Abstract
Erythroid Krüppel-like factor (EKLF [KLF1]) is a transcriptional regulator that plays a critical role within a specific subset of hematopoietic cells, particularly in the erythroid lineage and its immediate precursor, the megakaryocyte-erythroid progenitor (MEP). We find that EKLF is posttranslationally modified by sumoylation at a single site near its amino terminus and that PIAS1 plays a critical role in this process. Mutation of this site has little effect on EKLF's ability to function as a transcriptional activator; however, it has a dramatic effect on its repressive abilities. The mechanism of repression likely involves a novel small ubiquitin-related modifier (SUMO)-dependent EKLF interaction with the Mi-2β component of the NuRD repression complex. Mutated EKLF is attenuated in its ability to repress megakaryocyte differentiation, implicating EKLF sumoylation status in differentiative decisions emanating from the MEP. These studies demonstrate a novel mechanism by which transcription factor sumoylation can alter protein-protein interactions and bipotential lineage decisions.
Erythroid Krüppel-like factor (EKLF [KLF1]) is a transcription factor that plays a critical role in activation of the β-globin gene during erythropoietic differentiation (4, 44). Its activity is important for the switch from fetal γ-globin to adult β-globin expression. At the supramolecular level, EKLF is also involved in formation of the correct chromatin configuration at the β-globin locus (2, 13).
Genetic ablation studies revealed that the absence of EKLF leads to embryonic death at the time of the switch to adult β-globin (embryonic day 14.5 [E14.5] to E15) due to a profound anemia caused by an α- to β-globin chain imbalance (39, 45). However, restoration of globin function did not completely rescue the phenotype, leading to a wider examination of EKLF function. Since then, EKLF has emerged as a transcription factor involved in the regulation of global erythroid gene expression that is critical for red cell development (14, 25, 47).
Murine EKLF is 358 amino acids long and consists of two functional domains: an N-terminal proline-rich transactivation domain and a C-terminal DNA binding domain created by three C2H2 zinc fingers, which recognize and interact with the CACCC core motif within promoters (5, 6, 37, 47). Naturally occurring mutations within this CACCC box result in β-thalassemias in humans (42) caused by reduced levels of β-globin expression due to decreased DNA binding and transcriptional activation by EKLF (15).
The function of EKLF is tightly regulated by interactions with different cofactors and also by posttranslational modifications. EKLF associates with transcriptional activators such as p300, CBP, and P/CAF that have intrinsic histone acetyltransferase (HAT) activity. EKLF itself is acetylated by p300 and CBP at two sites which results in its transcriptional superactivation (59, 60). Phosphorylation of threonine 41 is essential for optimal EKLF activity (43), and EKLF stability is regulated by its ubiquitination status (48).
Although most functional evidence describes EKLF as a transcriptional activator, we have demonstrated that EKLF can also interact with corepressors Sin3A and histone deacetylase 1 (HDAC1) and may behave as a transcriptional repressor as well, depending on the stage of erythroid development maturation (8, 9).
Since, at the cellular level, EKLF expression is mainly restricted to the erythroid lineage (55), most assays have been performed within that compartment. Thus, EKLF is known to regulate the transcription of genes such as α-hemoglobin stabilizing protein (AHSP), cytoskeletal proteins, heme synthesis enzymes, transcription factors, and blood group antigens (14, 25, 47). However, recent results from gain- and loss-of-function studies have revealed an unexpected role of EKLF as an inhibitor of megakaryopoiesis, suggesting a novel function of this transcription factor in lineage commitment during hematopoiesis (16).
SUMO (small ubiquitin-related modifier) consists of 101 amino acids that after C-terminal maturation can be covalently linked to protein substrates. Sumoylation of proteins proceeds via a multienzymatic pathway that shares similarity with the ubiquitin-conjugation system but uses a SUMO-specific enzymatic machinery (30). First, SUMO is activated by an E1-activating enzyme, the heterodimer Aos1/Uba2 in the presence of ATP, and then is transferred to the E2-conjugating enzyme Ubc9, and subsequently is attached to a specific lysine in the target protein. This final step is facilitated by E3-ligases that are required for efficient sumoylation in vivo. Proteins such as PIAS (protein inhibitor of activated STAT) family, Polycomb, and RanBP have been identified as E3 ligases. At the present time, four SUMO family members are known to exist in mammals. Sumoylation is a highly dynamic process with substrates undergoing rapid conjugation and deconjugation (for reviews, see references 19, 20, 24, 28, and 62). In contrast to ubiquitination, sumoylation does not target a protein for degradation but may affect its subcellular localization (3, 46) and its stability (57), may antagonize other modifications (11, 26), or may affect target protein activity (22, 50). Transcription factors are the largest group of target proteins whose activities are affected by sumoylation (22).
Here, we show that EKLF is modified with SUMO-1 in vivo. We have mapped the single amino acid residue involved in the covalent SUMO conjugation to lysine 74, identified PIAS1 as the E3 ligase that enhances its sumoylation, and shown that its RING finger domain is essential for these interactions. Sumoylation leads to repression by EKLF, such that mutation of its target amino acid dramatically converts it from a repressor to a strong activator when tested on the same promoter. Consistent with these data, coexpression of a dominant-negative Ubc9 or a SUMO protease negates EKLF repression activity, and direct fusion of SUMO to the EKLF amino terminus yields a constitutively repressive molecule irrespective of a mutation at the natural target site. Sumoylation leads to two novel molecular and biological effects. First, this modification enables EKLF to interact with Mi-2β via an interaction that is distinct from that seen previously between EKLF and Sin3A. Second, it alters EKLF's ability to repress megakaryocyte cell differentiation. These observations generally suggest that SUMO modification plays a critical role in transcription factor-chromatin-remodeling complex interactions and that this subtle change can lead to the functional altering of crucial differentiative decisions.
MATERIALS AND METHODS
Cell lines.
Cells of the murine erythroleukemia (MEL) line MEL 745A and human embryonic kidney cell-derived line 293T were cultured in Dulbecco's modified Eagle's medium, and K562 (human erythroleukemia) cells were cultured in RPMI medium, both supplemented with 10% fetal calf serum and antibiotics. Stable K562 and MEL 745A cells were established after transfection with pSVneoHMTIIA-Ter containing wild-type (WT) or K74R EKLF under a zinc-inducible promoter (43) using DMRIE (Invitrogen) or Tfx 50 (Promega) reagent, respectively. Selection was with G418 at final concentrations of 400 μg/ml for K562 cells and 800 μg/ml for MEL 745A cells.
Assessment of erythroid differentiation of a MEL stable cell line.
MEL stable cells were induced with 80 μM Zn for 18 h to express WT and K74R EKLF. For erythroid differentiation, cells were treated with 5 mM hexamethylene bisacetamide (Sigma catalog no. H6260) for 4 days. Each day, RNA samples were collected for quantitative PCR (qPCR) analysis.
Assessment of megakaryocytic or erythroid differentiation of K562 stable cell line.
K562 stable cells were induced with 160 μM Zn for 18 h to express WT and K74R EKLF. For megakaryocytic differentiation cells were diluted to 10E5 cells/ml and treated with 3 nM TPA (phorbol 12-myristate 13-acetate [Sigma; catalog no. P8139]) for 4 days. Each day, cells were monitored by flow cytometry for the presence of megakaryocytic marker CD41 (10E5 [antibody labeled with Alexa 647 was a kind gift from B. Coller]) and analyzed by FACScalibur (Becton Dickinson, San Jose, CA), and RNA samples were collected for qPCR analysis.
For erythroid differentiation, cells were diluted to 2 × 10E5 and treated with 30 μM hemin (Sigma H5533) for 4 days. Each day, cells were monitored by staining with benzidine and RNA samples were collected for qPCR analysis.
Immunofluorescence studies and analysis of subcellular localization.
K562 cells were used to examine the expression and subcellular localization of the EKLF-yellow fluorescent protein (YFP) fusion together with green fluorescent protein (GFP)-SUMO fusion. Cells were additionally cotransfected with Ubc9 and PIAS1. After 36 h, cells were cytospun onto slides and fixed for 10 min at room temperature in 4% formaldehyde (diluted in phosphate-buffered saline [PBS]). Three subsequent wash steps were performed in PBS. The slides were then mounted in Vectashield (Vector Laboratories, Inc.) supplemented with DAPI (4′,6′-diamidino-2-phenylindole) to identify cell nuclei. YFP, GFP, and DAPI were visualized on a Zeiss LSM 510 META confocal laser-scanning microscope.
RT-qPCR.
Total RNA was isolated from K562, MEL, or lineage-depleted bone marrow cells using TRIzol reagent (Sigma). One microgram was then reverse transcribed using a Promega reverse transcription (RT) system kit with random hexamers. Real-time PCR was performed using a SYBR green kit (Applied Biosystems 4304886) and primer sequences as described in the supplemental material. Results were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Analysis was performed on an ABI Prism 7900 HT on 384-well plates.
Generation of transgenic mice with EKLF under PF4 promoter control.
EKLF expression was placed under the control of the megakaryocyte-specific platelet factor 4 (PF4) promoter by subcloning its cDNA (WT or K74R mutant) into the Nhe1 site of pPF4 (kind gift of K. Ravid [61]). Transgenic mice were obtained after injection of the linearized (NdeI) fragment into the pronucleus of one-cell-stage fertilized eggs (Mount Sinai Genetics Shared Research Facility). Genotyping was performed by genomic PCR using transgene-specific primers pF4 (AGAAAGTCACAGGAGCCACTG) and EKLF R2.75 (TCTGAGGAGACGCAGGATTTG). Expression was monitored by RT-PCR analysis of transgenic bone marrow RNA using EKLF/transgene-specific primers that spanned an intron within the transgene: pF4-beta3 (CAGCCACCACTTTCTGATAG) and pF4-EKLF5 (CTGCCCACGTGCTTTTTCAC). We obtained three founder mice for pF4-WT-EKLF and five founder mice for pF4-K74R-EKLF. The results of the experiments consist of the average obtained from two founders of each type.
Colony assays.
Bone marrow cells from the tibia and fibula of 6- to 12-week-old mice were isolated by flushing the marrow cavity in Iscove's modified Dulbecco's medium. Colony assays in methylcellulose or Megacult (Stem Cell Technologies) were performed as described previously (16) using transgenic or nontransgenic bone marrow cells that had been enriched by lineage depletion with StemSep magnetic beads (Stem Cell Technologies) that included interleukin-7R antibodies (eBiosciences) (34). Acetylcholinesterase assays were performed as recommended by the manufacturer (Stem Cell Technologies).
Bone marrow cells were suspended in PBS, cytospun onto a glass slide, air dried, and stained with May-Grunwald Giemsa (MGG) stains (Sigma) as per the manufacturer's recommendations shortly after harvesting and lineage depletion. Slides were scored by examination of 500 cells in duplicate under a ×100 oil immersion objective. Images were taken using a Zeiss Axiophot microscope.
RESULTS
EKLF is covalently modified by SUMO-1 at lysine 74.
Close examination of the EKLF amino acid sequence revealed one lysine that matched perfectly within the consensus sumoylation motif (i.e., ΨKXE, where “Ψ” represents a hydrophobic amino acid and “X” represents any amino acid). Within EKLF it is LKSE, where lysine 74 is a potential acceptor site for SUMO modification. This motif is located in the N-terminal transactivation domain of EKLF and is highly conserved (Fig. 1A). To determine whether EKLF undergoes sumoylation, we transiently transfected 293T cells with constructs encoding Flag-tagged EKLF, hemagglutinin (HA)-tagged SUMO-1, E2 enzyme-Ubc9, and one of the possible E3 ligases (PIAS1).
FIG. 1.
Sumoylation of EKLF in vivo. (A) Schematic representation of EKLF domains with posttranslational modifications and potential sumoylation site. Below is the alignment of amino acid sequences of EKLF from humans, chimps, mice, and rats spanning the SUMO consensus motif. (B) Mouse EKLF is sumoylated on lysine 74. 293T cells were cotransfected with constructs encoding Flag-EKLF (WT or K74R), Ubc9, Flag-PIAS1, and HA- or GFP-tagged SUMO. Lysates were subjected to immunoprecipitation (IP) with M2-agarose, and precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Western blotted (WB), and probed with anti-EKLF antibody (Ab). (C) Sumoylation of endogenous EKLF in erythroid cells. MEL cells were stably transfected with plasmids encoding Flag-WT EKLF or Flag-K74R EKLF. IP was carried out as for panel B, and the blots were probed with anti-EKLF or anti-SUMO antibodies as indicated. (D) Sumoylation of endogenous EKLF from fetal liver at E13.5. Protein extracts form fetal liver were subjected to IP with monoclonal EKLF antibody 7B2, and precipitated proteins were separated by SDS-PAGE, blotted, and probed with either polyclonal anti-EKLF or anti-SUMO antibodies.
In the presence of the components of SUMO machinery, a band migrating slower than EKLF is detected that is missing in the sample with EKLF alone, suggesting that EKLF can be sumoylated (Fig. 1B, lanes 1 and 2).
To confirm that the LKSE motif contains the SUMO acceptor site, we generated a mutant in which Lys 74 was changed to a similarly charged Arg residue (K74R EKLF) and tested for its ability to be modified. Western blot analysis revealed that even in the presence of components of the SUMO machinery, the K74R mutation abolished sumoylation (Fig. 1B, lane 3). This suggests that K74 is indeed the sole functional sumoylation site.
To further prove that EKLF is sumoylated and that the slower-migrating band is due to SUMO modification, we used GFP-tagged SUMO-1 protein instead of HA-tagged SUMO-1. Upon cotransfection, we noticed that the slower-migrating band was retarded even more due to the presence of the larger GFP tag (Fig. 1B, compare lanes 4 and 6). As expected, this band did not appear in the sample containing K74R EKLF (lane 5). These data provide evidence that the slower-migrating form of EKLF originates from covalently conjugated SUMO-1 to lysine 74.
EKLF is sumoylated in erythroid cell lines and primary fetal liver cells.
As EKLF is an erythroid-specific transcription factor, we investigated whether it can be posttranslationally modified in MEL cells by the endogenous sumoylation machinery. We generated two stably transfected MEL cell lines: one with Flag-WT EKLF and a second with Flag-K74R EKLF as a negative control. After immunoprecipitation with anti-Flag antibodies, the proteins were immunoblotted with anti-EKLF and anti-SUMO antibodies. As shown in Fig. 1C, only WT EKLF is sumoylated by the endogenous SUMO machinery. In addition we examined the status of endogenous EKLF in E13.5 fetal liver cells by preparing protein extracts followed by immunoprecipitation with monoclonal anti-EKLF antibody. The subsequent Western blot probed with polyclonal anti-EKLF reveals two slower-migrating EKLF bands that are also recognized by the anti-SUMO antibody (Fig. 1D). Although the reason for the appearance of two bands is not clear, this is only observed in primary erythroid and not in transfected cell extracts. These results indicate that endogenous EKLF is sumoylated by endogenous proteins in vivo.
PIAS1 serves as an E3 ligase for EKLF sumoylation.
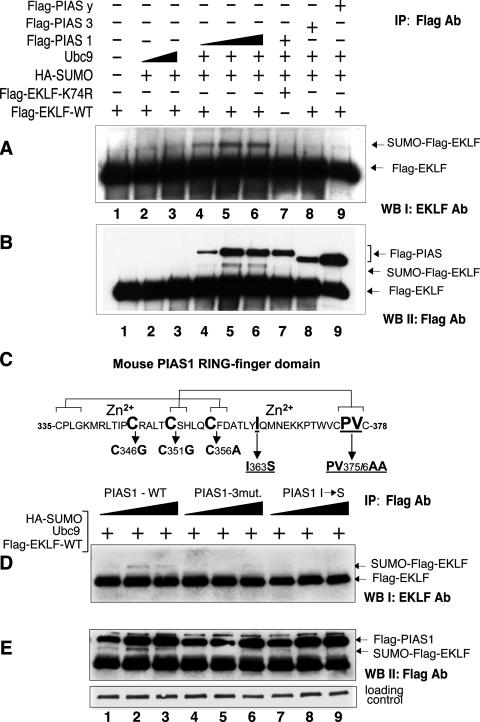
Previous studies have shown that proteins from the PIAS family can act as SUMO E3 ligases, and as such, they significantly enhance the level of sumoylation of the substrate (33). To examine whether PIAS1, PIAS3, or PIASy could act as a specific ligase for EKLF, we transfected 293T cells with constructs encoding Flag-EKLF, HA-SUMO-1, Ubc9, and Flag-tagged proteins from the PIAS family. After immunoprecipitation with anti-Flag antibodies, the proteins were subjected to Western blot analysis, first with anti-EKLF antibodies to detect the level of EKLF with its modification and second with anti-Flag antibody to detect the level of expression of PIAS proteins. As shown in Fig. 2A, we find that the slower-migrating form of EKLF appears only in the presence of PIAS1. Figure 2B confirms that all PIAS proteins were properly expressed. Comparison of lanes 4, 5, and 6 in Fig. 2A reveals that the presence of increasing amounts of cotransfected PIAS1 correlates with an increase in the intensity of the slower-migrating EKLF bands corresponding to sumoylated EKLF. No such bands are observed with the K74R mutant. In addition, in the presence of PIAS3 or PIASy, we do not detect any SUMO-conjugated EKLF. Thus, we conclude that PIAS1 promotes sumoylation of EKLF in a dose-dependent manner and that PIAS1 is the specific E3 ligase for EKLF. This also supports the idea that E3 SUMO ligases display substrate specificity.
FIG. 2.
PIAS1 as an E3 ligase for EKLF. (A) 293T cells were cotransfected with plasmids encoding indicated proteins, lysates were immunoprecipitated (IP) with M2-agarose, proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and blotted. EKLF was detected by Western blotting (WB) with anti-EKLF antibodies (Ab). Various levels of Ubc9 or PIAS1 were tested as indicated. (B) The blot from panel A was reprobed with anti-Flag antibodies to reveal the level of expression of PIAS family proteins, which migrate to a region of the gel distinct from EKLF or SUMO-EKLF. (C) Schematic representation of WT RING finger domain of mouse PIAS1. The “cross-brace” arrangement of the zinc coordinating residues is based on the c-cbl ubiquitin ligase structure (40, 63). Mutations in the RING finger (C346G, C351G, and C356A) are indicated. Residues important in PIAS1 interaction with E2 (Ubc9) mutated in this experiment are underlined (I363S and PV375/6AA). (D) 293T cells were cotransfected with constructs encoding Flag-WT EKLF, Ubc9, and HA-SUMO together with increasing amounts (0.5, 1, and 1.5 μg) of Flag-WT PIAS1 or indicated mutants. IP carried out with M2-agarose and the blot was probed with anti-EKLF antibody. (E) The blot from Fig. 2D was reprobed with anti-Flag antibody to reveal the level of expression of PIAS1 proteins.
Sumoylation of EKLF depends on the intact RING finger domain of PIAS1 and its interactions with Ubc9.
To further characterize the function of PIAS1 in EKLF sumoylation, first we determined whether the PIAS1 RING finger domain is important for its E3 ligase activity. We introduced three point mutations at positions C346G, C351G, and C356A within the RING finger domain. Cysteines 351 and 356 are involved in Zn2+ coordination, and changes of these amino acids will disturb the RING finger structure (Fig. 2C). The effect of these mutations was tested in vivo in 293T cells, which were cotransfected with constructs encoding Flag-EKLF, HA-SUMO-1, Ubc9, and increasing amounts of mutated Flag-PIAS1. Whole-cell extracts were subjected to immunoprecipitation with M2-agarose, and proteins were analyzed with anti-EKLF antibodies. As shown in Fig. 2D, the RING finger mutant fails to enhance sumoylation of EKLF as compared to the WT-PIAS1 (Fig. 2D, lanes 1 to 6). These data suggest that a functional RING domain of PIAS1 is important for its E3 ligase function in EKLF sumoylation.
To examine if PIAS1 needs to interact with Ubc9 during the ligation step, two additional mutants of PIAS1 were constructed. Amino acids located at the surface of PIAS1 that interact with Ubc9 were changed (40). We generated mutants PIAS1-I363S and PIAS1-PV375/6AA and analyzed them the same way as mutants of the RING finger. Both of these mutants are defective in promoting sumoylation of EKLF (Fig. 2D, lanes 7 to 9) (data not shown). The levels of expression of the particular PIAS1 mutants and WT PIAS1 are similar, as shown in Fig. 2E. Thus, we conclude that the interaction between Ubc9 and PIAS1 is essential for SUMO modification of EKLF.
The subcellular localization of EKLF is not altered upon SUMO modification.
Sumoylation has been shown to regulate the intracellular localization of a number of proteins (3, 46). We analyzed the subcellular localization of EKLF in K562 cells, a human erythroleukemia cell line that does not contain endogenous EKLF (12). We transfected these cells with YFP-WT EKLF or mutant YFP-K74R EKLF together with GFP-SUMO, Ubc9, and PIAS1. YFP was fused to the C terminus of EKLF and did not impair its sumoylation, as shown in Fig. 3C. The distribution of both YFP and GFP fusion proteins was revealed by direct fluorescence and shows that EKLF is localized into nucleus, predominantly as distinct, nuclear dot-like structures, independent of its sumoylation status. However, only WT EKLF overlaps with SUMO, unlike K74R EKLF (Fig. 3A and B). Taken together, these results indicate that sumoylation of EKLF is not likely involved in altering its intracellular localization.
FIG. 3.
Localization of the EKLF and SUMO within K562 cells. K562 cells were cotransfected with YFP-Flag-WT EKLF (A) or YFP-Flag K74R EKLF (B) together with GFP-tagged SUMO-1, Ubc9, and PIAS1. After 36 h of incubation, cells were cytospun, fixed, permeabilized, and stained with DAPI to visualize nuclei. Only WT EKLF colocalized with SUMO in nuclei, as indicated by merged yellow signal. (C) Sumoylation of YFP-EKLF to test whether the C-terminal fusion of YFP does not interfere with SUMO-1 modification. 293T cells were cotransfected with constructs encoding YFP-Flag-WT EKLF or YFP-Flag-K74R EKLF together with Ubc9, PIAS1, and HA-SUMO. After 36 h of incubation, cells were lysed and subjected to immunoprecipitation (IP) with M2-agarose, and precipitated proteins were visualized by Western blotting (WB) after detection with anti-EKLF antibodies (Ab).
Sumoylation affects EKLF transcriptional activity and promotes repression.
Because the SUMO acceptor site for EKLF is located within its transactivation domain, we focused our attention on determining whether this covalent protein modification can alter EKLF transcriptional activity. To address this question, we carried out a series of transactivation assays using a luciferase reporter assay. First we compared the sumoylation-deficient mutant K74R EKLF with WT EKLF protein with respect to their ability to activate the β-globin gene promoter, the major natural target of EKLF (12). K562 cells were cotransfected with this reporter plasmid and with either empty vector or constructs encoding WT or K74R EKLF alone or in the presence of SUMO machinery (Ubc9 and HA-SUMO-1). Cell extracts were then analyzed for luciferase activity. As shown in Fig. 4A, there is little difference in activity between WT and K74R EKLF with or without the presence of SUMO and Ubc9. To enhance the efficiency of sumoylation, we also included PIAS1 (as the E3 ligase) but its inclusion causes a dramatic decrease of activation of both WT and K74R EKLF (Fig. 4A), indicating that this inhibition is SUMO independent. Then, in a similar transactivation assay, we tested other known EKLF native targets, the promoter of the AHSP (α-hemoglobin stabilizing protein) gene and the promoters of the BKLF (basic Kruppel-like factor) (18, 47). We did not detect any difference in activity of either WT or K74R EKLF (Fig. 4B) (data not shown). We also did not detect any effect on endogenous chromosomal β-globin promoter activity in hexamethylene bisacetamide-differentiated MEL cells that had been stably transfected with WT or K74R EKLF and analyzed by quantitative RT-PCR (data not shown).
FIG. 4.
Comparison of transcriptional activity of the WT EKLF and K74R EKLF. K562 cells were cotransfected with plasmids expressing the luciferase reporter gene under control of β-globin promoter (A) or AHSP promoter (B) along with WT EKLF or K74R EKLF alone or together with Ubc9, HA-SUMO, and PIAS1 as indicated. A plasmid expressing Renilla luciferase was included as a control for normalization of transfection efficiency. Black and gray bars depict transcriptional activity by WT EKLF and the K74R EKLF mutant, respectively. The Western blot (WB) shows the level of expression of WT and K74R EKLF used in lanes 2 and 5 of the luciferase assay. Ab, antibody.
Having established that sumoylation does not alter the transcriptional capacity of EKLF towards activation of the β-globin or AHSP promoters, we next tested the functional consequence of sumoylation on the repression activity of EKLF. To test for this property EKLF was fused to an exogenous DNA binding domain (GAL4) because its own DNA binding domain, overlapping the zinc fingers, is involved in interactions with corepressors (9). To monitor whether repression activity of EKLF is affected by sumoylation, we again carried out a luciferase reporter assay. K562 cells were cotransfected with a reporter plasmid (5xGAL-TK-Luc) and with expression constructs encoding GAL domain alone or fusion proteins GAL-WT EKLF or GAL-K74R EKLF with or without Ubc9 and HA-SUMO-1.
The basal level of luciferase activity, driven by the thymidine kinase (TK) promoter in the presence of GAL domain alone, is repressed 60% in the presence of GAL-WT EKLF (Fig. 5A), as seen previously (9). Unexpectedly, the sumoylation-deficient mutant (GAL-K74R EKLF) does not behave as a repressor at all, but instead yields a 15-fold-higher transcriptional activity than the GAL-WT EKLF (Fig. 5A). Cotransfection with the components of the SUMO machinery (Ubc9 and HA-SUMO-1) has little effect, likely due to the already high level of endogenous SUMO-1 in K562 cells that allows for sufficient endogenous sumoylation of EKLF (data not shown).
FIG. 5.
Sumoylation of EKLF promotes repression. K562 cells were cotransfected with the 5xGAL-TK-Luc reporter gene (shown above) together with plasmids expressing the indicated proteins. A plasmid expressing Renilla luciferase was included as a control for transfection efficiency. Black and gray bars depict transcriptional activities by WT EKLF and the K74R EKLF mutant, respectively. The comparisons of activities are as follows. (A) GAL-WT EKLF versus GAL-K74R EKLF alone. The Western blot (WB) shows the level of expression of GAL-WT/K74R EKLF. (B) GAL-WT EKLF or GAL-K74R EKLF in the presence of HA-SUMO-1 and WT Ubc9 or DN Ubc9. Levels are normalized to that of the control experiment without DN Ubc9. (C) GAL-WT EKLF or GAL-K74R EKLF in the presence of Ubc9 and HA-SUMO1, with or without WT SSP. Levels are normalized to that of the control experiment without SSP. (D) GAL-WT EKLF and GAL-K74R EKLF activities are compared versus those of their constitutively sumoylated fusion GAL-SUMO-WT EKLF and GAL-SUMO-K74R EKLF.
To confirm that the effect of the K74R mutation on repression was a result of a lack of SUMO-1 modification rather than the lack of another potential lysine modification, we repeated the same experiment in two ways. First, we included the dominant-negative (DN), catalytically inactive form of Ubc9 that contains a single amino acid change (C93S) (23). DN Ubc9 suppresses function of the SUMO modification pathway, and thus GAL-WT EKLF should not undergo sumoylation and should behave like the mutant GAL-K74R EKLF. Indeed, when normalized to the level of reporter expression in the presence of WT EKLF (and thus in a repressed state), inclusion of DN Ubc9 leads to derepression and a dramatically higher level of reporter activity (Fig. 5B). The importance of the sumoylation site for this effect is shown by the fact that the K74R EKLF mutant level of activity is not affected by the addition of DN Ubc9. Second, we carried out an analogous experiment in the presence of WT SSP (SUMO-specific isoprotease), which cleaves off SUMO from its substrate proteins and thus leaves GAL-WT EKLF unsumoylated. This also leads to a more active (27-fold) GAL-WT EKLF when normalized to the control experiment performed in the absence of SUMO isopeptidase (Fig. 5C). Again, the GAL-K74R EKLF mutant is barely affected by SSP expression (Fig. 5C).
As a final test, we prepared plasmids that express a chimeric protein by fusion of GAL-WT or GAL-K74R EKLF with SUMO at its N terminus to mimic constitutively sumoylated EKLF irrespective of the K74R mutation. We analyzed them with the same luciferase reporter assay and found that both SUMO-containing fusions efficiently repress the activity as compared to the GAL domain alone (Fig. 5D) or even to GAL-WT EKLF (Fig. 5D, compare lane 2 with lanes 3 and 5).
Taken together, these studies demonstrate two roles for EKLF sumoylation. One is that sumoylation can directly and reversibly regulate EKLF activity as a repressor and suggests that SUMO modification promotes repression activity of EKLF, a result seen with other transcription factors. But the second observation was unexpected, as disruption of the SUMO acceptor site in EKLF does not simply lead to an EKLF protein with a basal, nonrepressive level of activity but rather to its dramatic conversion to a superactivation phenotype, showing that there is a direct correlation between the increase of transcriptional activity and reduction in the degree of sumoylation.
Sumoylation of the transactivation domain of EKLF promotes efficient binding of Mi-2β, the subunit of NuRD complex.
From previous work, it is known that the zinc finger domain of EKLF (amino acids 287 to 376) interacts with Sin3A/HDAC1 (9). However, as the sumoylation site is located in its transactivation (proline-rich) domain, we considered whether another repressor complex could be involved in SUMO-dependent repression. Prior to testing this idea, we first confirmed that a construct containing only the EKLF transactivation region fused to the GAL DNA binding domain could be (at least) derepressed or even superactivated by mutation of its SUMO site (Fig. 6A). Indeed, the derepression caused by the SUMO-deficient proline domain alone (18-fold) is comparable to that seen with full-length EKLF (15-fold), demonstrating that the zinc finger domain is not required for the SUMO-dependent repression. Next, using trichostatin A (TSA), an inhibitor of HDACs, we examined the possible involvement of an HDAC in SUMO-dependent repression. Indeed, in the presence of TSA, the proline domain of GAL-WT EKLF is derepressed (Fig. 6B), indicating that sumoylation and deacetylase activities both play a role in EKLF's repressive properties.
FIG. 6.
Sumoylation of the proline domain of EKLF enhances its interaction with the Mi-2β/HDAC repression complex. (A) K562 cells were cotransfected with 5xGAL-TK-Luc reporter gene together with a construct encoding the full-length or proline domain alone of GAL-WT or K74R EKLF. Both GAL-K74R EKLF full-length and GAL-K74R-Pro derepress SUMO-dependent repression of the parent construct to the level (fold) indicated. (B) Using a similar luciferase assay as in panel A, TSA treatment of cells transfected with the proline domain of GAL-WT EKLF relieves transcriptional repression. (C) Immunoprecipitation (IP) assay (M2-agarose) of 293T cells after cotransfection with plasmids encoding the indicated proteins. Precipitated proteins were resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and EKLF was detected by Western blotting (WB) with anti-EKLF antibodies (Ab).
In light of these observations, and based on the published properties and similar behavior of the LIN-1 sumoylated protein from Caenorhabditis elegans or p73 (35, 38), we tested whether the Mi-2β subunit of NuRD complex is involved in sumoylated EKLF repression by monitoring whether they interact in vivo. The GAL-WT or K74R proline domain of EKLF together with Flag-tagged Mi-2β were expressed in 293T cells and subjected to immunoprecipitation using M2-agarose. We find that only the sumoylated proline domain shows any affinity towards the Mi-2β subunit of NuRD complex, unlike the SUMO-deficient point mutant (Fig. 6C). These data suggest that the Mi-2β/HDAC complex is involved in the SUMO-dependent repression property of EKLF and that this is specifically dependent on protein-protein interactions between SUMO-modified EKLF and Mi-2β.
Sumoylation of EKLF is involved in inhibition of megakaryocytic differentiation.
To explore the biological relevance of sumoylation of EKLF, we considered the processes whereby EKLF could play role as a repressor. We have recently found that EKLF exerts a negative effect upon the early stage of megakaryocytic differentiation, likely due to inhibition of critical megakaryocytic factors such as Fli-1 (16). Erythroid and megakaryocytic lineages share a common precursor, and the subsequent onset of committed erythro- or megakaryocytic progenitors depends on the balance between the transcription factors involved in this lineage determination. EKLF together with Fli-1, a megakaryocytic transcription factor, may be involved in cross-antagonistic interactions for the final lineage determination (16).
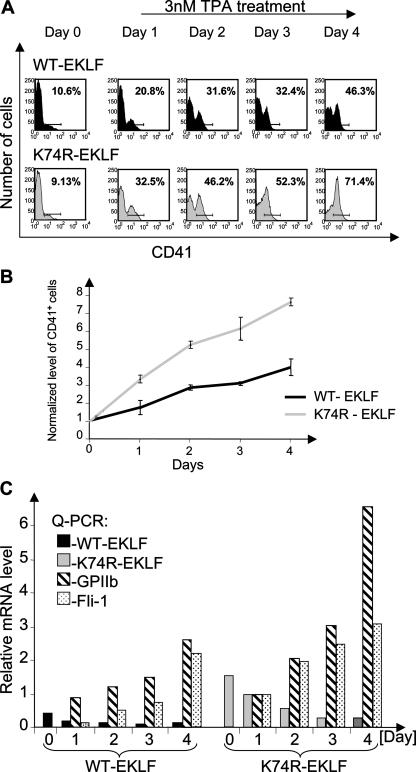
We tested for the possible involvement of sumoylation of EKLF in this process by using K562 cells. Although this is an erythroleukemic cell line, it can be induced to differentiate towards a megakaryocytic phenotype in response to specific chemical inducers (56 and see the supplementary material). To this end, we prepared stable K562 cell lines with either WT or K74R EKLF under the control of a zinc-inducible promoter. These were treated with ZnCl2 for 18 h prior to induction of megakaryocytic differentiation with TPA and monitored for 4 days. Using flow cytometry, we measured the level of cell surface antigen CD41 as a marker specific for megakaryocytic development. The flow cytometry results show that initially both cell lines yield comparable histogram profiles for CD41 (Fig. 7A). However, the subsequent dynamics of megakaryocytic differentiation are significantly different in the two lines, as both the kinetics and extent of CD41+ accumulation are faster and greater in the SUMO-deficient mutant of EKLF than the WT (Fig. 7A and B). This suggests that the K74R EKLF is unable to inhibit megakaryocytic differentiation as efficiently as WT EKLF.
FIG. 7.
Sumoylation of EKLF inhibits TPA-induced differentiation of K562 cell towards megakaryocytes. K562 cells stably transfected with WT or K74R-EKLF were induced to differentiate towards megakaryocytes with a subsaturating amount (3 nM) of TPA, and samples were analyzed at each of 4 days. Representative results are shown for one of four experiments. (A) Flow cytometry analysis of CD41 expression at the indicated time points for a typical experiment. (B) Rate of progress towards megakaryocyte differentiation upon TPA treatment. The level of CD41 at day 0 was used to normalize the levels seen at later time points and was given a value of 1. Results are the average of six experiments. (C) Quantitative RT-PCR analysis for EKLF, GPIIb, and Fli-1 mRNA expression (coded as indicated) from total RNA collected at the indicated time points after TPA induction. The mRNA level of K74R EKLF at day 1 was given an arbitrary value of 1, and all other levels were normalized to that value for comparison. Expression of GAPDH was used to standardize the particular expression levels. All experiments were performed three times; the qPCR values are the average of triplicates. A representative experiment is shown.
In parallel, we carried out qPCR analyses of the mRNA levels for EKLF, GPIIb (glycoprotein IIb, another megakaryocyte-specific marker), and Fli-1, which further confirm difference between WT and K74R EKLF (Fig. 7C). Overexpression of WT EKLF decreases normal Fli-1 message levels during megakaryopoiesis of embryonic stem cells in culture (16). In the present experiment, although the mRNA levels of the K74R mutant are more pronounced than those of WT EKLF at simultaneous time points (Fig. 7C), the levels of expression of megakaryocytic markers GPIIb and Fli-1 are much higher for K74R mutant than in the presence of the lower level of WT EKLF (Fig. 7C). These data suggest a significant functional consequence of the mutation in the SUMO acceptor site of EKLF: an inability to exert its inhibitory effect on megakaryocytic differentiation.
K562 cells can be also differentiated towards an erythroid phenotype. However, erythroid differentiation after hemin treatment of the induced WT or K74R EKLF stable K562 cell lines was unaffected, as judged by benzidine staining and analysis by RT-qPCR of γ-globins (data not shown). This is not surprising as γ-globins are not the natural target of EKLF (12).
The data obtained from differentiation of K562 cells with TPA led us to postulate that the constitutive expression of WT or K74R EKLF will not have equivalent effects in vivo within the megakaryocytic lineage, where EKLF is no longer expressed (16). To examine this prediction we cloned EKLF downstream of the megakaryocyte-specific PF4 promoter and established transgenic lines from these DNA constructs. PF4 is a protein expressed in megakaryocyte granules whose promoter has been successfully used in a number of studies to drive restricted expression (61). Lines that expressed the transgenes in the bone marrow were examined in more detail by cellular and molecular analyses, and results from these lines are more fully described below.
Lineage-depleted bone marrow cells were cytospun onto slides and monitored for the presence of megakaryocytes by acetylcholinesterase staining. Visual inspection of the field made it readily apparent that, already at harvest, bone marrow cells from transgenic mice expressing WT EKLF contain significantly less megakaryocytes than those from a nontransgenic line (Fig. 8A). Quantitation of MGG-stained slides revealed a threefold decrease in megakaryocyte cellularity in WT EKLF transgenic bone marrow (Fig. 8A). This demonstrates that misexpression of EKLF in the megakaryocyte lineage has a repressive effect on megakaryocyte formation, consistent with the present studies in K562 cells and with our recently published analyses (16). However, of particular relevance to EKLF sumoylation, visual inspection and quantitative analysis of transgenic bone marrow from the K74R EKLF line revealed no effect of mutant EKLF transgene expression on megakaryocyte cellularity (Fig. 8A).
FIG. 8.
Constitutive expression of WT but not sumoylation-mutant EKLF in megakaryocytes impairs megakaryocyte formation in vivo. Lineage-depleted bone marrow cells from nontransgenic (non-txgn) or PF4 EKLF transgenic lines (WT or K74R) were analyzed as follows. (A) Freshly harvested cells were spun onto slides and visualized by acetylcholinesterase stain alone (top right) or in combination with MGG stain (bottom right). Note the lower number of megakaryocytes per field seen in either case. Megakaryocyte abundance was quantitated within MGG-stained fields by morphology (at least 500 cells per individual count). Data are the average of six assays from two separate experiments (P < 0.001 when comparing nontransgenic versus WT EKLF or WT EKLF versus K74R EKLF). (B) The presence of megakaryocyte precursors was assayed morphologically by colony formation in Megacult after 12 to 14 days of culture and staining with acetylcholinesterase (typical colony shown on left). Colony numbers were normalized to that seen with the nontransgenic control, which was given a value of 100 (middle). Megakaryocyte colony data are the average of 8 to 10 assays from four separate experiments (P < 0.001 when comparing nontransgenic versus WT EKLF or WT EKLF versus K74R EKLF). Erythroid CFU-E colonies were monitored after 3 days of culture in methylcellulose and similarly normalized (right). Erythroid colony data are the average of 7 to 10 assays from four separate experiments performed at the same time and from the same samples as the megakaryocyte colony assays. (C) Total EKLF (right) or transgenic EKLF (left) mRNA was quantitated by RT-PCR. Levels were normalized to that seen in the WT EKLF material, which was given a value of 1. Values are the average of two assays each in triplicate.
We next determined whether transgene expression also altered megakaryocyte colony formation. Megacult colony assays (Fig. 8B) of lineage-depleted bone marrow cells show a similar trend to that seen with the cell assay: WT EKLF transgenic bone marrow contains fivefold less megakaryocyte colony-forming potential than the nontransgenic control, while the K74R-EKLF transgenic bone marrow is not affected (Fig. 8B). CFU-erythroid (CFU-E) colony formation is not significantly affected in any of these bone marrow samples. Quantitative RT-PCR analysis shows that K74R EKLF is expressed at an even higher level than that of WT EKLF (Fig. 8C), thus ruling out the trivial possibility that its lack of effect on megakaryocyte cell number and colony formation is a result of absent transgene expression. As expected, total EKLF levels are unaffected in any of the samples. Together with our data from K562 cells, we conclude that availability of the SUMO modification site in EKLF is absolutely critical for it to exert its normal cellular function during hematopoiesis related to inhibition of megakaryopoiesis.
DISCUSSION
Sumoylation of protein substrates has been implicated in a variety of important cellular and molecular control mechanisms. Our discovery that EKLF, a critical erythroid transcriptional regulator, is also modified by SUMO raises novel molecular and biological implications for red cell regulation. In particular, these follow from our additional observations that EKLF repression, but not activation, is the key functional property that is altered by sumoylation; that sumoylation affects a previously undescribed molecular interaction between EKLF and Mi-2β; and that the biological role of this modification may relate to bipotential lineage decisions established during later stages of hematopoiesis.
We identified a unique SUMO acceptor site (lysine 74) within the SUMO consensus motif (LKSE) in the EKLF sequence that is also conserved among its mammalian orthologues. As EKLF is sumoylated within its transactivation domain, it is not surprising that the modification alters its transcriptional activity, in particular conferring a repression phenotype to EKLF. Despite reports that sumoylation can prevent ubiquitin-mediated degradation of proteins (11), we propose that the differences in activity between WT and K74R EKLF (∼15-fold) are not the consequence of different stabilities, as our own report on EKLF ubiquitination (48) shows that mutation at K74 does not affect EKLF stability. Additionally, K74 is not one of the lysines modified by p300/CBP acetylation (59) that play a role in EKLF transcriptional activation.
SUMO related repression can be executed in at least two ways. One is by altering the cellular localization of the modified protein. Using immunofluorescence, we have shown that the distribution of sumoylated or SUMO-deficient forms of EKLF was not measurably influenced by the presence of components of SUMO machinery. Moreover, both forms of EKLF were localized in the nucleus, mostly in well-distinguished speckles, but remarkably only WT EKLF colocalized exclusively with SUMO.
As a result, we speculate that sumoylation is involved in conferring the repressive phenotype of EKLF by promoting its interaction with corepressors. Recruitment of SUMO to a promoter in the context of a GAL4-SUMO fusion is sufficient for repression, suggesting that SUMO itself can directly bind transcriptional corepressors (35, 52). Sumoylation may induce conformational changes that likely alter the interaction interface of the protein. SUMO-modified proteins can directly recruit HDAC: for example, sumoylated p300 interacts with HDAC6 (21), Elk-1-SUMO interacts with HDAC2 (58), and sumoylated histone H4 recruits HDAC1 and heterochromatin protein 1 (HP1) (53). Sumoylation can also promote interactions with complexes that contain HDAC as a component, for instance the LIN-1 or p73 interaction with NuRD/HDAC (35, 38). Inhibition of HDAC activity with TSA relived the repression by EKLF and led to superactivation of the promoter, up to a level comparable to that of the EKLF SUMO-deficient mutant, supporting the contention that HDAC activity is involved in EKLF repression. Together with the results showing that EKLF and Mi-2β interact in vivo, we infer that EKLF possibly interacts with the NuRD repression complex, one whose components include HDAC and Mi-2β (1, 17, 35). The EKLF-NuRD interaction has not been previously described, nor has an interaction between a sumoylated transcription factor and Mi-2β.
Given the dynamic and transient nature of sumoylation, it may be required only to initiate assembly of EKLF repressor complex but not to maintain it. Studies of the mechanisms by which SUMO modification regulates activity of transcription factors are complicated by the fact that in many cases the associated transcription factor coactivators and/or corepressors are also modified by SUMO. For example, sumoylation of HDAC1 and HDAC4 leads to enhancement of their repressive activities as compared to nonmodified forms (10, 32). We suggest that SUMO-1 regulates the transcriptional activity of EKLF both directly through EKLF modification, leading to recruitment of a repression complex through the Mi2β subunit, and then also indirectly by affecting the activity of the coregulators within that complex. This could be part of the “mark” that is left at the chromatin even after the SUMO modification is removed from EKLF (24).
EKLF also interacts with the SWI/SNF chromatin-remodeling complex, particularly the ATPase-containing BRG1 subunit, leading to hypersensitive site formation at the adult β-globin promoter prior to its transcriptional activation (2). We have now shown an interaction with Mi-2β, which is the ATPase-containing subunit of the NuRD-remodeling complex, as being important for repression. Not only are these separate functional properties, but they also map to different regions of EKLF, as the zinc finger domain at the EKLF carboxyl terminus is responsible for the interaction with SWI/SNF (2, 29) but the amino-terminal region near K74 is critical for the interaction with Mi-2β. Whether the SWI/SNF and Mi-2β interactions are antagonistic to each other, as recently shown for a subset of inflammatory response genes (49), is not addressed by the present studies, since the target genes (activation in erythroid and repression in megakaryocyte cells) are not overlapping. In light of this, however, the novel interaction with Mi-2β raises the possibility that EKLF's role in chromatin remodeling may be more complex in the erythroid cell and not limited to SWI/SNF and activation.
The NuRD complex has been previously implicated in erythroid gene regulation. For example, it is found at and is recruited by its interaction with FOG1 to GATA1-bound repressed genes in erythroid cells (27, 51) and also associates with NFE2/MafK (7, 54). It is part of an Ikaros-containing complex (PYR) that contains a mixture of NuRD and SWI/SNF subunits and may play a role in γ - to β-globin switching (41). Finally, again together with SWI/SNF, it forms part of a large remodeling complex at the β-globin locus control region (36). Given EKLF's critical requirement in β-globin locus chromatin structure and expression (2, 13), it will be of interest to see whether its sumoylation status plays any role in formation of these other complexes also found at the locus.
In an effort to identify natural target genes for EKLF repression, we attended to our ongoing studies of EKLF's unexpected role in erythroid-megakaryocyte lineage decisions. Microarray analyses showed that megakaryocyte-expressed molecules, including Fli-1, GPIIb, and vWF, are upregulated in EKLF-defective fetal liver cells (16). Given the results of the present study that demonstrate the critical role of SUMO in EKLF repressive actions, we tested whether these observations are related by using a simplified system (K562 cell line), which upon TPA treatment can differentiate towards the megakaryocytic phenotype (56). Using derivatives of these cells that contained inducible WT or K74R EKLF, we observed that the dynamics of differentiation were not equivalent: the cells expressing WT EKLF differentiated more slowly than the ones that expressed the SUMO-deficient mutant of EKLF. Quantitative gene expression analysis revealed that levels of the megakaryocyte targets implicated in the microarray data were more highly repressed in the presence of the WT than in the K74R mutant EKLF. Although these data do not prove a direct interaction between EKLF and these targets genes, they do suggest that the sumoylation status of EKLF (and its interaction with NuRD) is a major biological determinant in the mechanism controlling cell differentiation and, possibly, lineage commitment from the common megakaryocyte-erythroid precursor. The involvement of the NuRD complex in controlling cell differentiation has been shown for lymphocyte function, where separate Ikaros interactions with NuRD or SWI/SNF lead to their compartmentalization into distinct nuclear chromatin regions and are thought to play a role in T-cell proliferation and differentiation (31).
Our results from the in vivo experiments, where we forced the expression of WT or K74R EKLF in the megakaryocytic lineage, additionally support the involvement of SUMO modification of EKLF in the final outcome of megakaryocytic differentiation. Expression of sumoylated EKLF significantly blocks the number of megakaryocytes and also decreases the potential of bone marrow precursors to form megakaryocytic colonies, whereas the SUMO-deficient mutant of EKLF does not.
EKLF has been proposed to regulate transcription by associating with chromatin-remodeling complexes and other transcription regulators. Our previous studies have implicated EKLF association with p300/CBP, and its subsequent acetylation by these factors, as having a role in transcriptional activation, enhancing its association with SWI/SNF remodelers (60). On the other hand, SUMO conjugation to EKLF may have a dramatically different result by specifically facilitating its ability to associate with other factors, especially those involved in transcriptional repression. The subsequent epigenetic results of these types of interactions would markedly differ. Further studies are essential to more completely elucidate the complexity of the molecular mechanism involved in SUMO-mediated gene repression by EKLF and its biological role in lineage decisions.
Supplementary Material
Acknowledgments
We are grateful to D. Manwani for hematological expertise (in the cytology and colony assay analyses) and also to D. Manwani, F. Lohmann, P. Frontelo, T. Sengupta, M. Galdass, H. Fathallah, and C. Iancu-Rubin for helpful discussions and/or critical reading of the manuscript.
This work was supported by NIH PHS grant R01 DK46865 (to J.J.B.). Confocal laser-scanning microscopy was performed at the MSSM-Microscopy Shared Resource Facility, supported with funding from NIH-NCI shared resources grant (5R24 CA095823-04) and NSF Major Research Instrumentation grant (DBI-9724504). The Flow Cytometry and the Quantitative PCR Shared Research Facility are supported by MSSM.
Footnotes
Published ahead of print on 15 October 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahringer, J. 2000. NuRD and SIN3 histone deacetylase complexes in development. Trends Genet. 16:351-356. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, J. A., J. J. Bieker, and B. M. Emerson. 1998. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell 95:93-104. [DOI] [PubMed] [Google Scholar]
- 3.Besnault-Mascard, L., C. Leprince, M. T. Auffredou, B. Meunier, M. F. Bourgeade, J. Camonis, H. K. Lorenzo, and A. Vazquez. 2005. Caspase-8 sumoylation is associated with nuclear localization. Oncogene 24:3268-3273. [DOI] [PubMed] [Google Scholar]
- 4.Bieker, J. 2001. EKLF and the development of the erythroid lineage, p. 71-84. In K. Ravid and J. D. Licht (ed.), Transcription factors: normal and malignant development of blood cells. Wiley Interscience, Hoboken, NJ.
- 5.Bieker, J. J. 2001. Kruppel-like factors: three fingers in many pies. J. Biol. Chem. 276:34355-34358. [DOI] [PubMed] [Google Scholar]
- 6.Bieker, J. J., and C. M. Southwood. 1995. The erythroid Krüppel-like factor transactivation domain is a critical component for cell-specific inducibility of a β-globin promoter. Mol. Cell. Biol. 15:852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand, M., J. A. Ranish, N. T. Kummer, J. Hamilton, K. Igarashi, C. Francastel, T. H. Chi, G. R. Crabtree, R. Aebersold, and M. Groudine. 2004. Dynamic changes in transcription factor complexes during erythroid differentiation revealed by quantitative proteomics. Nat. Struct. Mol. Biol. 11:73-80. [DOI] [PubMed] [Google Scholar]
- 8.Chen, X., and J. J. Bieker. 2004. Stage-specific repression by the EKLF transcriptional activator. Mol. Cell. Biol. 24:10416-10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, X., and J. J. Bieker. 2001. Unanticipated repression function linked to erythroid Krüppel-like factor. Mol. Cell. Biol. 21:3118-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David, G., M. A. Neptune, and R. A. DePinho. 2002. SUMO-1 modification of histone deacetylase 1 (HDAC1) modulates its biological activities. J. Biol. Chem. 277:23658-23663. [DOI] [PubMed] [Google Scholar]
- 11.Desterro, J. M., M. S. Rodriguez, and R. T. Hay. 1998. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol. Cell 2:233-239. [DOI] [PubMed] [Google Scholar]
- 12.Donze, D., T. M. Townes, and J. J. Bieker. 1995. Role of erythroid Kruppel-like factor in human gamma- to beta-globin gene switching. J. Biol. Chem. 270:1955-1959. [DOI] [PubMed] [Google Scholar]
- 13.Drissen, R., R. J. Palstra, N. Gillemans, E. Splinter, F. Grosveld, S. Philipsen, and W. de Laat. 2004. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev. 18:2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drissen, R., M. von Lindern, A. Kolbus, S. Driegen, P. Steinlein, H. Beug, F. Grosveld, and S. Philipsen. 2005. The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol. Cell. Biol. 25:5205-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, W. C., C. M. Southwood, and J. J. Bieker. 1994. Analyses of beta-thalassemia mutant DNA interactions with erythroid Kruppel-like factor (EKLF), an erythroid cell-specific transcription factor. J. Biol. Chem. 269:1493-1500. [PubMed] [Google Scholar]
- 16.Frontelo, P., D. Manwani, M. Galdass, H. Karsunky, F. Lohmann, P. G. Gallagher, and J. J. Bieker. 22 August 2007. Novel role for EKLF in megakaryocyte lineage commitment. Blood [Epub ahead of print.] doi: 10.1182/blood-2007-03-082065. [DOI] [PMC free article] [PubMed]
- 17.Fujita, N., D. L. Jaye, C. Geigerman, A. Akyildiz, M. R. Mooney, J. M. Boss, and P. A. Wade. 2004. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell 119:75-86. [DOI] [PubMed] [Google Scholar]
- 18.Funnell, A. P. W., C. A. Maloney, L. J. Thompson, J. Keys, M. Tallack, A. C. Perkins, and M. Crossley. 2007. Erythroid Krüppel-like factor directly activates the basic Krüppel-like factor gene in erythroid cells. Mol. Cell. Biol. 27:2777-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill, G. 2003. Post-translational modification by the small ubiquitin-related modifier SUMO has big effects on transcription factor activity. Curr. Opin. Genet. Dev. 13:108-113. [DOI] [PubMed] [Google Scholar]
- 20.Gill, G. 2004. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 18:2046-2059. [DOI] [PubMed] [Google Scholar]
- 21.Girdwood, D., D. Bumpass, O. A. Vaughan, A. Thain, L. A. Anderson, A. W. Snowden, E. Garcia-Wilson, N. D. Perkins, and R. T. Hay. 2003. P300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11:1043-1054. [DOI] [PubMed] [Google Scholar]
- 22.Girdwood, D. W., M. H. Tatham, and R. T. Hay. 2004. SUMO and transcriptional regulation. Semin. Cell Dev. Biol. 15:201-210. [DOI] [PubMed] [Google Scholar]
- 23.Gong, L., T. Kamitani, K. Fujise, L. S. Caskey, and E. T. Yeh. 1997. Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9. J. Biol. Chem. 272:28198-28201. [DOI] [PubMed] [Google Scholar]
- 24.Hay, R. T. 2005. SUMO: a history of modification. Mol. Cell 18:1-12. [DOI] [PubMed] [Google Scholar]
- 25.Hodge, D., E. Coghill, J. Keys, T. Maguire, B. Hartmann, A. McDowall, M. Weiss, S. Grimmond, and A. Perkins. 2006. A global role for EKLF in definitive and primitive erythropoiesis. Blood 107:3359-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoege, C., B. Pfander, G. L. Moldovan, G. Pyrowolakis, and S. Jentsch. 2002. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419:135-141. [DOI] [PubMed] [Google Scholar]
- 27.Hong, W., M. Nakazawa, Y. Y. Chen, R. Kori, C. R. Vakoc, C. Rakowski, and G. A. Blobel. 2005. FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J. 24:2367-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, E. S. 2004. Protein modification by SUMO. Annu. Rev. Biochem. 73:355-382. [DOI] [PubMed] [Google Scholar]
- 29.Kadam, S., and B. M. Emerson. 2003. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell 11:377-389. [DOI] [PubMed] [Google Scholar]
- 30.Kerscher, O., R. Felberbaum, and M. Hochstrasser. 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22:159-180. [DOI] [PubMed] [Google Scholar]
- 31.Kim, J., S. Sif, B. Jones, A. Jackson, J. Koipally, E. Heller, S. Winandy, A. Viel, A. Sawyer, T. Ikeda, R. Kingston, and K. Georgopoulos. 1999. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 10:345-355. [DOI] [PubMed] [Google Scholar]
- 32.Kirsh, O., J. S. Seeler, A. Pichler, A. Gast, S. Muller, E. Miska, M. Mathieu, A. Harel-Bellan, T. Kouzarides, F. Melchior, and A. Dejean. 2002. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO J. 21:2682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotaja, N., U. Karvonen, O. A. Jänne, and J. J. Palvimo. 2002. PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol. Cell. Biol. 22:5222-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhl, C., A. Atzberger, F. Iborra, B. Nieswandt, C. Porcher, and P. Vyas. 2005. GATA1-mediated megakaryocyte differentiation and growth control can be uncoupled and mapped to different domains in GATA1. Mol. Cell. Biol. 25:8592-8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leight, E. R., D. Glossip, and K. Kornfeld. 2005. Sumoylation of LIN-1 promotes transcriptional repression and inhibition of vulval cell fates. Development 132:1047-1056. [DOI] [PubMed] [Google Scholar]
- 36.Mahajan, M. C., G. J. Narlikar, G. Boyapaty, R. E. Kingston, and S. M. Weissman. 2005. Heterogeneous nuclear ribonucleoprotein C1/C2, MeCP1, and SWI/SNF form a chromatin remodeling complex at the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 102:15012-15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, I. J., and J. J. Bieker. 1993. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol. Cell. Biol. 13:2776-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minty, A., X. Dumont, M. Kaghad, and D. Caput. 2000. Covalent modification of p73alpha by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif. J. Biol. Chem. 275:36316-36323. [DOI] [PubMed] [Google Scholar]
- 39.Nuez, B., D. Michalovich, A. Bygrave, R. Ploemacher, and F. Grosveld. 1995. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature 375:316-318. [DOI] [PubMed] [Google Scholar]
- 40.Ohi, M. D., C. W. Vander Kooi, J. A. Rosenberg, W. J. Chazin, and K. L. Gould. 2003. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat. Struct. Biol 10:250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Neill, D. W., S. S. Schoetz, R. A. Lopez, M. Castle, L. Rabinowitz, E. Shor, D. Krawchuk, M. G. Goll, M. Renz, H.-P. Seelig, S. Han, R. H. Seong, S. D. Park, T. Agalioti, N. Munshi, D. Thanos, H. Erdjument-Bromage, P. Tempst, and A. Bank. 2000. An Ikaros-containing chromatin-remodeling complex in adult-type erythroid cells. Mol. Cell. Biol. 20:7572-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orkin, S. H., H. H. Kazazian, Jr., S. E. Antonarakis, S. C. Goff, C. D. Boehm, J. P. Sexton, P. G. Waber, and P. J. Giardina. 1982. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature 296:627-631. [DOI] [PubMed] [Google Scholar]
- 43.Ouyang, L., X. Chen, and J. J. Bieker. 1998. Regulation of erythroid Kruppel-like factor (EKLF) transcriptional activity by phosphorylation of a protein kinase casein kinase II site within its interaction domain. J. Biol. Chem. 273:23019-23025. [DOI] [PubMed] [Google Scholar]
- 44.Perkins, A. 1999. Erythroid Kruppel like factor: from fishing expedition to gourmet meal. Int. J. Biochem. Cell. Biol. 31:1175-1192. [DOI] [PubMed] [Google Scholar]
- 45.Perkins, A. C., A. H. Sharpe, and S. H. Orkin. 1995. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature 375:318-322. [DOI] [PubMed] [Google Scholar]
- 46.Pichler, A., and F. Melchior. 2002. Ubiquitin-related modifier SUMO1 and nucleocytoplasmic transport. Traffic 3:381-387. [DOI] [PubMed] [Google Scholar]
- 47.Pilon, A. M., D. G. Nilson, D. Zhou, J. Sangerman, T. M. Townes, D. M. Bodine, and P. G. Gallagher. 2006. Alterations in expression and chromatin configuration of the alpha hemoglobin-stabilizing protein gene in erythroid Krüppel-like factor-deficient mice. Mol. Cell. Biol. 26:4368-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quadrini, K. J., and J. J. Bieker. 2006. EKLF/KLF1 is ubiquitinated in vivo and its stability is regulated by activation domain sequences through the 26S proteasome. FEBS Lett. 580:2285-2293. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez-Carrozzi, V. R., A. A. Nazarian, C. C. Li, S. L. Gore, R. Sridharan, A. N. Imbalzano, and S. T. Smale. 2006. Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 20:282-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez, M. S., J. M. Desterro, S. Lain, C. A. Midgley, D. P. Lane, and R. T. Hay. 1999. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 18:6455-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez, P., E. Bonte, J. Krijgsveld, K. E. Kolodziej, B. Guyot, A. J. Heck, P. Vyas, E. de Boer, F. Grosveld, and J. Strouboulis. 2005. GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J. 24:2354-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross, S., J. L. Best, L. I. Zon, and G. Gill. 2002. SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol. Cell 10:831-842. [DOI] [PubMed] [Google Scholar]
- 53.Shiio, Y., and R. N. Eisenman. 2003. Histone sumoylation is associated with transcriptional repression. Proc. Natl. Acad. Sci. USA 100:13225-13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shyu, Y.-C., T.-L. Lee, C.-Y. Ting, S.-C. Wen, L.-J. Hsieh, Y.-C. Li, J.-L. Hwang, C.-C. Lin, and C.-K. Shen. 2005. Sumoylation of p45/NF-E2: nuclear positioning and transcriptional activation of the mammalian β-like globin gene locus. Mol. Cell. Biol. 25:10365-10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Southwood, C. M., K. M. Downs, and J. J. Bieker. 1996. Erythroid Kruppel-like factor exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev. Dyn. 206:248-259. [DOI] [PubMed] [Google Scholar]
- 56.Tetteroo, P. A., F. Massaro, A. Mulder, R. Schreuder-van Gelder, and A. E. von dem Borne. 1984. Megakaryoblastic differentiation of proerythroblastic K562 cell-line cells. Leuk. Res. 8:197-206. [DOI] [PubMed] [Google Scholar]
- 57.Weger, S., E. Hammer, and R. Heilbronn. 2004. SUMO-1 modification regulates the protein stability of the large regulatory protein Rep78 of adeno associated virus type 2 (AAV-2). Virology 330:284-294. [DOI] [PubMed] [Google Scholar]
- 58.Yang, S. H., and A. D. Sharrocks. 2004. SUMO promotes HDAC-mediated transcriptional repression. Mol. Cell 13:611-617. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, W., and J. J. Bieker. 1998. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl. Acad. Sci. USA 95:9855-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, W., S. Kadam, B. M. Emerson, and J. J. Bieker. 2001. Site-specific acetylation by p300 or CREB binding protein regulates erythroid Krüppel-like factor transcriptional activity via its interaction with the SWI-SNF complex. Mol. Cell. Biol. 21:2413-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, Y., Y. Nagata, G. Yu, H. G. Nguyen, M. R. Jones, P. Toselli, C. W. Jackson, M. Tatsuka, K. Todokoro, and K. Ravid. 2004. Aberrant quantity and localization of Aurora-B/AIM-1 and survivin during megakaryocyte polyploidization and the consequences of Aurora-B/AIM-1-deregulated expression. Blood 103:3717-3726. [DOI] [PubMed] [Google Scholar]
- 62.Zhao, J. 4 September 2007. Sumoylation regulates diverse biological processes. Cell Mol. Life Sci. [Epub ahead of print.] doi: 10.1007/s00018-007-7137-4. [DOI] [PMC free article] [PubMed]
- 63.Zheng, N., P. Wang, P. D. Jeffrey, and N. P. Pavletich. 2000. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell 102:533-539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.