Exhaled breath condensate (EBC) is a promising source of biomarkers of lung disease. It is important to note that EBC is not a biomarker, but rather a matrix in which biomarkers may be identified, in that way equivalent to blood, sweat, tears, urine and saliva. EBC may be thought of either as a body fluid or as a condensate of exhaled gas (and therefore not a body fluid). This issue is relevant because of potential government regulatory issues involved with laboratory assessment of “body fluids”.
There are three principal contributors to EBC(1). First are variable-sized particles or droplets that are aerosolized from the airway lining fluid—such particles presumably reflecting the fluid itself. Second is distilled water that condenses from gas phase out of the nearly water-saturated exhalate, substantially diluting the aerosolized airway lining fluid. Third are water soluble volatiles that are exhaled and absorbed into the condensing breath. Interest lies both in the non-volatile constituents mostly derived from the airway lining fluid particles and in the water-soluble volatile constituents which are found in substantially higher concentrations and are therefore more readily assayed than the non-volatile compounds.
The field of EBC research has advanced gradually, with the debates surrounding an emerging field helping to pose questions and gradually leading to answers. There are several key issues that are listed below.
1. Source of EBC biomarkers
Very little work has yet been done to help understand the nature and source of the exhaled particles/droplets that are part of the EBC matrix. That micron and sub-micron sized droplets emanate from the mouth or endotracheal tube in exhaled breath has been confirmed by laser particle counters(2, 3), and indeed such particles serve as the only explanation for the presence of clearly non-volatile constituents in EBC such as cytokines(4) and sodium ion(5). However, how these particles form and change during exhalation before leaving the body is the subject only of speculation. Forwarded theories include that small amounts of airway lining fluid are torn from the airway surface when turbulence provides energy to the airway wall, similar to spray arising from whitecaps on the ocean on a windy day. Energy to overcome surface tension also may be applied to the wall when closed airways/alveoli pop open during inspiration, likewise potentially creating exhalable particles. Although surfactant and surfactant proteins found in EBC(6) have been suggested to indicate an alveolar origin of the exhaled particles, this is not convincing, for alveolar fluids can move more proximal in the airway.
The size of particles that are measured exiting the mouth during expiration rapidly may be affected by condensation or evaporation. Size and numerical measurements by laser particle counters therefore reflect the particle size entering the counter, not necessarily the particle size initially generated from the airway lining surface.
2. Particle size
One 10 micron particle entering a sample of EBC can supply 1,000,000 times the quantity of non-volatiles to a sample of EBC as one 0.1 micron particle. However, there is skewing of the particle sizes exhaled towards the smaller particles(3). Overall, the relative contribution to EBC non-volatile constituent of the different sized particles remains unknown.
3. Oropharyngeal contribution to EBC
In oral EBC collections, there is no reason to suspect that particles can not be released from the oral and retropharyngeal mucosa into the airstream, with potential variably to contaminate what might otherwise be a pure lower airway sample. Furthermore, depending in part on the EBC collection equipment, gross or microscopic salivary contamination of EBC can and does occur(7). Some subjects simply drool during collection, affirming the need for salivary trapping systems to be in place. Measures of salivary amylase are often used to test for the presence of salivary contamination. Most investigators find that no amylase is identified in the great majority of samples, although certainly those using higher sensitivity assays tend to report the presence of measurable amylase in a subset of samples(7). It is important to mention that the amylase assays used are far from perfect and, similar to all other protein assays in EBC, suffer from some—potentially substantial—amount of false positivity and negativity. Overconfident reliance on any protein assay in EBC not uncommonly has led to mistaken conclusions, and this may be the case for amylase measures as well. Measurable phosphate has been suggested to be reliable indicators of salivary contamination as well(8). One group concluded that saliva is the source of less than 10% of respiratory droplets(9).
The ratios among various non-volatile compounds in EBC has been found to be substantially different than the ratio of compounds in saliva, suggesting a dominant (but not entire) lower airway source of EBC constituents(9). In oral collections, there is currently no certainty that oral contribution can be completely excluded from the sample. In samples collected by endotracheal tube, there is confidence that the immediate source of volatiles and non-volailtes is the lower airway and lungs (although aspiration of saliva and gastric fluid can contaminate the lower airway fluid, of course).
4. Dilution
The airway lining fluid component of EBC is highly diluted by condensing vapor phase water. Estimates of the dilution of ALF particles in EBC range from 20-fold to 30,000- fold(10). 2000 to 10000 fold seems to be a generally accepted number(11). There may be relevant day-to-day and sample-to sample intrasubject variability in dilution, although debate occurs because the assays used for assessment of dilution are themselves a source of variability. As is common in much of medicine and biomedical science, in terms of a confident dilution marker for EBC there is as yet no gold standard. Within a given study, it seems worthwhile to attempt to standardize against a relevant additional EBC component, such as the conductivity of a lyophilized sample or ion measurements (10, 12), total protein, or urea measurement(9). Indeed, in comparison to bronchoalveolar lavage, it may be easier to obtain a reliable dilution indicator for EBC, because unlike BAL, there is no reasonable mechanism by which collection of EBC significantly alters the airway lining fluid(11).
There are two times when dilution markers are unnecessary. First are when multiple biomarkers are measured concurrently and their ratios considered. Ratios among inter-reactive or biologically related biomarkers can be of particular interest and eliminate the need for dilution markers. Examples of such ratios include IFN gamma (“Th1”) to IL4 (Th2) ratio(13, 14), nitrite:nitrate (NO2− : NO3−) ratio(15), and pH (which can be considered a ratio of acids and bases)(16). The second time when dilution markers are unnecessary is when there is a confident assay for a substance which serves as an on-off indicator of an abnormality. Examples might include the presence of M tuberculosis DNA (by PCR), gastric pepsin, rhinovirus RNA (by RT-PCR) and anthrax toxin. False positivity in such assays needs to be nil.
5. Lack of gold standards of lung disease assessment or diagnosis with which to compare EBC measurements
There is currently no gold-standard invasive or non-invasive method of determining absolute concentrations of airway lining fluid non-volatile constituents with which EBC can be readily compared. For example, bronchoalveolar lavage is subject to its own dilution concerns. Microsampling techniques that draw fluid from the airway wall by capillary action or suction alter the fluid itself, creating a lung biomarker equivalent to the Heisenberg Uncertainty Principle. Induced sputum suffers similarly to microsampling techniques in that the fluid expectorated appears at least somewhat affected by the sputum production process, at least on subsequent sampling. As a result, there is no consensus as to whether airway lining fluid is isotonic, hypo- or hypertonic in comparison to blood. The concentration of sodium ion in the human ALF remains subject to some uncertainty.
Our general disdain of invasively collecting samples from healthy lungs additionally limits our knowledge of normal airway fluid components, and normal variability. Invasiveness or discomfort of collection drastically limits our ability to study airway components. These issues underlie the attractiveness of EBC as a research and clinical tool. Importantly, the lack of ability to well access unadulterated airway lining fluid using other means should prompt caution if we are overly critical of EBC in light of the above delineated concerns. EBC may well be better than other alternatives; it may well have fewer drawbacks than other methods.
6. Validation
EBC is often lumped together with exhaled nitric oxide in review articles and insurance company briefings, but from a validation standpoint technically is far behind exhaled nitric oxide (eNO)(1). However this is not because exhaled NO is a “better” biomarker than EBC. We must remember that EBC is not a biomarker at all. Exhaled NO is one biomarker, whereas EBC is a matrix in which so many biomarkers have been identified that there is simply not the concentration of investigators studying any one EBC biomarker as there has been for eNO. A search of PubMed reveals approximately 426 EBC papers, with the first paper in 1980, but with a fairly steady increase in publications over the past 8 years (see Figure 1). These papers cover multiple diseases and over 100 biomarkers, with more being identified monthly.
Figure 1.
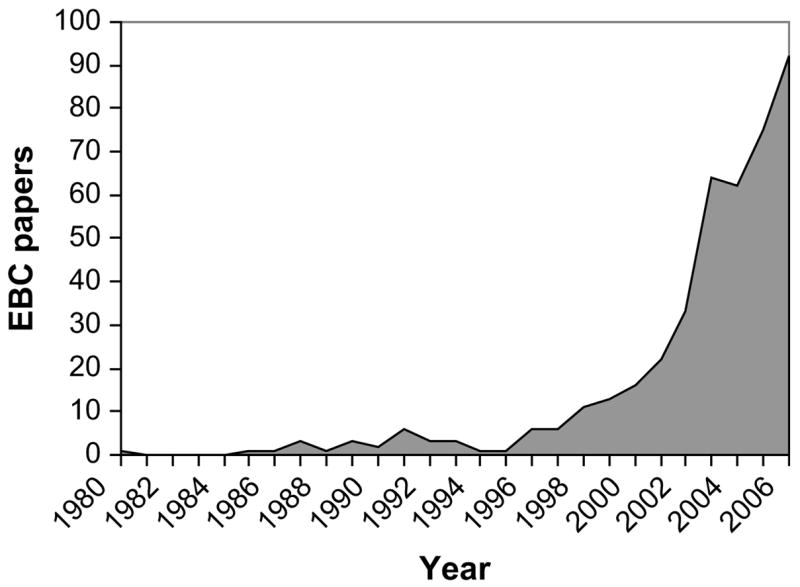
Exhaled breath condensate publications by year in the peer-reviewed literature. Perhaps 100 more publications are identified in the non-peer-reviewed literature.
7. Collection of EBC
As noted, interest in EBC lies first and foremost with its ease of collection in nearly any setting. Entirely non-invasive, it takes as little as 5 breaths to collect sufficient sample for assay, although in research practice, substantially longer collection times are often used to assure sufficient sample is available for repeated analysis of multiple biomarkers. Ten minutes of tidal breathing yields 1–2 milliliters of sample, and is well tolerated. Some centers, including ours, focus on 1 or 2 biomarkers at a time allowing for smaller sample sizes. At the University of Virginia, the most common EBC collection duration is 5 minutes.
Several options exist for collection of EBC samples. Multiple custom devices have been used throughout the years, using various cooling techniques and device shapes and materials. In terms of parts-which are often found about a respiratory laboratory-such home made systems often can be made cheaply, although the expense in terms of personnel time may be surprisingly substantial. Commercially available equipment is also available (see Table 1). Certain biomarkers are seemingly best collected under set condensation conditions, but these conditions are markedly different for various biomarkers(11). Although standardized methods of collection and storage for certain individual biomarkers are developing, there is no expectation that there will ever be a standardized EBC collection procedure that will be uniform for all biomarkers. Therefore any collection method that satisfies the needs of the user is acceptable, but there is no one-size fit all standardized methodology.
Table 1.
Commercially available exhaled breath condensate collection systems.
| EBC collection system | Manufacturer | Advantages | Disadvantages |
|---|---|---|---|
| ECoScreen I and ECoScreen II | Viasys, USA, Europe | Most commonly published EBC collection system. More common in European centers. Optional package for determination of total exhaled volume. | Not readily portable. Cleaning between patients may need to be extensive to abide by standard respiratory care practices. No ability to control condensation temperature (Eco1). |
| RTube | Respiratory Research, USA | More total EBC collections performed using RTube than other systems. Multiple collections can be performed concurrently. More common in North American centers. Disposable (no cleaning between patients). Portable. Can be prepared for use in a standard freezer. | Choice and maintenance of set condensing temperature requires optional cooling unit, otherwise condensation temperature is chosen by cooling sleeve preparation temperature and rises during collection. |
| Anacon | Biostec, Spain | Controllable temperature of collection. Designed for use on ventilated patients | Few publications |
| TurboDeccs | Italchil, Italy | Has both non-disposable and disposable portions. Controllable collection temperature. Moderately portable. Readily cleanable because of disposable components. | Few publications. Simple system. One collection at a time. |
8. Range of EBC biomarkers
Categorization of EBC biomarkers has been done in the past (Horvath Task Force(11)) although is open to change. There are several potential categorizations, and biomarkers may fall into one or more of the following groups:
Categorization group 1.
1. Volatile compounds
2. Non-volatile compounds
3. Non-volatile compounds derived from volatile compounds
Categorization group 2
4. Very low molecular weight compounds
5. Low molecular weight compounds
6. Polypeptides
7. Proteins
8. Nucleic acids
Miscellaneous differentiation
9. Lipid mediators
10. Inorganic molecules
11. Organic molecules
12. Redox relevant molecules
13. pH relevant molecules.
14. Cytokines, chemokines.
There is no reason to suspect that anything more than a tiny minority of potentially relevant compounds have been reported as yet. Given a sufficiently sensitive assay, it is likely that any reasonably stable molecule in the airway lining fluid can be found in EBC. More useful EBC biomarker categories will result from new findings.
The most substantive difference among these categories is that between volatile and non-volatile constituents. As an introductory caveat, it is important to note that some clearly non-volatile compounds found in EBC may be derivatives of volatiles. For example, nitrate (NO3−) and nitrite (NO2−)—ionized and therefore not volatile—may arise in EBC in part from a reaction of volatile gas nitric oxide (NO) after reaction with oxygen(17). Chloride ion (Cl−), another non volatile, can be at least in part delivered as the volatile hydrochloric acid (HCl).
Volatiles such as acetic acid, formic acid and ammonia are found in much higher concentrations in EBC than non-volatile constituents, and tend, therefore, to be much easier to measure. Volatile biomarkers may be identified in the high micromolar or even low millimolar range. Their arrival and concentration in EBC is controlled by entirely different factors than the non-volatile biomarkers. Indeed, the amount and size of particles formed by turbulence (or other means) and dilution factors are irrelevant for volatile biomarkers. That dilution markers are not needed may enhance the value of the volatile biomarker assays.
However, other factors are important in regards to interpretation of volatile biomarker levels, including water solubility, gas-liquid partition coefficients, temperature of the source fluid (airway lining fluid), temperature of the condenser, pH of the source fluid and EBC, and the opportunity to react within (and therefore be captured by) the EBC matrix. So, what does this mean? It means we need to be careful about interpretation. An elevated level of formic acid in EBC may well not mean more formic acid production in the airway lining fluid, but rather may well indicate a lower pH of the airway lining fluid (and therefore enhanced volatility because non-volatile formate ion is protonated in acidic fluid to form the somewhat volatile species formic acid. The importance of EBC pH as an indicator of airway lining fluid pH is because of this feature of volatile acids and bases: Acids tend to be volatile from, while bases tend to be trapped by, acidic source fluid. When EBC pH is lower than normal, more acid and/or less base has been delivered to and captured in the EBC, primarily because more acid and less base has been volatilized from an acidic airway. Although EBC pH does not equal airway pH, an acidic EBC is indeed created from an acidic airway source fluid, so qualitative non-invasive assessments of airway pH deviation become achievable.
Some of the issues so far determined to be relevant to control for when planning to assay volatiles include:
Condensation temperature that is sufficiently cold to freeze the EBC may diminish the amount of volatiles (which are more readily absorbed into the liquid phase)(18).
Frozen storage may protect reactive or unstable compounds, but may also allow sublimation of the volatiles into the airspace above the frozen EBC (unpublished observation). These volatiles will be lost when the storage container is opened, unless efforts are made to thaw and remix the sample before opening.
Volatile substances respond differently to sample manipulation. Each substance of interest should be studied well to control for potential effects of collection duration, temperature, storage conditions, and assay system.
Non-volatile constituents of EBC make up a broad category containing molecules as small as sodium ion (Na+) and as large as immunoglobulins. There are numerous publications in the literature presenting an individual compound that has been found in EBC (relying often on one assay) with levels depending upon disease state, with speculations added that the biomarker may be valuable in managing the disease of interest. There is optimism, but the more experienced researchers have also learned certain lessons:
Confirm the results of your assay with other assays using different methodology.
Assure that assay controls are performed appropriately and thoroughly. This is perhaps the single most important point for investigators studying EBC. EBC is a highly dilute, low-protein aqueous matrix. If one uses commercially available assay kits for EBC, it is important to assure that the standards used for comparison (standard curve generation) are done in a matrix as similar to that EBC sample as possible. The artifact-producing effect of using improper standards is often called a “matrix effect” and can be substantial in EBC assays. Using proteinaceous standards (“cytokine X in BSA”), and attempting to compare to unaltered EBC will assuredly lead to misleading assay values. One choice is for the EBC sample to be altered so as to be substantially similar to the standards (such as by adding albumin, as the case may be). Because of inadequate recognition of matrix effect, it is likely that some of the published data are contaminated by artifacts sufficiently to invalidate their attached conclusions.
The pH of EBC does vary in disease states substantially, with pH values as low as 3.5 and has high as 9.0 reported(16, 19). Because the reactivities and stabilities of many of the other biomarkers of interest are affected by the pH of the fluid in which they are found, and because accuracy of some assays can be affected as well, investigators needs to be aware that EBC pH can cause assay artifact as well as loss (or gain) of biomarkers in EBC during storage.
Beware that, in the absence of dilution assessment different levels of a single EBC biomarker in a disease state may be interpreted either to represent different levels of the biomarker in the airway lining fluid, or a different amount or size of particles evolved from an otherwise identical airway lining fluid. To reiterate an earlier point, ratios among more than one related biomarker do not require dilution markers to be of more confident value.
Many if not most of the non-volatile constituents found within EBC are identified by assays pushing their lower limits of accuracy. On the one hand, great care should be taken to assure that the assay is reporting correctly. On the other hand, expectations for assay reproducibility at these low levels cannot be overly high. EBC biomarkers have often been critiqued as suffering from high intrasubject variability, and therefore of marginal value. In many cases, however, this variability may greatly result from assay variability as opposed to biological or EBC collection system variability. Such assay variability is found for dilution assessment efforts as well, which can mathematically compound the overall non-biologic variability and if not accounted for can lead to incorrect conclusions exaggerating the apparent biologic variability or EBC collection system variability.
Concentration of EBC samples by lyophilization/dehydration/freeze drying with resuspension of the lyophilate in small volumes of highly pure water has seemingly allowed for biomarker assessment by immunoassays in the many cases where the levels were previously simply too low to be measurable. A 1 ml EBC sample (collected in 7 minutes for example), can be lyophilized and resuspended into 50 uL—a 20-fold concentration. The advent of multiplex bead arrays has allowed for the tiny reconstituted volumes to be used for multiple immunoassays concurrently, opening up an exciting potential, with proof of concept already in place(20), to gain a broad window on the balance among cytokines (or other mediators) in the airway lining fluid during any respiratory state. The issue of variable dilution remains (unless dilution factors are analysed), but the ratios among the cytokines are likely valid as long as sufficient assay controls have been performed.
It is beyond the scope of this chapter to provide details regarding each of the non-volatile biomarkers that has been reported in EBC, and the field is advancing with new discoveries weekly. The reader is referred to the Report of the European Respiratory Society/American Thoracic Society Joint Task Force on Exhaled Breath Condensate(11) for a relatively recent comprehensive compendium of expert opinion on many of the published EBC biomarkers. A key point is that conscientious assay technique will likely find in EBC any substance of substantially high concentration in the airway lining fluid.
Footnotes
Conflict of Interest: JH is a cofounder of Respiratory Research, Inc., which manufactures exhaled breath condensate collection equipment.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt J. Exhaled breath condensate: An evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002;110(1):28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 2.Fairchild CI, Stampfer JF. Particle concentration in exhaled breath. Am Ind Hyg Assoc J. 1987;48(11):948–9. doi: 10.1080/15298668791385868. [DOI] [PubMed] [Google Scholar]
- 3.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10(2):105–16. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 4.Tufvesson E, Bjermer L. Methodological improvements for measuring eicosanoids and cytokines in exhaled breath condensate. Respir Med. 2005 doi: 10.1016/j.rmed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Zacharasiewicz A, Wilson N, Lex C, Li A, Kemp M, Donovan J, Hooper J, Kharitonov SA, Bush A. Repeatability of sodium and chloride in exhaled breath condensates. Pediatr Pulmonol. 2004;37(3):273–5. doi: 10.1002/ppul.10431. [DOI] [PubMed] [Google Scholar]
- 6.Sidorenko GI, Zborovskii EI, Levina DI. Surface-active properties of the exhaled air condensate (a new method of studying lung function) Ter Arkh. 1980;52(3):65–8. [PubMed] [Google Scholar]
- 7.Gaber F, Acevedo F, Delin I, Sundblad BM, Palmberg L, Larsson K, Kumlin M, Dahlen SE. Saliva is one likely source of leukotriene B4 in exhaled breath condensate. Eur Respir J. 2006;28(6):1229–35. doi: 10.1183/09031936.00151905. [DOI] [PubMed] [Google Scholar]
- 8.Griese M, Noss J, Bredow CCv. Protein pattern of exhaled breath condensate and saliva. Proteomics. 2002;2(6):690–6. doi: 10.1002/1615-9861(200206)2:6<690::AID-PROT690>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Effros RM, Peterson B, Casaburi R, Su J, Dunning M, Torday J, Biller J, Shaker R. Epithelial lining fluid solute concentrations in chronic obstructive lung disease patients and normal subjects. J Appl Physiol. 2005;99(4):1286–92. doi: 10.1152/japplphysiol.00362.2005. [DOI] [PubMed] [Google Scholar]
- 10.Effros RM, Hoagland KW, Bosbous M, Castillo D, Foss B, Dunning M, Gare M, Lin W, Sun F. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med. 2002;165(5):663–9. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 11.Horvath I, Hunt J, Barnes PJ. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26(3):523–48. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 12.Effros RM, Biller J, Foss B, Hoagland K, Dunning MB, Castillo D, Bosbous M, Sun F, Shaker R. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med. 2003;168(12):1500–5. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 13.Shahid SK, Kharitonov SA, Wilson NM, Bush A, Barnes PJ. Increased interleukin-4 and decreased interferon-gamma in exhaled breath condensate of children with asthma. Am J Respir Crit Care Med. 2002;165(9):1290–3. doi: 10.1164/rccm.2108082. [DOI] [PubMed] [Google Scholar]
- 14.Robroeks CM, Jobsis Q, Damoiseaux JG, Heijmans PH, Rosias PP, Hendriks HJ, Dompeling E. Cytokines in exhaled breath condensate of children with asthma and cystic fibrosis. Ann Allergy Asthma Immunol. 2006;96(2):349–55. doi: 10.1016/S1081-1206(10)61247-1. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen TA, Woo-Park J, Hess M, Goins M, Urban P, Vaughan J, Smith A, Hunt J. Assaying all of the nitrogen oxides in breath modifies the interpretation of exhaled nitric oxide. Vascul Pharmacol. 2005;43(6):379–84. doi: 10.1016/j.vph.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Paget-Brown AO, Ngamtrakulpanit L, Smith A, Bunyan D, Hom S, Nguyen A, Hunt JF. Normative data for pH of exhaled breath condensate. Chest. 2006;129(2):426–30. doi: 10.1378/chest.129.2.426. [DOI] [PubMed] [Google Scholar]
- 17.Hunt J, Byrns RE, Ignarro LJ, Gaston B. Condensed expirate nitrite as a home marker for acute asthma [letter] Lancet. 1995;346(8984):1235–6. doi: 10.1016/s0140-6736(95)92947-9. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan J, Ngamtrakulpanit L, Pajewski TN, Turner R, Nguyen TA, Smith A, Urban P, Hom S, Gaston B, Hunt J. Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur Respir J. 2003;22(6):889–94. doi: 10.1183/09031936.03.00038803. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaou NC, Lowe LA, Murray CS, Woodcock A, Simpson A, Custovic A. Exhaled breath condensate pH and childhood asthma: unselected birth cohort study. Am J Respir Crit Care Med. 2006;174(3):254–9. doi: 10.1164/rccm.200601-140OC. [DOI] [PubMed] [Google Scholar]
- 20.Gessner C, Scheibe R, Wotzel M, Hammerschmidt S, Kuhn H, Engelmann L, Hoheisel G, Gillissen A, Sack U, Wirtz H. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir Med. 2005;99(10):1229–40. doi: 10.1016/j.rmed.2005.02.041. [DOI] [PubMed] [Google Scholar]


