Abstract
Hemeoxygenase-2 (HO-2) is an antioxidant enzyme that can modulate recombinant maxi-K+ channels and has been proposed to be the acute O2 sensor in the carotid body (CB). We have tested the physiological contribution of this enzyme to O2 sensing using HO-2 null mice. HO-2 deficiency leads to a CB phenotype characterized by organ growth and alteration in the expression of stress-dependent genes, including the maxi-K+ channel α-subunit. However, sensitivity to hypoxia of CB is remarkably similar in HO-2 null animals and their control littermates. Moreover, the response to hypoxia in mouse and rat CB cells was maintained after blockade of maxi-K+ channels with iberiotoxin. Hypoxia responsiveness of the adrenal medulla (AM) (another acutely responding O2-sensitive organ) was also unaltered by HO-2 deficiency. Our data suggest that redox disregulation resulting from HO-2 deficiency affects maxi-K+ channel gene expression but it does not alter the intrinsic O2 sensitivity of CB or AM cells. Therefore, HO-2 is not a universally used acute O2 sensor.
INTRODUCTION
The carotid body (CB), a neural crest–derived organ, constitutes part of the homeostatic acute oxygen-sensing system required for mammalian adaptation to hypoxic environments and extrauterine life (Weir et al., 2005). Glomus cells are the chemoreceptor elements in the CB and sense hypoxemia through inhibition of membrane K+ channel activity. This leads to cell depolarization and Ca2+ channel opening, neurosecretion of transmitters, and activation of afferent nerve fibers signaling the brainstem respiratory center to evoke hyperventilation (López-Barneo et al., 1988, 2001; Weir et al., 2005). Whereas several K+ channel types regulated by O2 tension have been described in CB glomus cells of different mammalian species, the molecular mechanism underlying O2 sensing has been elusive (Prabhakar, 2000; López-Barneo et al., 2001). Recently, it was proposed that hemeoxygenase-2 (HO-2), an antioxidant enzyme that uses O2 to convert heme into biliverdin, iron, and carbon monoxide (CO) (Shibahara et al., 1985; Poss et al., 1995), could be an O2 sensor (Williams et al., 2004). HO-2 was found to coimmunoprecipitate with heterologously expressed maxi-K+ channels, and inhibition of this enzyme with siRNA abolished the O2 modulation of recombinant channels (Williams et al., 2004). Native maxi-K+ channels recorded in patches excised from glomus cells were activated by HO-2 substrates (heme and NADPH); thus it was proposed that HO-2 acts as an O2 sensor through the production of CO (Hoshi and Lahiri, 2004; Williams et al., 2004), which is by itself a maxi-K+ channel activator (Wang and Wu, 1997). Given the broad biological relevance of acute O2 sensing and its possible relationship with redox regulation (Weir et al., 2005), we considered of major interest to evaluate the actual physiological role of HO-2 in O2 homeostasis. Mice strains with genetic deficiencies have previously been used to study the mechanisms of O2 sensing (Archer et al., 1999; Fu et al., 2000; Piruat et al., 2004), so we tested to see whether O2 sensitivity of acutely responding chemoreceptor organs was altered in the HO-2 knockout mouse (Poss et al., 1995). We show that although HO-2 null animals have a previously unnoticed molecular and morphological phenotype affecting the CB, they exhibit a completely normal acute cellular responsiveness to hypoxia.
MATERIALS AND METHODS
Animals
Hemeoxygenase-2−/− mice in C57BL-6J background were a gift of J. Davis Clark (Stanford University, Stanford, CA). HO-2−/− mice were crossed with C57BL-6J to generate heterozygous animals. HO-2+/− were crossed and the progeny genotyped by two independent PCRs, using the following primers: wild-type allele (370 bp), 5′-TTCATAGCCATCTGTAGTGA-3′ and 5′-ATACTTCATGTCCTTGATCA-3′; mutant allele (287 bp), 5′-CCCGGTTCTTTTTGTCAAGA-3′ and 5′-CGATGTTTCGCTTGGTGGTC-3′. Animal care and experimentation were according to the institutional animal care committee guidelines.
Quantitative RT-PCR
Four groups of five to seven animals for every genotype were killed by chloral hydrate overdose (i.p.) and the carotid bodies were dissected, pooled, and stored in liquid nitrogen. mRNA was extracted using Dynabeads mRNA DIRECT micro kit (Dynal). First strand cDNA was synthesized from total mRNA extraction using the SuperscriptTM first strand synthesis system for reverse transcriptase PCR (Invitrogen). PCR amplifications of HO-2 and GAPDH mRNAs were performed using the following primers: HO-2 (Hmox2, 430 bp), 5′-ACTACTCAGCCACAATGTCT-3′ and 5′-GTGAATCCGATCCACATACT-3′; GAPDH (255 bp), 5′-CAAAATGGTGAAGGTCGGTGTG-3′ and 5′-TTTGATGTTAGTGGGGTCTCGC-3′.
Real-time PCR was performed in an ABI Prism 7500 Sequence Detection System (Applied Biosystems) using SYBR Green PCR Master mix (Applied Biosystems) and the thermocycler conditions recommended by the manufacturer. PCRs were performed in triplicate in a total volume of 25 μl containing 0.2 or 0.5 μl of the reverse transcriptase reaction. Each sample was analyzed for β-actin to normalize for RNA input amounts and to perform relative quantifications. Primers were designed using the computer program Primer Express (Applied Biosystems). The following primers were used: β-actin (Actb, 75 bp), 5′-GGCCCAGAGCAAGAGAGGTA-3′ and 5′-CATGTCGTCCCAGTTGGTAACA-3′; cyclophilin A (Ppia, 75 bp), 5′-ATGGCAAATGCTGGACCAAA-3′ and 5′-TGCCATCCAGCCATTCAGT-3′; tyrosine hydroxylase (Th, 75 bp), 5′-GGCTTCTCTGACCAGGCGTAT-3′ and 5′-GCTCACCCTGCTTGTATTGGA-3′; maxi K+ channel α subunit (Kcnma1, 76 bp), 5′-CATGGCTTTCAACGTGTTCTTC-3′ and 5′GCCAGAACCACAGCTTATCATT-3′. Melting curve analysis showed a single sharp peak with the expected Tm for all samples.
Amperometric Recording of Single Cell Catecholamine Secretion in Slices
Carotid body slices were used because the most reproducible single glomus cell responses to hypoxia are obtained in this preparation (Pardal and López-Barneo, 2002). Mice carotid body dissection, slicing, and culture, as well as the measurement of catecholamine secretion were performed following the same procedures described previously (Piruat et al., 2004). Slices from adrenal medulla were done using the carotid body protocol and recorded 1–4 h after slicing. Slices were transferred to a recording chamber and continuously perfused with a solution containing (in mM) 117 NaCl, 4.5 KCl, 23 NaHCO3, 1 MgCl2, 2.5 CaCl2, 5 glucose, and 5 sucrose. The “normoxic” solution was bubbled with a gas mixture of 5% CO2, 20% O2, and 75% N2 (O2 tension ∼145 mm Hg). The “hypoxic” solution was bubbled with 5% CO2 and 95% N2 to reach an O2 tension in the chamber of ∼15 mm Hg. To perform dose–response curves, the solutions were also bubbled with 12% and 6% O2, keeping CO2 at 5% and decreasing proportionally the amount of N2. When these solutions were used, the approximate values of O2 tension in the chamber were, respectively, 90 and 50 mm Hg. All the experiments were made at a temperature in the chamber of ∼36°C. The cumulative secretion signal is the sum of the time integral of successive amperometric events (Pardal and López-Barneo, 2002). Secretion rate (femtocoulombs [fC]/min) was calculated as the amount of charge transferred to the recording electrode during a given time period. Iberiotoxin (150–300 nM) was directly dissolved into the standard external solution.
Patch Clamp Recordings
Macroscopic and single-channel K+ currents were recorded from dispersed mouse glomus cells using either the whole-cell or inside-out excised patch configurations of the patch clamp technique as adapted to our laboratory (López-Barneo et al., 1988; Navarro-Antolín et al., 2005). Preparation of dispersed mouse CB cells was performed as described in Piruat et al. (2004). For macroscopic K+ current experiments, solution composition was as follows (in mM): internal, 80 potassium glutamate, 50 KCl, 1 MgCl2, 10 HEPES, 4 MgATP, 0.1 EGTA, pH 7.2; external, 117 NaCl, 4.5 KCl, 23 NaCO3H, 1 MgCl2, 2.5 CaCl2, 5 glucose, 5 sucrose, pH 7.4. Holding potential was −80 mV. For single channel current experiments, solution composition was as follows (in mM): internal (bath), 150 KCl, 5 HEDTA, 10 HEPES, adjusted to either 5 or 1.5 μM free Ca2+, pH 7.2; external (pipette), 150 KCl, 5 HEDTA, 10 HEPES, adjusted to 5 μM free Ca2+, pH 7.2.
Immunocytochemistry and Morphological Studies
For every genotype, three to five animals were killed by chloral hydrate overdose (i.p.), perfused with PBS and 4% paraformaldehyde in PBS, and the carotid bifurcations were dissected, washed with PBS, fixed 1 h at 4°C in 4% paraformaldehyde, and equilibrated for 12 h in a 30% sucrose solution. Bifurcations were included in OCT and snap-frozen by quenching in liquid nitrogen. Slices 10 μm thick were cut with a cryostat. Sections were stained with either the anti–HO-1 monoclonal antibody (1:1,000; Stressgen) or the anti-TH (tyrosine hydroxylase) polyclonal antibody (1:1,000; Pel-Freez). Signal was detected using a fluorescent secondary antibody (anti–rabbit IgG Alexa 568, 1:700 [Molecular Probes] and an anti–mouse fluorescein-conjugated IgG, 1:200; Pierce Chemical Co.). Images were acquired under an Olympus Bx-61 microscope and carotid body surfaces measured with the Image J program (NIH).
Statistical Analysis
Unless otherwise specified, data are expressed as mean ± SEM with the number (n) of experiments indicated. Statistical analysis was performed by unpaired Student's t test. A value of P < 0.05 was considered as statistically significant.
RESULTS
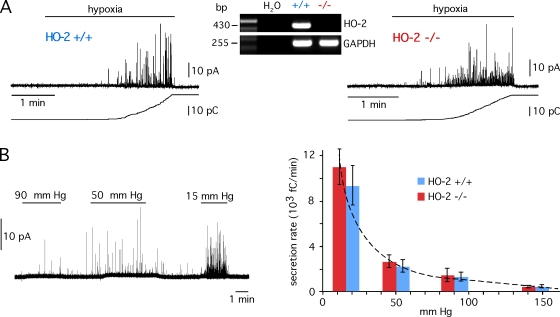
We have monitored responsiveness of CB glomus cells to hypoxia in animals with deletion of the HO-2 gene and control littermates (Fig. 1 A). CB thin slices (Pardal and López-Barneo, 2002; Piruat et al., 2004) were used to study the intrinsic O2 sensitivity of intact glomus cells separately from the other steps along the chemosensory pathway involved in the hypoxic ventilatory response. Catecholamine release (in femtocoulombs/min) from individual glomus cells subjected to low O2 tension (PO2 ∼ 15 mm Hg) (10442 ± 1139, mean ± SEM, n = 15) was unaltered in HO-2 null mice (9893 ± 1413, n = 13) (Fig. 1 A). The dose–response relation obtained from cells exposed to different PO2 levels almost perfectly matched the characteristic hyperbolic correlation between arterial PO2 and glomus cell cytosolic Ca2+ concentration or the afferent discharges of the CB sinus nerve (see Weir et al., 2005). Dose–response curves were indistinguishable in HO-2–deficient and wild-type mice, which rules out the existence of any subtle difference in O2 sensitivity between the two animal types (Fig. 1 B).
Figure 1.
Responsiveness of carotid body glomus cells to acute hypoxia in hemeoxigenase-2 null mice. (A) Amperometric recordings and corresponding cumulative secretion signals (in pC) of catecholamine release induced by hypoxia (∼15 mm Hg) carotid body glomus cells from control (HO-2+/+) and HO-2–deficient (HO-2−/−) mice. Inset, RT-PCR analysis showing the absence of HO-2 expression in the carotid body of HO-2 null mice. (B, left) Catecholamine secretion in an HO-2–deficient glomus cell exposed to solutions equilibrated with various levels of O2 tension. Control O2 tension was 145 mm Hg. (B, right) Dose–response curves estimated from glomus cells of HO-2 null mice and control littermates. Each data point is the mean ± SEM of six to eight experiments. The dotted line has been fitted by eye. The average secretion rate values in HO-2+/+ and HO-2−/− glomus cells at each O2 tension were not significantly different (P > 0.05).
The persistence of acute O2 sensing in HO-2 null mice was confirmed in neonatal adrenal medulla (AM) slices, another chemoreceptor organ (Seidler and Slotkin, 1985; Thompson et al., 1997). For unknown reasons, basal secretion rate showed a great variability among AM cells (range from 20 to 5,500 fC/min, n = 4, in HO-2+/+ and from 116 to 1336 fC/min, n = 3, in HO-2−/− mice). However, as shown in Fig. 2 (A and B), hypoxia (PO2 ∼ 15 mm Hg) induced in both HO-2+/+ and HO-2−/− chromaffin cells a quite similar secretory response, characterized by a respective average increase of 10.1- and 10.0-fold the basal secretion rate. These data strongly suggest that acute responsiveness to low PO2 in AM cells of HO-2–deficient animals was essentially unaltered.
Figure 2.
Responsiveness to acute hypoxia of neonatal (P2) adrenal medulla chromaffin cells in control and hemeoxygenase-2 null mice. (A, top) Typical amperometric recording of catecholamine release induced by hypoxia and corresponding cumulative secretion signal in a HO-2+/+ glomus cell. (A, bottom) Secretion rate in basal conditions (O2 tension, 145 mm Hg) and in hypoxia (O2 tension, ∼15 mm Hg). Statistical significance, P < 0.05, n = 4 HO-2+/+ cells. (B, top) Typical amperometric recording of catecholamine release induced by hypoxia and corresponding cumulative secretion signal in an HO-2−/− glomus cell. (B, bottom) Secretion rate in basal conditions (O2 tension, 145 mm Hg) and in hypoxia (O2 tension, ∼15 mm Hg). Statistical significance, P < 0.05, n = 3 HO-2−/− cells.
HO-2 null mice developed normally, without alteration in hematocrit (46 ± 0.9 for HO-2+/+, n = 14; 44 ± 1 for HO-2−/−, n = 15) or signs of respiratory distress during the first postnatal 2–3 mo. However, we investigated whether even in early life HO-2 deficiency causes any CB phenotypic alteration since this enzyme, constitutively expressed in most tissues, including neurons (Maines, 1997; Doré, 2002) and CB glomus cells (Williams et al., 2004), appears to have an important antioxidant role (Doré et al., 1999, 2000), and HO-2 knockout animals manifest cardio-respiratory alterations at advanced age (Adachi et al., 2004). HO-2 null young adults (<3 mo) showed a marked up-regulation of cyclophilin (Cyc) and tyrosine hydroxylase (TH), the rate-limiting enzyme for catecholamine synthesis highly expressed in CB glomus cells (Fig. 3 A). The mRNA level of hemeoxygenase 1 (HO-1), an inducible hemeoxygenase, appeared to be also slightly increased. In contrast, CB Slo1 mRNA (the maxi-K+ channel α-subunit gene) was significantly down-regulated in HO-2 null mice with respect to controls (Fig. 3 A). These alterations in the CB gene expression profile, as shown above unrelated with the mechanisms of CB O2 sensing, were probably consequence of a subclinical cellular oxidative stress (Kroll and Czyzyk-Krzeska, 1998; Jin et al., 2000), which could also be responsible for the CB growth observed in HO-2 null animals. As illustrated by the representative example in Fig. 3 B, in HO-2−/− animals (n = 5), CB volume increased from 39 ± 5 × 105 μm3 (control HO-2+/+, n = 3) to 81 ± 6 × 105 μm3. Although HO-1 mRNA increase in CB of HO-2–deficient animals was not statistically significant, we tested for changes in the protein levels of this inducible enzyme. Within the CB (a highly vascularized organ), HO-1 was, as expected, confined to blood vessels and, even in HO-2−/− animals, it seemed to be absent from the clusters (glomeruli) of TH+ glomus cells (Fig. 3 C).
Figure 3.
Carotid body phenotype in HO-2 null mice. (A) Quantitative changes in mRNA expression of cyclophilin (Cyc) tyrosine hydroxylase (TH), heme-oxygenase-1 (HO-1), and maxi-K+ channel α-subunit (Slo1) genes in the carotid body of HO-2–deficient animals with respect to control littermates (n = 6 measurements from four experiments). Statistically significant changes are indicated by asterisks. (B) Carotid body growth in young HO-2 null animals. The bar diagram indicates the number and surface area of sections in the carotid bifurcation that contained carotid body tissue in HO-2 null mice and control littermates. The photographs at the inset illustrate representative sections with the carotid body area surrounded by a white dashed line. (C) Expression of HO-1 and TH in the carotid artery bifurcation of HO-2 null mice. (C, left) Immunoreactivity against HO-1 localized in the large blood vessels and in small arteries inside the carotid body. (C, right) Immunostaining of TH+ glomus cell clusters in the carotid body. The white dashed lines indicate the area within the CB section occupied by medium-sized blood vessels. In B and C the internal (IC) and external (EC) carotid arteries are indicated.
As in other mammalian species, mouse glomus cells expressed the maxi-K+ channel gene (Fig. 3 A), and in consequence single maxi-K+ channel activity was readily recorded in patches excised from cultured CB cells (Fig. 4). Maxi-K+ channels of both, HO-2+/+ and −/− glomus cells, were modulated by intracellular Ca2+ (Fig. 4 A), and single-channel current amplitude and slope single-channel conductance were similar in the two animal types (Fig. 4, B and C). In symmetrical high K+ solutions, single-channel current amplitudes at +40 mV were 13.1 ± 0.18 pA (n = 11) for HO-2+/+ and 13.0 ± 0.13 pA (n = 9) for HO-2−/− animals. In these same experiments, average slope conductances were, respectively, 317 and 308 pS (see Fig. 4 C). Although not studied in detail, we observed, however, a trend toward a smaller number of channels per patch in HO-2−/− glomus cells compared with control wild type, which is consistent with the decrease of the maxi-K+ channel gene expression in HO-2–deficient animals (see Fig. 3 A).
Figure 4.
Maxi-K+ channel activity in mouse carotid body glomus cells. (A) Modulation of maxi-K+ channel activity by internal Ca2+ in HO-2+/+ and HO-2−/− glomus cells. Depolarizing pulses from 0 to 40 mV. (B and C) Single channel current–voltage relationships for maxi-K+ channels from HO-2+/+ and HO-2−/− glomus cells. Depolarizing pulses from 0 mV to the indicated membrane potentials. Internal [Ca2+], 5 μM. Note that current amplitude and slope conductance are similar for the two channel types and that the number of simultaneous channels openings in HO-2+/+ cells is higher than in HO-2−/− cells.
In accord with the single channel data, a component of the macroscopic K+ current recorded in dialyzed cells was inhibited (by 15.7 ± 2.7% at Vm = +20 mV, n = 6) after addition of the selective maxi-K+ blocker iberiotoxin (150–300 nM) (Fig. 5, A and B). In intact mouse CB glomus cells, iberiotoxin failed to prevent a strong secretory response upon subsequent exposure to low PO2. A similar lack of effect of iberiotoxin was observed in rat glomus cells, despite O2-sensitive maxi-K+ channels have been reported in this animal species (Wyatt and Peers, 1995; Williams et al., 2004) (Fig. 5 C).
Figure 5.
Maintenance of the secretory response to hypoxia after blockade of maxi-K+ channels with iberiotoxin. (A) Reversible inhibition of macroscopic K+ currents by iberiotoxin (Ibtx, 300 nM) in a dyalized mouse glomus cell. Membrane depolarization to +20 mV from −80 mV. (B) Average peak outward current–voltage relationship in control conditions and 5 min after addition of Ibtx (150–300 nM, mean ± SD, n = 6). (C, top) Secretory response to hypoxia (∼15 mm Hg) in an HO-2−/− mouse glomus cell in the presence of Ibtx (300 nM). Secretion rate during hypoxia was, respectively, 15,075 and 14,440 fC/min before and during exposure to Ibtx. (C, bottom) Secretory response to hypoxia (∼15 mm Hg) in a rat glomus cell in the presence of Ibtx (300 nM). Secretion rate during hypoxia was, respectively, 3,300 and 3,740 fC/min before and during exposure to Ibtx.
DISCUSSION
We have shown that the lack of HO-2, a ubiquitously expressed and powerful antioxidant enzyme, produces a notable CB phenotype, characterized by hypertrophy and alteration in the level of expression of some stress-dependent genes (including down-regulation of the maxi-K+ channel α-subunit). More importantly, we have observed that acute O2 sensing by neurosecretory CB or AM cells is practically unaltered in HO-2−/− animals.
Regarding the molecular phenotype observed in the CB of HO-2 null animals, we tested for a possible compensatory up-regulation of HO-1, an inducible hemeoxygenase. It has been shown that HO-1 is normally expressed in vascular smooth muscle and barely represented in neural tissue, so it does not prevent the deleterious effects of HO-2 deficiency in an ischemic/reperfusion stroke animal model (Maines, 1997; Doré, 2002). In accord with these data, the expression of HO-1, slightly up-regulated in CB tissue of HO-2–deficient mice, was restricted to the CB blood vessel wall and was nearly absent from the clusters of glomus cells.
The decreased expression of maxi-K+ channels in the CB of young HO-2 null animals, a phenomenon likely accentuated with animal age, resembles the reduction of K+ current amplitude observed in glomus cells from the hypertrophic CB of chronically hypoxic animals (Wyatt et al., 1995). Interestingly, decrease of glomus cell maxi-K+ current amplitude and CB hypertrophy have also been observed in mouse with partial deletion of the SDHD gene (Piruat et al., 2004; López-Barneo et al., 2006), a component of succinate dehydrogenase in mitochondrial complex II whose mutation produces CB tumors in humans (Baysal et al., 2000). Thus, our experiments might indicate that CB hypertrophy can occur without major alteration of the chemosensitive properties of the organ. The fact that CB glomus cells respond to hypoxia in the presence of iberiotoxin strongly suggest that maxi-K+ channels, although contributing to the membrane potential of rat glomus cells (Wyatt and Peers, 1995; Pardal et al., 2000), do not appear to be absolutely necessary for the hypoxic excitation of either mouse (wild type or HO-2 null) or rat CB cells.
Besides in the long-term regulation of some stress-dependent genes, HO-2 activity could also play an important regulatory role in CB cells by decreasing intracellular heme concentration and, thus, mitigate heme inhibition of maxi-K+ channel activity (Tang et al., 2003; López-Barneo and Castellano, 2005). Nevertheless, HO-2 deficiency does not alter the exquisite intrinsic O2 responsiveness of CB or AM cells, and, therefore, HO-2 does not seem to be a universal acute O2 sensor. Naturally, it must kept in mind that several O2-sensitive K+ channels, with interspecies differences, have been reported in rodent CB glomus cells (see López-Barneo et al., 2001), and these channels could be up-regulated in HO-2–deficient animals. In mouse glomus cells, for example, voltage-dependent channels of the Kv3 subfamily have been proposed to be O2 sensitive (Pérez-Garcia et al., 2004). Our results do, however, support the idea that HO-2 is unlikely to mediate regulation by O2 of whichever K+ channels are important for acute hypoxia sensitivity in mouse CB or AM cells.
Acknowledgments
We thank Dr. J.D. Clark for the generous gift of the HO-2 null mice.
A. Pascual is an investigator of the “Ramón y Cajal” program. J. López-Barneo received the “Ayuda a la Investigación 2000” of the Juan March Foundation. Research was supported by the Spanish Ministry of Health, the Lilly Foundation, and the Andalusian Government.
Olaf S. Andersen served as editor.
P. Ortega-Sáenz and A. Pascual contributed equally to this work.
Abbreviations used in this paper: AM, adrenal medulla; CB, carotid body; HO-2, hemeoxygenase-2; TH, tyrosine hydroxylase.
References
- Archer, S.L., H.L. Reeve, E. Michelakis, L. Puttagunta, R. Waite, D.P. Nelson, M.C. Dinauer, and E.K. Weir. 1999. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc. Natl. Acad. Sci. USA. 96:7944–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi, T., K. Ishikawa, W. Hida, H. Matsumoto, T. Masuda, F. Date, K. Ogawa, K. Takeda, K. Furuyama, Y. Zhang, et al. 2004. Hypoxemia and blunted hypoxic ventilatory response in mice lacking heme-oxygenase-2. Biochem. Biophys. Res. Commun. 320:514–522. [DOI] [PubMed] [Google Scholar]
- Baysal, B.E., R.E. Ferrell, J.E. Willett-Brozick, E.C. Lawrence, D. Myssiorek, A. Bosch, A. van der Mey, P.E. Taschner, W.S. Rubinstein, E.N. Myers, et al. 2000. Mutations in SDHD, a mitochondrial complex II gene in hereditary paraganglioma. Science. 287:848–851. [DOI] [PubMed] [Google Scholar]
- Doré, S. 2002. Decreased activity of the antioxidant heme oxygenase enzyme: implications in ischemia and in Alzheimer's disease. Free Radic. Biol. Med. 32:1276–1282. [DOI] [PubMed] [Google Scholar]
- Doré, S., M. Takahashi, C.D. Ferris, R. Zakhary, L.D. Hester, D. Guastella, and S.H. Snyder. 1999. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl. Acad. Sci. USA. 96:2445–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré, S., S. Goto, K. Sampei, S. Blackshaw, L.D. Hester, T. Ingi, A. Sawa, R.J. Traystman, R.C. Koehler, and S.H. Snyder. 2000. Heme oxygenase-2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neuroscience. 99:587–592. [DOI] [PubMed] [Google Scholar]
- Fu, X.W., D. Wang, C.A. Nurse, M.C. Dinauer, and E. Cutz. 2000. NADPH oxidase is an O2 sensor in airway chemoreceptors: evidence from K+ current modulation in wild-type and oxidase-deficient mice. Proc. Natl. Acad. Sci. USA. 97:4374–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi, T., and S. Lahiri. 2004. Oxygen sensing: it's a gas! Science. 306:2050–2051. [DOI] [PubMed] [Google Scholar]
- Jin, Z.G., M.G. Melaragno, D.F. Liao, C. Yan, J. Haendeler, Y.A. Suh, J.D. Lambeth, and B.C. Berk. 2000. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ. Res. 87:789–796. [DOI] [PubMed] [Google Scholar]
- Kroll, S.L., and M.F. Czyzyk-Krzeska. 1998. Role of H2O2 and heme-containing O2 sensors in hypoxic regulation of tyrosine hydroxylase gene expression. Am. J. Physiol. 274:C167–C174. [DOI] [PubMed] [Google Scholar]
- López-Barneo, J., J.R. López-López, J. Ureña, and C. González. 1988. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 241:580–582. [DOI] [PubMed] [Google Scholar]
- López-Barneo, J., R. Pardal, and P. Ortega-Sáenz. 2001. Cellular mechanisms of oxygen sensing. Annu. Rev. Physiol. 63:259–287. [DOI] [PubMed] [Google Scholar]
- López-Barneo, J., and A. Castellano. 2005. Multiple facets of maxi-K+ channels: the heme connection. J. Gen. Physiol. 126:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo, J., P. Ortega-Saenz, J.I. Piruat, and M. Garcia-Fernandez. 2006. Oxygen-sensing by ion channels and mitochondrial function in carotid body glomus cells. Novartis Found. Symp. 272:54–64. [DOI] [PubMed] [Google Scholar]
- Maines, M.D. 1997. The hemo oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37:517–554. [DOI] [PubMed] [Google Scholar]
- Navarro-Antolín, J., C. Levitsky, E. Calderón, A. Ordoñez, and J. López-Barneo. 2005. Decreased expression of maxi-K+ channel β1-subunit and altered vasoregulation in hypoxia. Circulation. 112:1309–1315. [DOI] [PubMed] [Google Scholar]
- Pardal, R., U. Ludewig, J. García-Hirschfeld, and J. López-Barneo. 2000. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc. Natl. Acad. Sci. USA. 97:2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal, R., and J. López-Barneo. 2002. Low glucose-sensing cells in the carotid body. Nat. Neurosci. 5:197–198. [DOI] [PubMed] [Google Scholar]
- Pérez-García, M.T., O. Colinas, E. Miguel-Velado, A. Moreno-Domínguez, and J.R. López-López. 2004. Characterization of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen sensing. J. Physiol. 557:457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piruat, J.I., C.O. Pintado, G.P. Ortega-Saenz, M. Roche, and J. Lopez-Barneo. 2004. Mitochondrial SDHD-deficient mice show persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol. Cell. Biol. 24:10933–10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss, K.D., M.J. Thomas, A.K. Ebralidze, T.J. O'Dell, and S. Tonegawa. 1995. Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron. 15:867–873. [DOI] [PubMed] [Google Scholar]
- Prabhakar, N.R. 2000. Oxygen sensing by the carotid body chemoreceptors. J. Appl. Physiol. 88:2287–2295. [DOI] [PubMed] [Google Scholar]
- Seidler, F.J., and T.A. Slotkin. 1985. Adrenomedullary function in the neonatal rat: responses to acute hypoxia. J. Physiol. 358:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara, S., R. Muller, H. Taguchi, and T. Yoshida. 1985. Cloning and expression of cDNA for rat heme oxygenase. Proc. Natl. Acad. Sci. USA. 82:7865–7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X.D., R. Xu, M.F. Reynolds, M.L. Garcia, S.H. Heinemann, and T. Hoshi. 2003. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 425:531–535. [DOI] [PubMed] [Google Scholar]
- Thompson, R.J., A. Jackson, and C.A. Nurse. 1997. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J. Physiol. 498:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R., and L. Wu. 1997. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J. Biol. Chem. 272:8222–8226. [DOI] [PubMed] [Google Scholar]
- Weir, E.K., J. López-Barneo, K.J. Buckler, and S.L. Archer. 2005. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 353:2042–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, S.E., P. Wootton, H.S. Mason, J. Bould, D.E. Iles, D. Riccardi, C. Peers, and P.J. Kemp. 2004. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 306:2093–2097. [DOI] [PubMed] [Google Scholar]
- Wyatt, C.N., and C. Peers. 1995. Ca2+-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J. Physiol. 483:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt, C.N., C. Wright, D. Bee, and C. Peers. 1995. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc. Natl. Acad. Sci. USA. 92:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]