Abstract
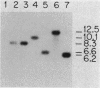
Genes involved in the production of phaseolotoxin by Pseudomonas syringae pv. "phaseolicola" NPS3121 were identified by Tn5 mutagenesis and cosmid cloning. A total of 5,180 kanamycin-resistant colonies were screened for the loss of phaseolotoxin production by a microbiological assay. Six independent, prototrophic, Tox- mutants were isolated that had Tn5 insertions in five different EcoRI fragments. All six mutants had Tn5 inserted in the same KpnI fragment, which had a length of ca. 28 kilobases including Tn5. The mutants produced residual toxin in vitro. An EcoRI fragment containing Tn5 and flanking sequences from mutant NPS4336 was cloned and used to probe a wild-type genomic library by colony hybridization. Seven recombinant plasmids showing homology to this probe were identified. Each Tox- mutant was restored in OCTase-specific toxin production by two or more of the recombinant plasmids. The data suggest that at least some of the genes involved in phaseolotoxin production were clustered in a large KpnI fragment. No homology was detected between the Tn5 target fragment cloned from mutant NPS4336 and the total genomic DNA from closely or distantly related bacteria that do not produce phaseolotoxin.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiale M. S., Mills D. Integration and partial excision of a cryptic plasmid in Pseudomonas syringae pv. phaseolicola. J Bacteriol. 1982 Nov;152(2):797–802. doi: 10.1128/jb.152.2.797-802.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Gantotti B. V., Patil S. S., Mandel M. Apparent Involvement of a Plasmid in Phaseotoxin Production by Pseudomonas phaseolicola. Appl Environ Microbiol. 1979 Mar;37(3):511–516. doi: 10.1128/aem.37.3.511-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Mitchell R. E., Bieleski R. L. Involvement of phaseolotoxin in halo blight of beans: transport and conversion to functional toxin. Plant Physiol. 1977 Nov;60(5):723–729. doi: 10.1104/pp.60.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. S. Inhibition of Ornithin Carbamyl Transferase from Bean Plants by the Toxin of Pseudomonas phaseolicola. Plant Physiol. 1970 Nov;46(5):752–753. doi: 10.1104/pp.46.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. S., Tam L. Q. Mode of Action of the Toxin from Pseudomonas phaseolicola: I. Toxin Specificity, Chlorosis, and Ornithine Accumulation. Plant Physiol. 1972 May;49(5):803–807. doi: 10.1104/pp.49.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. S., Youngblood P., Christiansen P., Moore R. E. Phaseotoxin A: an antimetabolite from Pseudomonas phaseolicola. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1019–1027. doi: 10.1016/0006-291x(76)90474-5. [DOI] [PubMed] [Google Scholar]
- Staskawicz B. J., Dahlbeck D., Keen N. T. Cloned avirulence gene of Pseudomonas syringae pv. glycinea determines race-specific incompatibility on Glycine max (L.) Merr. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6024–6028. doi: 10.1073/pnas.81.19.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B. J., Panopoulos N. J., Hoogenraad N. J. Phaseolotoxin-insensitive ornithine carbamoyltransferase of Pseudomonas syringae pv. phaseolicola: basis for immunity to phaseolotoxin. J Bacteriol. 1980 May;142(2):720–723. doi: 10.1128/jb.142.2.720-723.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B. J., Panopoulos N. J. Phaseolotoxin transport in Escherichia coli and Salmonella typhimurium via the oligopeptide permease. J Bacteriol. 1980 May;142(2):474–479. doi: 10.1128/jb.142.2.474-479.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo L. J., Mills D. Characterization of eight excision plasmids of Pseudomonas syringae pv. phaseolicola. Mol Gen Genet. 1984;195(1-2):90–95. doi: 10.1007/BF00332729. [DOI] [PubMed] [Google Scholar]
- Szabo L. J., Mills D. Integration and excision of pMC7105 in Pseudomonas syringae pv. phaseolicola: involvement of repetitive sequences. J Bacteriol. 1984 Mar;157(3):821–827. doi: 10.1128/jb.157.3.821-827.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]