipitated withdrawal. Tolerance, which was measured as a decrease in nicotine-induced hypolocomotion after subchronic nicotine treatment, was normal in α7 −/− mice. Finally, because anxiety and withdrawal symptoms are highly correlated in humans, we studied anxiety-like behaviors in α7 −/− mice using a battery of anxiety-related tests. The behavior of α7 −/− mice was indistinguishable from that of control mice. Our results point to the α7 subunit as one of the players in nicotine withdrawal, but not in nicotine tolerance or basal anxiety-like behavior.
Keywords: Nicotinic acetylcholine receptor, knock out mice, α7 subunit, nicotine, withdrawal, tolerance, anxiety, mecamylamine, methyllycaconitine
INTRODUCTION
Tobacco addiction is a prominent global health problem (Peto et al., 2000). The major addictive component of tobacco is nicotine, which acts at pentameric neuronal nicotinic acetylcholine receptors (nAChRs). These receptors comprise either α and β subunits, or α subunits only. To date, 9 α and 3 β subunits have been cloned (Ferrari et al., 2002). Certain subunit combinations, such as α4β2, α3β4, or α7 only, predominate in neurons. Among these, theα4β2- and α7-containing combinations are the most widely expressed in the central nervous system (CNS) (Dani and De Biasi, 2001; Hogg et al., 2003).
Presynaptic α7-containing (α7*) nAChRs facilitate neurotransmitter release (Alkondon et al., 2000). Pharmacological experiments have implicated these receptors in several effects of nicotine such as aversion and reward (Laviolette and van der Kooy, 2003), anxiety-like behavior (Tucci et al., 2003) and working memory (Levin, 2002). The generation of mice carrying an α7 null mutation provided a new tool for investigating the role of this subunit in the CNS (Orr-Urtreger et al., 1997). So far, few phenotypes have been associated with this mutation in both basal conditions (Franceschini et al., 2000; Morley and Rodriguez-Sierra, 2004; Paylor et al., 1998; Stolerman et al., 2004) and in the presence of nicotine (Franceschini et al., 2002; Stolerman et al., 2004). To date, nicotine withdrawal-enhanced nociception is the only nicotine-related phenotype shown to be affected in α7 −/− mice (Grabus et al., 2005).
The appearance of withdrawal symptoms upon cessation of chronic nicotine exposure is one of the major factors precluding the majority of people from successfully quitting tobacco use. Understanding which type of nAChR is involved in the mechanisms underlying nicotine withdrawal is important in the quest for drugs that can treat nicotine addiction. We have shown before that β4* nAChRs have a major role in nicotine withdrawal as mice carrying a β4 null mutation display greatly reduced nicotine withdrawal symptoms (Salas et al., 2004). However, nicotine withdrawal can be precipitated in mice chonically treated with nicotine by antagonists with partial selectivity not only for α3β4- (mecamylamine: MEC), but also for α4β2- (di-hydro-β-erytroidine) or α7* nAChRs (Damaj et al., 2003). In particular, a major role for the α7 subunit in nicotine withdrawal in mice was suggested by the fact that systemic MLA precipitates withdrawal after chronic nicotine treatment (Damaj et al., 2003). Those results suggest that either multiple nAChRs participate in the withdrawal syndrome, that the subunit specificity of some of these drugs is less than usually assumed, or both.
Tolerance to the effects of drugs of abuse is commonly observed when a drug is repeatedly used, and the link between tolerance, addiction and withdrawal has been studied for more than a century (Lacaille et al., 1987). It is widely believed that tolerance plays a critical role in the development and maintenance of nicotine addiction. The α4 subunit might mediate tolerance to some of the effects of nicotine (Tapper et al., 2004), while the α7 subunit seems to have no effect on tolerance (Naylor et al., 2005).
Anxiety relief is another reason often cited by smokers when asked why they continue smoking despite the adverse consequences (Parrott, 1998). Because nAChR β4 −/− mice are insensitive to the somatic signs of nicotine withdrawal (Salas et al., 2004) and they show decreased basal anxiety-like behavior (Salas et al., 2003), we wanted to further explore the link between anxiety-like behavior and nicotine withdrawal. Others suggested that α7 −/− mice might have decreased anxiety-like behavior in the open field (Paylor et al., 1998) and therefore, we examined anxiety-related behavior in α7 −/− mice in more detail.
In summary, the present study takes advantage of α7 −/− mice to address the role of α7* nAChRs in mediating the somatic signs of nicotine withdrawal, nicotine tolerance, and basal anxiety-related behavior.
MATERIALS AND METHODS
Animals
Experiments were conducted on two- to five-month old wild type mice and aged match mice lacking the α nAChR subunit (Orr-Urtreger et al., 1997). Mice were back-crossed 9 or 10 times into a C57BL/6 background. Male and female mice were housed under a 12-12 light-dark cycle, with access to food and water ad libitum, and experiments were performed during the light phase. Genotypes were confirmed at the end of each experiment and were disclosed to the experimenters after completion of data analysis. All procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee and followed the guidelines for animal intramural research from the National Institute of Health.
Nicotine withdrawal
Alzet pumps model 1002 (14 days, flow rate 0.25 μl/h, Durect, CA) were subcutaneously implanted according to manufacturer’s instructions. Pumps were filled with either saline or nicotine tartrate in saline to deliver a 24 mg/kg/day dose of nicotine (as free base) for 13 days. The mouse nicotine withdrawal protocol, which was previously used in our laboratory (Salas et al., 2004), was based on research by Berrendero (Balerio et al., 2004) and Damaj (Damaj et al., 2003). After 13 days of either nicotine or saline infusion, mice were given intraperitoneal (ip) injections of either MEC (3 mg/kg) or MLA (7.5 mg/kg), and immediately placed in a cage where withdrawal signs were recorded for 20 min. Grooming, scratching, chewing, and shaking were the main parameters monitored. Less common behaviors (“other” symptoms) such as cage scratching, head nodding, and wet dog shakes were also recorded (Salas et al., 2004).
Tolerance
For subchronic nicotine treatment in tolerance experiments, on day 1, mice were acclimated to the procedure by receiving 2 ip saline injections, 8 hours apart. On days 2, 3 and 4, mice received 3 ip injections of either saline or 0.5 mg/kg nicotine, 8 hours apart. On day 5, mice were injected with 0.5 mg/kg nicotine and locomotion was immediately measured over a 30 min session using a computer-operated tracking system (Any-maze, Stoelting, Wood Dale, Il).
Basal anxiety-related behavior
Open field activity test
Drug-naïve mice were placed in the open field (clear Plexiglas, 40 × 40 × 40 cm) for 10 minutes. Total locomotor activity and distance moved in a center square (20 × 20 cm) were measured using a computer-operated Ethovision system (Noldus, Wageningen, The Netherlands; this system was also used in all of the other basal behavior experiments). The total/center distance moved ratio was taken as a measure of anxiety-like behavior (Paylor et al., 1998). One hour after the first exposure to the apparatus, mice were returned to the open field, but this time an unfamiliar plastic block was added to the center of the open field to measure response to novelty.
Light/dark box
This test was performed by placing the mouse in a cage (44 × 21 × 21 cm) that has two chambers: one chamber was bigger and lit, and the other was smaller and dark (Crawley and Goodwin, 1980). The animal was initially placed in the lit side, and transitions between sides and the time spent in each division were recorded for 10 min.
Elevated-plus maze
This maze consisted of four arms (25 × 7 cm, elevated 50 cm from the floor), of which two had high black walls (15 cm high), and two were without walls (Pellow et al., 1985). Mice were placed at the intersection between the arms, and the number of entries into all arms, as well as the time spent in the open arms was recorded for 5 minutes.
Statistical analysis
Data were examined by two-way ANOVA (genotype × treatment) followed by Duncan’s post-hoc comparisons, or by Student’s t test, when appropriate using the Statistica (Statsoft, Tulsa, OK) package.
RESULTS
Alpha 7 −/− mice show impaired MEC-precipitated nicotine withdrawal
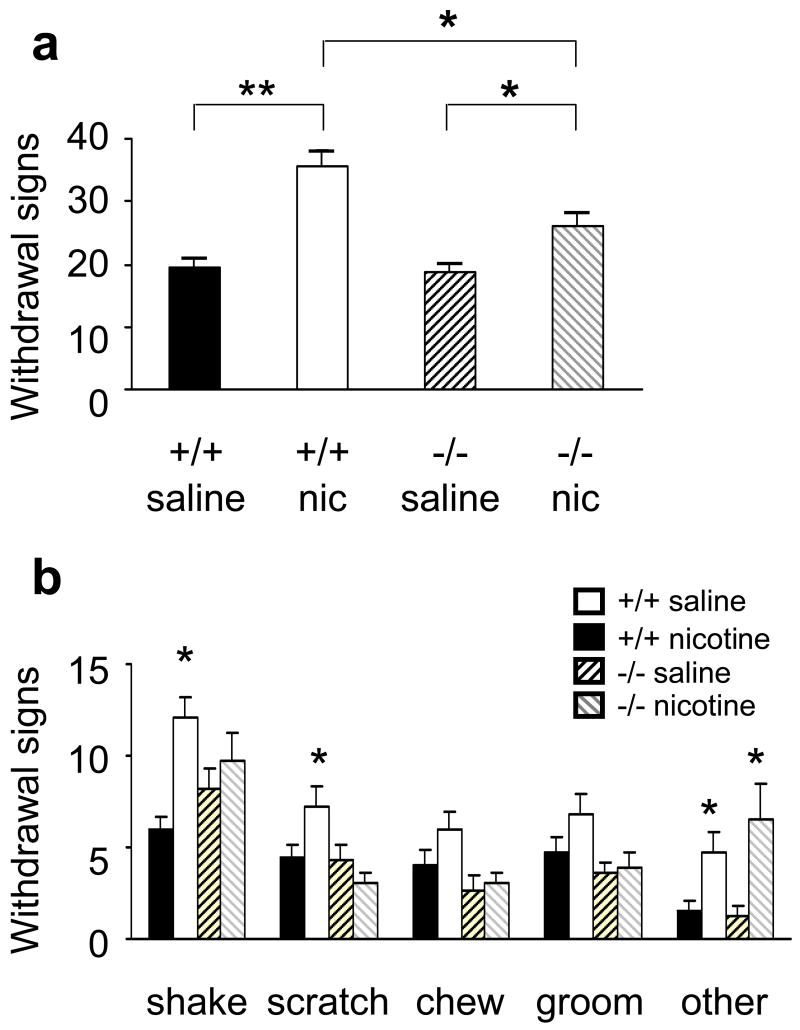
During the 20 minutes of observation after injection of 3 mg/kg MEC to precipitate withdrawal, control mice chronically treated with nicotine exhibited significantly more somatic signs of withdrawal than saline-treated control mice (Fig. 1a). The behavior of saline-treated α7 −/− mice was indistinguishable from that of saline-treated wild type mice. Although nicotine-treated α7 −/− mice showed significantly more signs than α7 −/− mice treated with saline, they displayed significantly less withdrawal signs than wild-type controls treated with nicotine (Fig 1a). There was a statistical effect of treatment (F1,75 = 22.9, p<0.01) and a significant effect of genotype (F1,75 = 4.45, p<0.05). In Duncan’s post hoc analysis, we found a significant difference between wild type mice treated with nicotine and mutant mice treated with nicotine (p<0.05).
Figure 1.
Decreased somatic signs of mecamylamine-induced nicotine withdrawal in α7 −/− mice. a) Total nicotine withdrawal signs in control mice treated with saline (+/+ saline, black bars, n=22) or nicotine (+/+ nic, white bars, n=31), α7 −/− mice treated with saline (−/− saline, dark striped bars n=15) or nicotine (−/− nic, light striped bars n=14), during the 20 min immediately after mecamylamine injection. b) Comparison of different withdrawal signs between wild type (black and white bars) and α7 −/− mice (striped bars). “Other” symptoms were wet dog shakes, head nods, and cage scratching. a)*p<0.05, **p<0.001; b) *p<0.05 vs. saline, same genotype.
When each of the four major withdrawal symptoms (shaking, grooming, scratching, and chewing) were studied separately, nicotine-treated α7 −/− mice had lower scores than nicotine-treated wild type mice in every case, except the less common “other” symptoms (cage scratching, head nodding and wet dog shakes). In the latter case, interestingly, α7 −/− mice were indistinguishable from control mice (Fig 1b).
In a separate experiment conducted on mice treated with nicotine for 13 days, withdrawal was precipitated with 6 mg/kg MEC. With this MEC dose, no significant differences were observed between the experimental groups (α7 +/+, 30 ± 2, n =14; α7 −/−, 27 ± 2, n=12; p>0.05, data not shown). One day later, the same mice were observed to make sure that behavioral signs had returned to pre-MEC levels. After that, the implants delivering nicotine were removed, and one day later withdrawal signs were observed for 20 min.. No difference between the two experimental groups was observed after spontaneous withdrawal (α7 +/+, 27 ± 2, n=14; α7 −/−, 32 ± 4, n=12; p>0.05, data not shown).
Alpha 7 −/− mice show normal MLA-precipitated nicotine withdrawal
In another series of experiments, withdrawal was precipitated with the injection of 7.5 mg/kg MLA. During the 20 minutes of observation after MLA injection, nicotine-treated wild type mice showed increased number of withdrawal signs. Interestingly, α7−/− mice also showed MLA-precipitated nicotine withdrawal (Fig 2a). When the different signs were analyzed, no differences were found between genotypes (Fig 2b). The behavior of saline-treated α7 −/− mice was indistinguishable from that of saline-treated wild type mice, indicating the MLA alone did not produce any obvious effect on mice not treated with nicotine.
Figure 2.
Normal MLA-induced nicotine withdrawal in α7 −/− mice. a) Total nicotine withdrawal signs in control mice treated with saline (+/+ saline, black bars, n=7) or nicotine (+/+ nic, white bars, n=6), α7−/− mice treated with saline (−/− saline, dark striped bars n=6) or nicotine (−/− nic, light striped bars n=6), during the 20 min immediately after MLA injection. b) Comparison of different withdrawal signs between wild type (black and white bars) and α7−/− mice (striped bars). “Other” symptoms were wet dog shakes, head nods, and cage scratching. *p<0.05 vs. saline, same genotype. No statistically significant differences were found between genotypes.
Alpha 7 −/− mice show normal tolerance to the hypolocomotor effect of nicotine
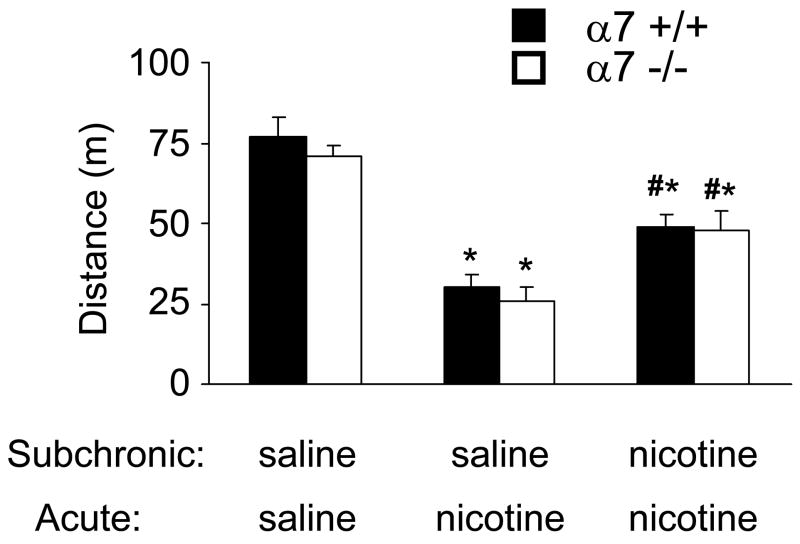
Wild type mice that received subchronic saline injections prior to behavioral testing showed significant hypolocomotion in the open field in response to acute nicotine administration (Fig 3). In contrast, wild type mice that received subchronic nicotine injections developed tolerance to the hypolocomotor effect of acute nicotine in the open field (Fig 3). As previously reported (Franceschini et al., 2002), the effects of acute nicotine were normal in α7 −/− mice. We now showed that the tolerance produced by subchronic injections of nicotine was similar in control and α7 −/− mice (Fig 3). ANOVA detected a clear effect of treatment (F2,49 = 50.8, p<0.01) but no effect of genotype (F1,49= 0.99, p>0.05) or interaction (F2,49 = 0.22, p>0.05).
Figure 3.
Normal tolerance to the hypolocomotor effect of nicotine in α7 −/− mice. Mice were injected for 3 days with saline or 0.5 mg/kg nicotine (Subchronic) and acutely with saline or 0.5 mg/kg nicotine (Acute) before behavioral testing. * p<0.05 vs. saline/saline for the same genotype, # p<0.05 vs. saline/nicotine for the same genotype. n= 7–11 per experimental group.
Alpha 7 −/− mice show normal basal anxiety-related behavior
In the open field, wild type and α7 −/− mice moved similar total distances and displayed similar center/total distance ratios (Fig 4a, b 1st trial). When novelty-induced exploration was examined by placing a plastic block in the center of the open field, the results again showed no difference between wild type and α7 −/− mice (Fig 4a, b 2nd trial). Similarly, the α7 null mutation had no effect on the behavior recorded in the elevated plus maze and light-dark box experiments (Fig 4c, d, e, and f).
Figure 4.
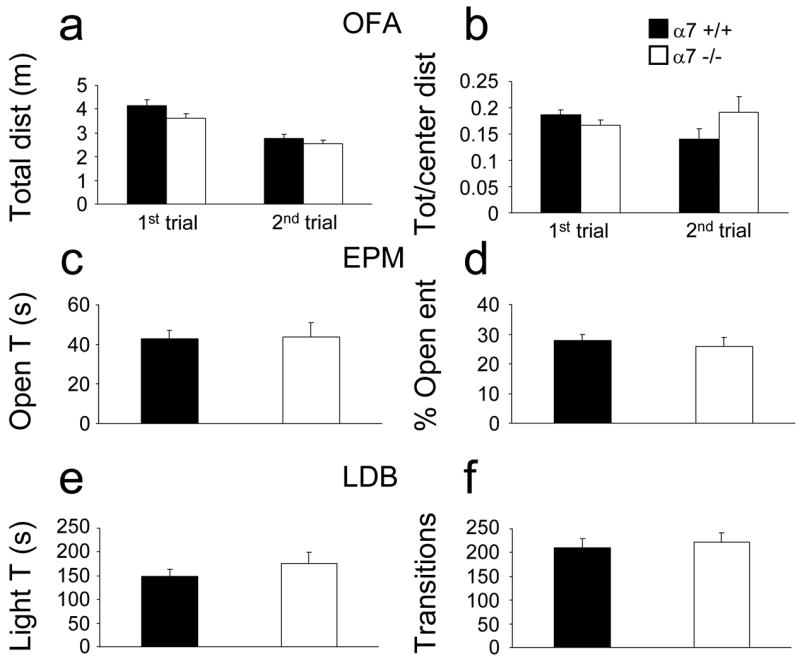
Normal anxiety-related behavior in α7 −/− mice. Mice (22 α7 +/+, 18 α7 −/−) were subjected to four anxiety related experiments. a) and b) Open field activity (OFA): total distance moved and center/total distance ratio, respectively. 1st trial, 10 minutes in the open field; 2nd trial, 10 min in the open field with a novel plastic block. c) and d) Elevated plus maze (EPM): time spent in open arms and percentage of entries in open arms, respectively. e) and f) Light/dark box (LDB): time in lit zone and total transitions between lit and dark sides, respectively. No statistically significant differences were found.
DISCUSSION
Prolonged nicotine use triggers neuroadaptations that contribute to the phenomena of dependence, tolerance, and reinforcement of nicotine use (Laviolette and van der Kooy, 2004). Withdrawal symptoms are among the major causes of failure when smokers try to quit (West et al., 1989), and therapeutic measures aimed at the minimization of withdrawal symptoms might aid smoking cessation.
In rodents, nAChR antagonists administered after long-term nicotine treatment precipitate the appearance of nicotine withdrawal symptoms. These include somatic signs such as shakes and increased grooming, as well as non-somatic signs such as hyperalgesia and anxiety-like responses (Damaj et al., 2003; David et al., 1994; Isola et al., 1999). MEC, which has a slightly higher selectivity for α3β4* nAChRs than for other α/β combinations and much lower affinity for α7* receptors (Papke et al., 2001), is the most effective nAChR antagonist at precipitating both somatic and non-somatic signs of withdrawal (Damaj et al., 2003; David et al., 1994; Malin et al., 1998).
Our data show that the lack of α7* nAChRs is sufficient to decrease the somatic signs of mecamylamine-induced withdrawal. It is important to note that this effect is only partial; while the number of somatic signs of withdrawal in nicotine-treated α7 −/− mice is significantly smaller than in wild type mice under nicotine withdrawal, it is also significantly higher than in nicotine-naïve mice. The decrease in withdrawal in α7 −/− mice is not as strong as that in β4 −/− mice, in which no evidence of withdrawal could be observed (Salas et al., 2004). It should also be noted that when a higher dose of MEC was used, no significant difference was observed between α7 +/+ and α7 −/− mice. This might point to the possibility that the lack of α7 receptors only shifts the dose response to MEC, but does not really preclude withdrawal. In addition, we found no difference between α7+/+ and α7 −/− mice during spontaneous withdrawal. These results could be due to the fact that spontaneous withdrawal produces fewer withdrawal signs at a given time compared to mecamylamine injections, making the identification of small differences in behavior harder. We also cannot exclude that the results on the spontaneous withdrawal were a consequence of the fact that the mice had undergone MEC-precipitated withdrawal two days earlier.
A recent report by Grabus and colleagues based on experiments with α7 −/− mice (Grabus et al., 2005) showed an effect of the α7 subunit in nicotine withdrawal-induced hyperalgesia but not on somatic signs. This discrepancy with our current observations could be explained by differences between the nicotine withdrawal protocols. While we used osmotic minipumps to deliver nicotine, and MEC or MLA to precipitate withdrawal, Grabus and colleagues used nicotine in the drinking water and spontaneous withdrawal. In fact, when we measured somatic withdrawal signs by removing the osmotic minipups, we did not find a significant difference between the genotypes.
The role of α7* receptors in nicotine withdrawal has been controversial even when investigated with nAChR-subtype specific drugs. For example, some investigators reported that the α7-specific blocker MLA does not induce somatic signs of nicotine withdrawal after prolonged nicotine exposure (Markou and Paterson, 2001), but a different group showed that MLA is indeed able to precipitate withdrawal (Damaj et al., 2003). Somewhat surprisingly, MLA-precipitated nicotine withdrawal was apparent in α7 −/− mice, at least when 7.5 mg/kg MLA are used to precipitate abstinence symptoms. We chose this concentration of MLA because it had been used before to precipitate nicotine withdrawal (Damaj et al., 2003), and because this and similar concentrations have been assumed in the in vivo rodent literature to be α7-specific (Chipana et al., 2006; Hildebrand et al., 1998; Markou and Paterson, 2001). Our results suggest that caution should be used when assigning roles to the α7 subunit based on MLA in vivo effects. In fact, in vitro experiments by other groups have already questioned the specificity of MLA. First, it was shown that MLA blocks two types of currents in ventral tegmental neurons in rats, and only one of these seems to be α7-mediated (Klink et al., 2001). In addition, MLA has been shown to block the α-conotoxin MII -sensitive and -insensitive components of nicotine-stimulated striatal synaptosomal dopamine release in mice, an effect not dependent on the α7 subunit (Salminen et al., 2004).
Tolerance is thought to play a crucial role in the development of drug abuse, by dampening sensitivity to repeated drug use with consequent enhancement of the negative emotional state during early withdrawal (Nestler, 2004). In the specific case of nicotine, however, the role of tolerance has been reported as non-related to withdrawal and quitting odds (Perkins et al., 2002). When we studied the influence of the α7 subunit on the development of tolerance to the hypolocomotor effect of nicotine, we did not find any difference between wild type and α7 −/− mice. This is in agreement with published data showing normal tolerance to nicotine in α7 −/− mice (Naylor et al., 2005). In that study, control and α7 −/− mice were trained to press levers under a schedule of food reinforcement. Acute injection of nicotine had a depressive effect on the number of lever presses, and this effect was diminished after repeated administration of nicotine. Control and α7 −/− mice showed similar tolerance to the effects of nicotine under those conditions. In addition, a dissociation between α7 nAChR and nicotine tolerance was first reported in a work describing the time course of tolerance to nicotine-induced seizures and nicotine-induced increases in α-bungarotoxin binding (an α7-specific toxin) in the hippocampus (Miner et al., 1988). In that report, it was shown that 2 days after chronic nicotine treatment was discontinued, α-bungarotoxin binding was back to normal but tolerance was still present.
Finally, we did not find any significant difference in anxiety-related behavior between wild type and α7 −/− mice. Deficits in this kind of behavior have been reported for several nAChR mutant mice (Booker et al., 2000; Labarca et al., 2001; Ross et al., 2000; Salas et al., 2003). Our current data suggest that the α7 subunit, despite its broad pattern of central expression, is not involved in anxiety-related behavior in mice.
In terms of potential implications for the development of anti-tobacco drugs, it should be noted that varenicline, a recently approved therapeutic agent to help quit tobacco, is marketed as a partial agonist at α4/β2 nAChRs (Coe et al., 2005) although it is a full agonist at α7* nAChRs, and a partial agonist at β4* nAChRs (Mihalak et al., 2006). Because we have shown that, at least in mice, the β4 and the α7, but not the β2 nAChR subunits are implicated in the somatic manifestations of nicotine withdrawal (Salas et al., 2004, and this work), it is tempting to speculate that varenicline’s effect on smoking behavior might be mediated not only by β2* but also by α7* and β 4* nAChRs. In particular, β2* nAChRs could be more important for the cognitive and affective aspects of withdrawal whereas α7* and β 4* nAChRs might be more important for the somatic manifestations of nicotine abstinence (see also Shoaib and Bizarro, 2005; Portugal et al., 2007). It must be noted, however, that SSR591813, another α4/β2 partial agonist with no measurable effect on α7 or β4-containing receptors, is also a potential aid to smoking cessation (Cohen et al., 2003). These data
In conclusion, we have shown that in mice, the α7 nAChR subunit influences the somatic signs of nicotine withdrawal, but not basal anxiety-like behaviors or tolerance to nicotine. In addition, we have shown a clear affect of MLA on α7 −/− mice, which raises questions about the specificity of this drug for the α7 subunit. These data can be of importance in the effort to develop new and more effective drugs to help smoking cessation.
Acknowledgments
We thank Tetyana Aleksenko and Fredalina Pieri for excellent technical support. This work was supported by a NIDA grant (DA017173) to MDB.
Abbreviations
- nAChRs
nicotinic acetylcholine receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. doi: 10.1016/s0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci. 2004;20:2737–2748. doi: 10.1111/j.1460-9568.2004.03714.x. [DOI] [PubMed] [Google Scholar]
- Booker TK, Tritto T, Colman J, Cui C, Collins ACFHS. The 20th Neuropharmacology Conference. Vol. 1. Elsevier Science; New Orleans: 2000. Beta3 subunits in regulation of anxiety: analysis of beta3 null mutant mice; p. 65. [Google Scholar]
- Chipana C, Camarasa J, Pubill D, Escubedo E. Protection against MDMA-induced dopaminergic neurotoxicity in mice by methyllycaconitine: involvement of nicotinic receptors. Neuropharmacology. 2006;51(4):885–895. doi: 10.1016/j.neuropharm.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Cohen C, Bergis OE, Galli F, Lochead AW, Jegham S, Biton B, Leonardon J, Avenet P, Sgard F, Besnard F, Graham D, Coste A, Oblin A, Curet O, Voltz C, Gardes A, Caille D, Perrault G, George P, Soubrie P, Scatton B. SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. J Pharmacol Exp Ther. 2003;306:407–420. doi: 10.1124/jpet.103.049262. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. The Journal of pharmacology and experimental therapeutics. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacology, Biochemistry & Behavior. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- David HM, Lake JR, Victoria AC, Cunningham JS, Kathleen MH, Delia LC, Owen BW. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology. 1994;V115:180–184. doi: 10.1007/BF02244770. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le Novere N, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Franceschini D, Orr-Urtreger A, Yu W, Mackey LY, Bond RA, Armstrong D, Patrick JW, Beaudet AL, De Biasi M. Altered barorreflex in αdeficient mice. Behavioral Brain Reseach. 2000;113:3–10. doi: 10.1016/s0166-4328(00)00195-9. [DOI] [PubMed] [Google Scholar]
- Franceschini D, Paylor R, Broide R, Salas R, Bassetto L, Gotti C, De Biasi M. Absence of alpha7-containing neuronal nicotinic acetylcholine receptors does not prevent nicotine-induced seizures. Brain Res Mol Brain Res. 2002;98:29–40. doi: 10.1016/s0169-328x(01)00309-6. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Imad Damaj M. Nicotine physical dependence in the mouse: involvement of the alpha7 nicotinic receptor subtype. European Journal of Pharmacology. 2005;515:90–93. doi: 10.1016/j.ejphar.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilstrèom B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Reviews of Physiology, Biochemistry and Pharmacology. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. 2003 147. [DOI] [PubMed] [Google Scholar]
- Isola R, Vogelsberg V, Wemlinger TA, Neff NH, Hadjiconstantinou M. Nicotine abstinence in the mouse. Brain Res. 1999;850:189–196. doi: 10.1016/s0006-8993(99)02131-9. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, Nashmi R, Kofuji P, Dang H, Shi W, Fidan M, Khakh BS, Chen Z, Bowers BJ, Boulter J, Wehner JM, Lester HA. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The motivational valence of nicotine in the rat ventral tegmental area is switched from rewarding to aversive following blockade of the alpha7-subunit-containing nicotinic acetylcholine receptor. Psychopharmacology (Berl) 2003;166:306–313. doi: 10.1007/s00213-002-1317-6. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Upchurch TP, Shenoi M, Rajan N, Schweinle WE. Nicotine abstinence syndrome precipitated by the competitive nicotinic antagonist dihydro-beta-erythroidine. Pharmacol Biochem Behav. 1998;60:609–613. doi: 10.1016/s0091-3057(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–373. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Miner LL, Collins AC. The effect of chronic nicotine treatment on nicotine-induced seizures. Psychopharmacology (Berl) 1988;95(1) doi: 10.1007/BF00212766. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Rodriguez-Sierra JF. A phenotype for the alpha 7 nicotinic acetylcholine receptor null mutant. Brain Res. 2004;1023:41–47. doi: 10.1016/j.brainres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Naylor C, Quarta D, Fernandes C, Stolerman IP. Tolerance to nicotine in mice lacking alpha7 nicotinic receptors. Psychopharmacology (Berl) 2005;180:558–563. doi: 10.1007/s00213-005-2187-5. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. The Journal of Neuroscience. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. Journal of Pharmacology & Experimental Therapeutics. 2001;297:646–656. [PubMed] [Google Scholar]
- Parrott AC. Nesbitt’s Paradox resolved? Stress and arousal modulation during cigarette smoking. Addiction. 1998;93:27–39. doi: 10.1046/j.1360-0443.1998.931274.x. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. α7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensimotor gating: a behavioral characterization of Acra7-deficient mice. Learning and Memory. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl) 2002;163:194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321:323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2007 doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. Journal of Neuroscience. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl) 2005;178:211–222. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor alpha 7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–371. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tucci SA, Genn RF, File SE. Methyllycaconitine (MLA) blocks the nicotine evoked anxiogenic effect and 5-HT release in the dorsal hippocampus: possible role of alpha7 receptors. Neuropharmacology. 2003;44:367–373. doi: 10.1016/s0028-3908(02)00391-x. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychological Medicine. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]