Abstract
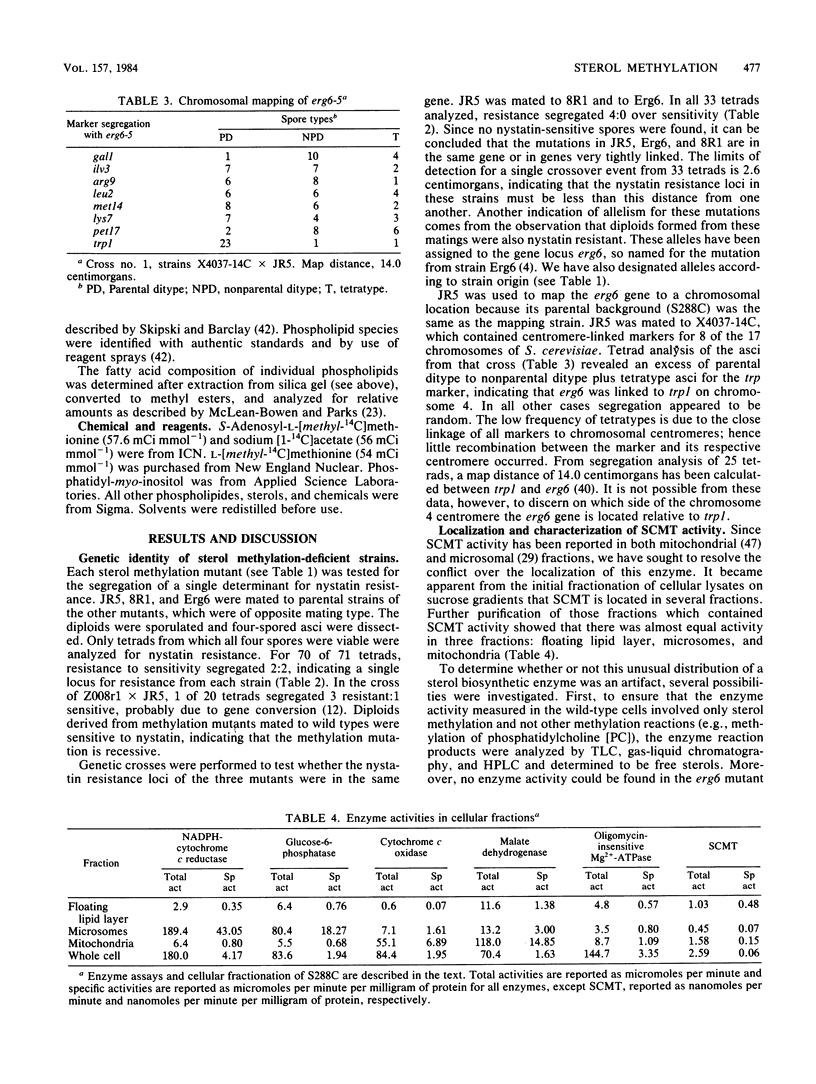
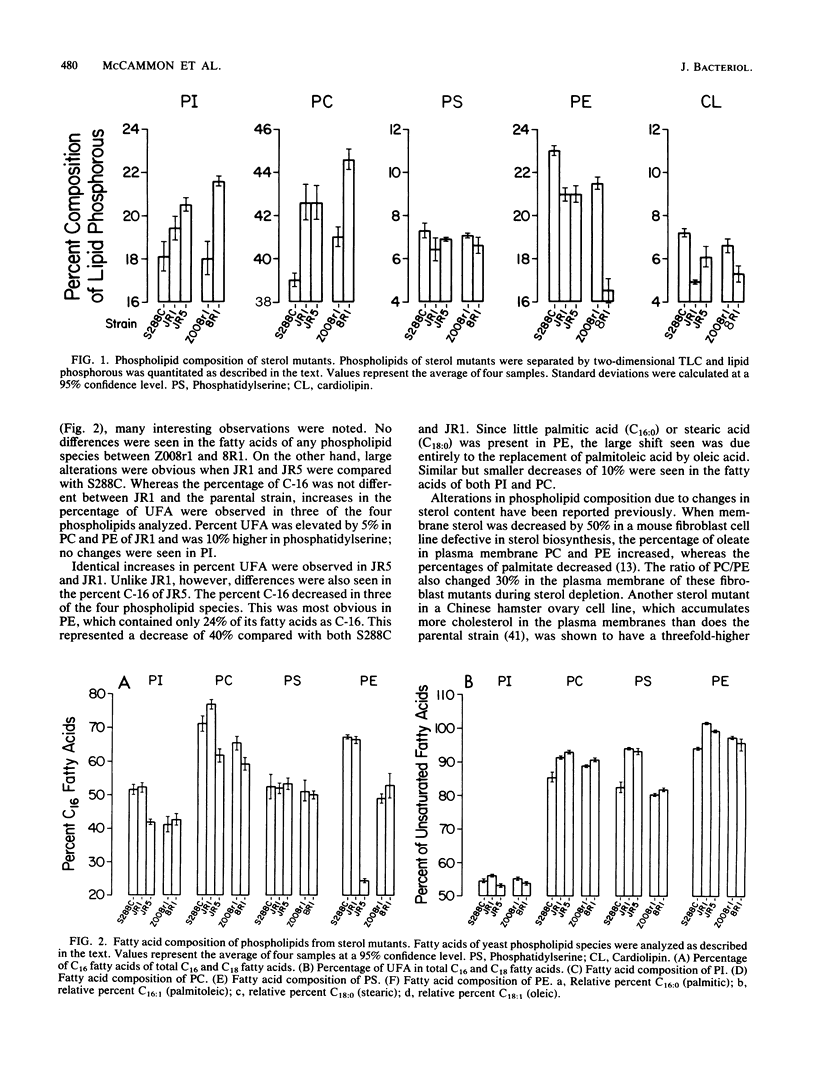
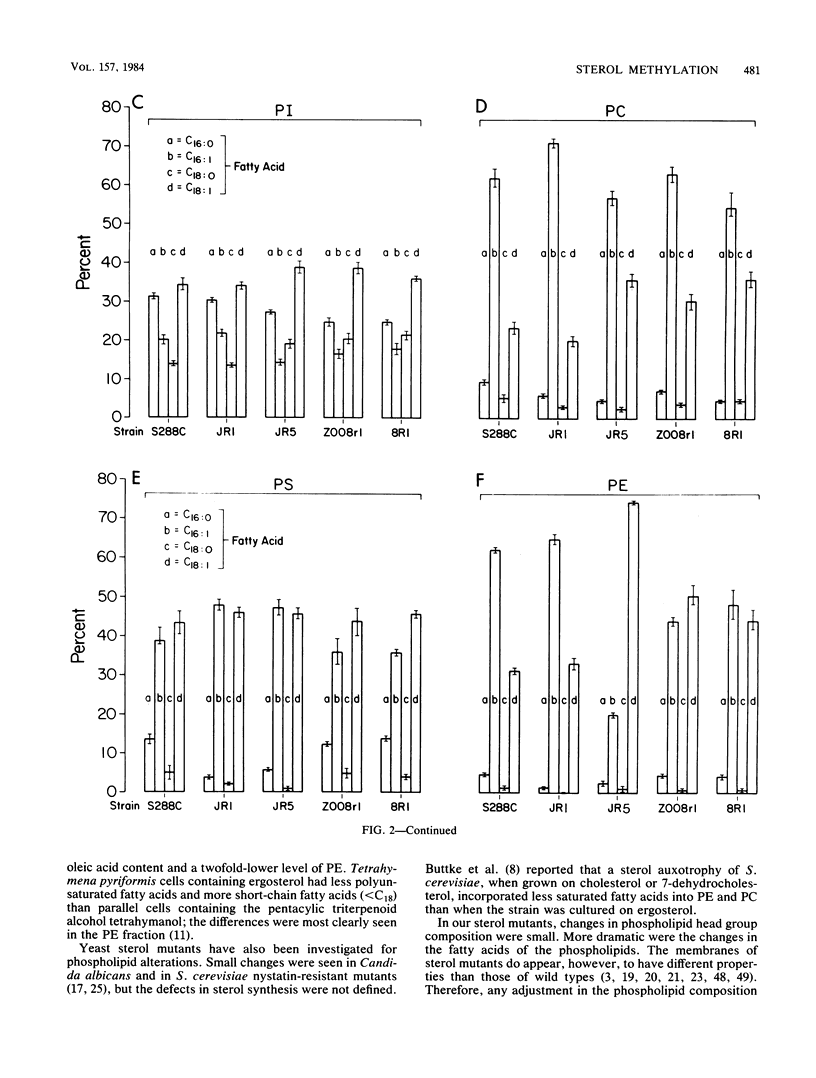
Various nystatin-resistant mutants defective in S-adenosylmethionine: delta 24-sterol-C-methyltransferase (EC 2.1.1.41) were shown to possess alleles of the same gene, erg6. The genetic map location of erg6 was shown to be close to trp1 on chromosome 4. Despite the single locus for erg6, S-adenosylmethionine: delta 24-sterol-C-methyltransferase enzyme activity was found in three separate fractions: mitochondria, microsomes, and the "floating lipid layer." The amount of activity in each fraction could be manipulated by assay conditions. The lipids and lipid synthesis of mutants of Saccharomyces cerevisiae defective in the delta 24-sterol-C-methyltransferase were compared with a C5(6) desaturase mutant and parental wild types. No ergosterol (C28 sterol) could be detected in whole-cell sterol extracts of the erg6 mutants, the limits of detection being less than 10(-11) mol of ergosterol per 10(8) cells. The distribution of accumulated sterols by these mutants varied with growth phase and between free and esterified fractions. The steryl ester concentrations of the mutants were eight times higher than those of the wild type from exponential growth samples. However, the concentration of the ester accumulated by the mutants was not as great in stationary-phase cells. Whereas the head group phospholipid composition was the same between parental and mutant strains, strain-dependent changes in fatty acids were observed, most notably a 40% increase in the oleic acid content of phosphatidylethanolamine of one erg6 mutant, JR5.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey R. B., Miller L., Parks L. W. Enzymatic analysis of C27 sterol-accumulating yeast strains. J Bacteriol. 1976 May;126(2):1012–1013. doi: 10.1128/jb.126.2.1012-1013.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. B., Parks L. W. Yeast sterol esters and their relationship to the growth of yeast. J Bacteriol. 1975 Nov;124(2):606–612. doi: 10.1128/jb.124.2.606-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard M., Lees N. D., Burrows L. S., Kleinhans F. W. Differences in crystal violet uptake and cation-induced death among yeast sterol mutants. J Bacteriol. 1978 Sep;135(3):1146–1148. doi: 10.1128/jb.135.3.1146-1148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard M., Woods R. A., Bartón D. H., Corrie J. E., Widdowson D. A. Sterol mutants of Saccharomyces cerevisiae: chromatographic analyses. Lipids. 1977 Aug;12(8):645–654. doi: 10.1007/BF02533759. [DOI] [PubMed] [Google Scholar]
- Barton D. H., Corrie J. E., Bard M., Woods R. A. Biosynthesis of terpenes and steroids. IX. The sterols of some mutant yeasts and their relationship to the biosynthesis of ergosterol. J Chem Soc Perkin 1. 1974;11(0):1326–1333. [PubMed] [Google Scholar]
- Barton D. H., Gunatilaka A. A., Jarman T. R., Widdowson D. A., Bard M., Woods R. A. Biosynthesis of terpenes and steroids. X. The sterols of some yeast mutants doubly defective in ergosterol biosynthesis. J Chem Soc Perkin 1. 1975;(1):88–92. doi: 10.1039/p19750000088. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Jones S. D., Bloch K. Effect of sterol side chains on growth and membrane fatty acid composition of Saccharomyces cerevisiae. J Bacteriol. 1980 Oct;144(1):124–130. doi: 10.1128/jb.144.1.124-130.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. L., Kohlbrenner W. E. The mode of inhibition of oxidative phosphorylation by efrapeptin (A23871). Evidence for an alternating site mechanism for ATP synthesis. J Biol Chem. 1978 Jul 25;253(14):4865–4873. [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- Ferguson K. A., Conner R. L., Mallory F. B. The effect of ergosterol on the fatty acid composition of Tetrahymena pyriformis. Arch Biochem Biophys. 1971 May;144(1):448–450. doi: 10.1016/0003-9861(71)90501-7. [DOI] [PubMed] [Google Scholar]
- Freter C. E., Ladenson R. C., Silbert D. F. Membrane phospholipid alterations in response to sterol depletion of LM cells. Metabolic studies. J Biol Chem. 1979 Aug 10;254(15):6909–6916. [PubMed] [Google Scholar]
- Fryberg M., Oehlschlager A. C., Unrau A. M. Sterol biosynthesis in antibiotic sensitive and resistant Candida. Arch Biochem Biophys. 1976 Mar;173(1):171–177. doi: 10.1016/0003-9861(76)90247-2. [DOI] [PubMed] [Google Scholar]
- Fryberg M., Oehlschlager A. C., Unrau A. M. Sterol biosynthesis in antibiotic-resistant yeast: nystatin. Arch Biochem Biophys. 1974 Jan;160(1):83–89. doi: 10.1016/s0003-9861(74)80011-1. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. Two types of resistance to polyene antibiotics in Candida albicans. Nature. 1974 Oct 18;251(5476):656–659. doi: 10.1038/251656a0. [DOI] [PubMed] [Google Scholar]
- Karst F., Lacroute F. Ertosterol biosynthesis in Saccharomyces cerevisiae: mutants deficient in the early steps of the pathway. Mol Gen Genet. 1977 Sep 9;154(3):269–277. doi: 10.1007/BF00571282. [DOI] [PubMed] [Google Scholar]
- Kleinhans F. W., Lees N. D., Bard M., Haak R. A., Woods R. A. ESR determinations of membrane permeability in a yeast sterol mutant. Chem Phys Lipids. 1979 Jan-Feb;23(2):143–154. doi: 10.1016/0009-3084(79)90042-2. [DOI] [PubMed] [Google Scholar]
- Lees N. D., Bard M., Kemple M. D., Haak R. A., Kleinhans F. W. ESR determination of membrane order parameter in yeast sterol mutants. Biochim Biophys Acta. 1979 Jun 2;553(3):469–475. doi: 10.1016/0005-2736(79)90302-x. [DOI] [PubMed] [Google Scholar]
- McCammon M. T., Parks L. W. Inhibition of sterol transmethylation by S-adenosylhomocysteine analogs. J Bacteriol. 1981 Jan;145(1):106–112. doi: 10.1128/jb.145.1.106-112.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean-Bowen C. A., Parks L. W. Corresponding changes in kynurenine hydroxylase activity, membrane fluidity, and sterol composition in Saccharomyces cerevisiae mitochondria. J Bacteriol. 1981 Mar;145(3):1325–1333. doi: 10.1128/jb.145.3.1325-1333.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. T., Jr, Gaylor J. L. Investigation of an S-adenosylmethionine: delta 24-sterol methyltransferase in ergosterol biosynthesis in yeast. Specificity of sterol substrates and inhibitors. J Biol Chem. 1970 Sep 25;245(18):4684–4688. [PubMed] [Google Scholar]
- Nagai J., Yokoe S., Tanaka M., Hibasami H., Ikeda T. Increased proportion of medium chain fatty acids in nystatin-resistant yeast mutants. Lipids. 1981 Jun;16(6):411–417. doi: 10.1007/BF02535007. [DOI] [PubMed] [Google Scholar]
- Neal W. D., Parks L. W. Sterol 24(28) methylene reductase in Saccharomyces cerevisiae. J Bacteriol. 1977 Mar;129(3):1375–1378. doi: 10.1128/jb.129.3.1375-1378.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes W. R. Role of sterols in membranes. Lipids. 1974 Aug;9(8):596–612. doi: 10.1007/BF02532509. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Sekula B. C., Nes W. D., Adler J. H. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978 Sep 10;253(17):6218–6225. [PubMed] [Google Scholar]
- Nishino T., Hata S., Taketani S., Yabusaki Y., Katsuki H. Subcellular localization of the enzymes involved in the late stage of ergosterol biosynthesis in yeast. J Biochem. 1981 May;89(5):1391–1396. doi: 10.1093/oxfordjournals.jbchem.a133330. [DOI] [PubMed] [Google Scholar]
- Parks L. W., Anding C., Ourisson G. Sterol transmethylation during aerobic adaptation of yeast. Eur J Biochem. 1974 Apr 16;43(3):451–458. doi: 10.1111/j.1432-1033.1974.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Parks L. W., Bond F. T., Thompson E. D., Starr P. R. 8(9),22 -Ergostadiene-3 -ol, an ergosterol precursor accumulated in wild-type and mutants of yeast. J Lipid Res. 1972 May;13(3):311–316. [PubMed] [Google Scholar]
- Parks L. W., McLean-Bowen C., Bottema C. K., Taylor F. R., Gonzales R., Jensen B. W., Ramp J. R. Aspects of sterol metabolism in the yeast Saccharomyces cerevisiae and in Phytophthora. Lipids. 1982 Mar;17(3):187–196. doi: 10.1007/BF02535102. [DOI] [PubMed] [Google Scholar]
- Parks L. W. Metabolism of sterols in yeast. CRC Crit Rev Microbiol. 1978;6(4):301–341. doi: 10.3109/10408417809090625. [DOI] [PubMed] [Google Scholar]
- Pierce A. M., Pierce H. D., Jr, Unrau A. M., Oehlschlager A. C. Lipid composition and polyene antibiotic resistance of Candida albicans mutants. Can J Biochem. 1978 Feb;56(2):135–142. doi: 10.1139/o78-023. [DOI] [PubMed] [Google Scholar]
- Pierce A. M., Unrau A. M., Oehlschlager A. C., Woods R. A. Azasterol inhibitors in yeast. Inhibition of the delta 24-sterol methyltransferase and the 24-methylene sterol delta 24(28)-reductase in sterol mutants of Saccharomyces cerevisiae. Can J Biochem. 1979 Mar;57(3):201–208. doi: 10.1139/o79-025. [DOI] [PubMed] [Google Scholar]
- Pinto W. J., Nes W. R. Stereochemical specificity for sterols in Saccharomyces cerevisiae. J Biol Chem. 1983 Apr 10;258(7):4472–4476. [PubMed] [Google Scholar]
- Rodriguez R. J., Parks L. W. Structural and physiological features of sterols necessary to satisfy bulk membrane and sparking requirements in yeast sterol auxotrophs. Arch Biochem Biophys. 1983 Sep;225(2):861–871. doi: 10.1016/0003-9861(83)90099-1. [DOI] [PubMed] [Google Scholar]
- SWANSON M. A. Phosphatases of liver. I. Glucose-6-phosphatase. J Biol Chem. 1950 Jun;184(2):647–659. [PubMed] [Google Scholar]
- Sinensky M. Adaptive alteration in phospholipid composition of plasma membranes from a somatic cell mutant defective in the regulation of cholesterol biosynthesis. J Cell Biol. 1980 Apr;85(1):166–169. doi: 10.1083/jcb.85.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobus M. T., Holmlund C. E., Whittaker N. F. Effects of the hypocholesteremic agent trifluperidol on the sterol, steryl ester, and fatty acid metabolism of Saccharomyces cerevisiae. J Bacteriol. 1977 Jun;130(3):1310–1316. doi: 10.1128/jb.130.3.1310-1316.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. Metabolic interconversion of free sterols and steryl esters in Saccharomyces cerevisiae. J Bacteriol. 1978 Nov;136(2):531–537. doi: 10.1128/jb.136.2.531-537.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. Triaglycerol metabolism in Saccharomyces cerevisiae. Relation to phospholipid synthesis. Biochim Biophys Acta. 1979 Nov 21;575(2):204–214. doi: 10.1016/0005-2760(79)90022-5. [DOI] [PubMed] [Google Scholar]
- Thompson E. D., Parks L. W. Effect of altered sterol composition on growth characteristics of Saccharomyces cerevisiae. J Bacteriol. 1974 Nov;120(2):779–784. doi: 10.1128/jb.120.2.779-784.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. D., Parks L. W. The effect of altered sterol composition on cytochrome oxidase and S-adenosylmethionine: delta 24 sterol methyltransferase enzymes of yeast mitochondria. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1207–1213. doi: 10.1016/0006-291x(74)90825-0. [DOI] [PubMed] [Google Scholar]
- Vary M. J., Edwards C. L., Stewart P. R. The biogenesis of mitochondria. IX. Formation of the soluble mitochondrial enzymes malate dehydrogenase and fumarase in Saccharomyces cerevisiae. Arch Biochem Biophys. 1969 Mar;130(1):235–243. doi: 10.1016/0003-9861(69)90029-0. [DOI] [PubMed] [Google Scholar]


