Abstract
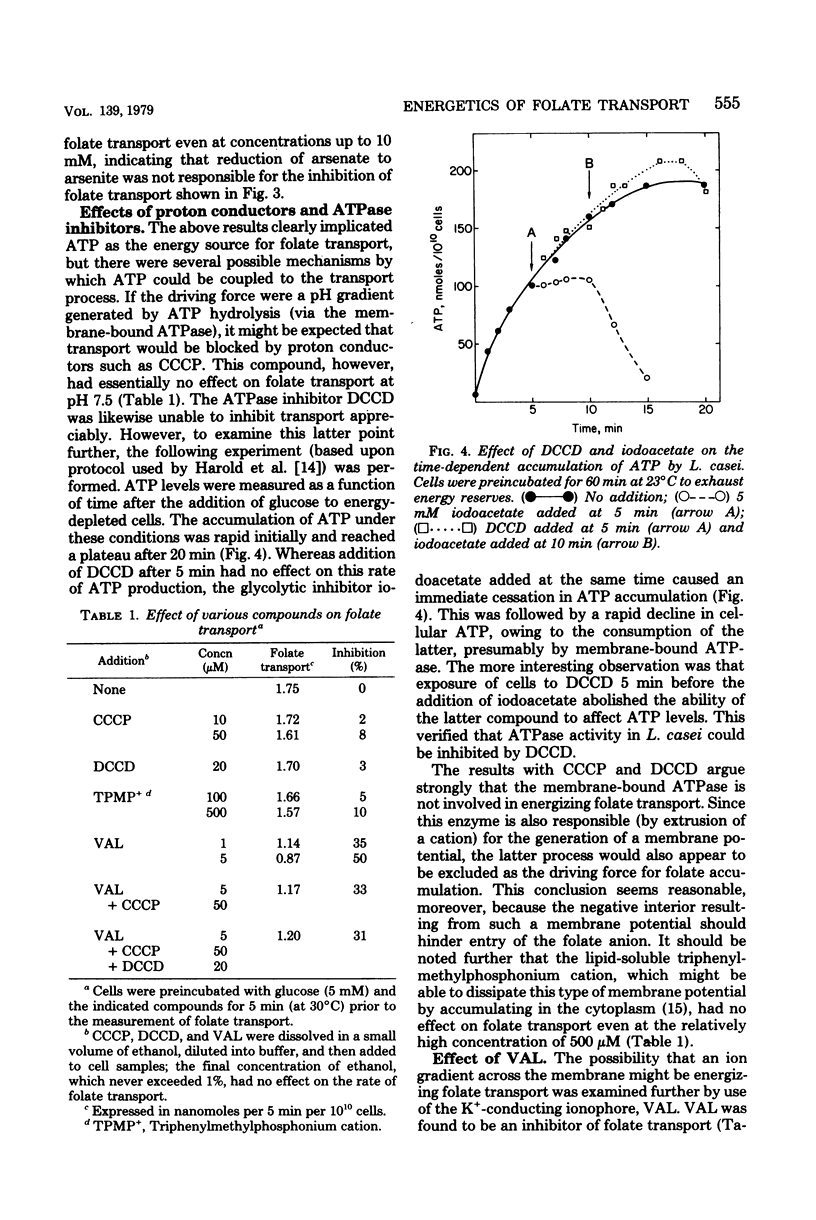
Lactobacillus casei cells can accumulate folate to an intracellular concentration in excess of 500 μM and to concentration gradients (relative to the extracellular compartment) of several thousand-fold. Maximum rates of folate transport are achieved rapidly (t1/2 < 1 min) after the addition of glucose to energy-depleted cells and occur at intracellular adenosine 5'-triphosphate concentrations above 625 μM. The rate of folate transport and the adenosine 5'-triphosphate content of cells are both extremely sensitive to arsenate and decrease in parallel with increasing concentrations of the inhibitor, indicating a requirement for phosphate-bond energy in the transport process. The energy source is not a membrane potential or a pH gradient generated via the membrane-bound adenosine triphosphatase, since dicyclohexylcarbodiimide (an adenosine triphosphatase inhibitor) and carbonyl cyanide m-chlorophenylhydrazone (a proton conductor) have little effect on the uptake process. The K+-ionophore, valinomycin, is an inhibitor of folate transport, but does not act via a mechanism involving dissipation of the membrane potential. This can be deduced from the facts that the inhibition by valinomycin is relatively insensitive to pH, is considerably greater in Na+- than in K+-containing buffers, and is not enhanced by the addition of proton conductors. Folate efflux is not affected by valinomycin, glucose, or various metabolic inhibitors, although a rapid release of the accumulated vitamin can be achieved by the addition of unlabeled folate together with an energy source (glucose). These results suggest that the active transport of folate into L. casei is energized by adenosine 5'-triphosphate or an equivalent energy-rich compound, and that coupling occurs not via the membrane-bound adenosine triphosphatase but by direct interaction of the energy source with a component of the transport system.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Hirata H., Harold F. M. Accumulation of lipid-soluble ions and of rubidium as indicators of the electrical potential in membrane vesicles of Escherichia coli. J Biol Chem. 1975 Feb 25;250(4):1405–1412. [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Cheer S., Gentile J. H., Hegre C. S. Improved methods for ATP analysis. Anal Biochem. 1974 Jul;60(1):102–114. doi: 10.1016/0003-2697(74)90134-1. [DOI] [PubMed] [Google Scholar]
- Cooper B. A. Studies of (3H)folic acid uptake by Lactobacillus casei. Biochim Biophys Acta. 1970 Apr 14;208(1):99–109. doi: 10.1016/0304-4165(70)90052-8. [DOI] [PubMed] [Google Scholar]
- FORREST W. W., WALKER D. J. SYNTHESIS OF RESERVE MATERIALS FOR ENDOGENOUS METABOLISM IN STREPTOCOCCUS FAECALIS. J Bacteriol. 1965 Jun;89:1448–1452. doi: 10.1128/jb.89.6.1448-1452.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg I. Phosphate transport in Micrococcus lysodeikticus. Biochim Biophys Acta. 1977 May 2;466(3):451–460. doi: 10.1016/0005-2736(77)90338-8. [DOI] [PubMed] [Google Scholar]
- Friedberg I. The effect of ionophores on phosphate and arsenate transport in Micrococcus lysodeikticus. FEBS Lett. 1977 Sep 15;81(2):264–266. doi: 10.1016/0014-5793(77)80531-0. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Dio 9 and chlorhexidine: inhibitors of membrane-bound ATPase and of cation transport in Streptococcus faecalis. Biochim Biophys Acta. 1969 Jun 3;183(1):129–136. doi: 10.1016/0005-2736(69)90136-9. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Interaction of arsenate with phosphate-transport systems in wild- type and mutant Streptococcus faecalis. J Bacteriol. 1966 Jun;91(6):2257–2262. doi: 10.1128/jb.91.6.2257-2262.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Ion currents and physiological functions in microorganisms. Annu Rev Microbiol. 1977;31:181–203. doi: 10.1146/annurev.mi.31.100177.001145. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. I. The membrane potential. J Membr Biol. 1972;8(1):27–44. doi: 10.1007/BF01868093. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B., Huennekens F. M. Transport of folate compounds into Lactobacillus Casei. Arch Biochem Biophys. 1974 Oct;164(2):722–728. doi: 10.1016/0003-9861(74)90085-x. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Folate transport in Lactobacillus casei: solubilization and general properties of the binding protein. Biochem Biophys Res Commun. 1976 Feb 9;68(3):712–717. doi: 10.1016/0006-291x(76)91203-1. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Mechanism of folate transport in Lactobacillus casei: evidence for a component shared with the thiamine and biotin transport systems. J Bacteriol. 1979 Mar;137(3):1308–1314. doi: 10.1128/jb.137.3.1308-1314.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Purification and properties of a membrane-associated, folate-binding protein from Lactobacillus casei. J Biol Chem. 1977 Jun 10;252(11):3760–3765. [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Kadner R. J., Huennekens F. M. The folate and thiamine transport proteins of Lactobacillus casei. J Supramol Struct. 1977;6(2):239–247. doi: 10.1002/jss.400060209. [DOI] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huennekens F. M., Vitols K. S., Henderson G. B. Transport of folate compounds in bacterial and mammalian cells. Adv Enzymol Relat Areas Mol Biol. 1978;47:313–346. doi: 10.1002/9780470122921.ch5. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- O'Brien T. A., Nieva-Gomez D., Gennis R. B. Complex formation between the uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) and valinomycin in the presence of potassium. J Biol Chem. 1978 Mar 25;253(6):1749–1751. [PubMed] [Google Scholar]
- Oxender D. L. Membrane transport. Annu Rev Biochem. 1972;41(10):777–814. doi: 10.1146/annurev.bi.41.070172.004021. [DOI] [PubMed] [Google Scholar]
- Rae A. S., Strickland K. P. Studies on phosphate transport in Escherichia coli. II. Effects of metabolic inhibitors and divalent cations. Biochim Biophys Acta. 1976 May 21;433(3):564–582. doi: 10.1016/0005-2736(76)90282-0. [DOI] [PubMed] [Google Scholar]
- Rayman M. K., Lo T. C., Sanwal B. D. Transport of succinate in Escherichia coli. II. Characteristics of uptake and energy coupling with transport in membrane preparations. J Biol Chem. 1972 Oct 10;247(19):6332–6339. [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Transport and metabolism of folates by bacteria. J Biol Chem. 1975 Mar 25;250(6):2243–2253. [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]


