Abstract
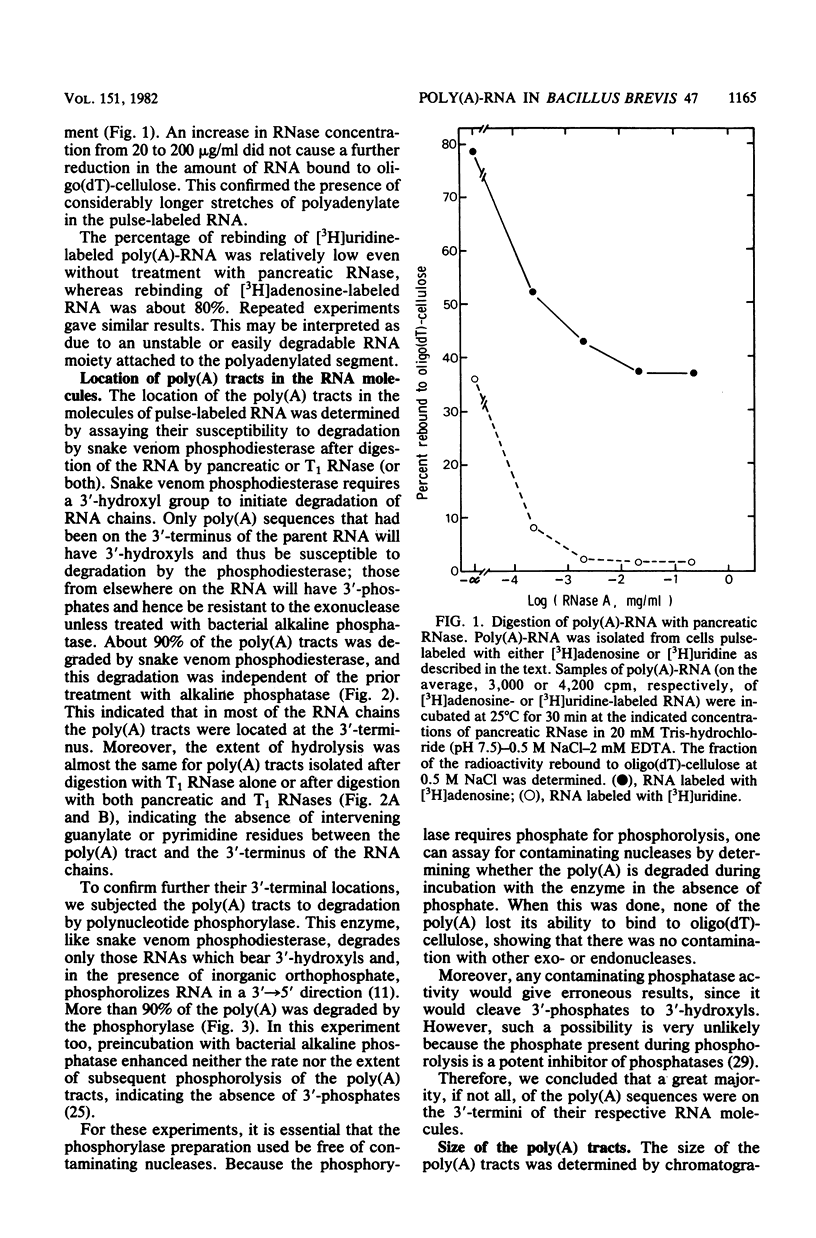
Up to about 50% of the total radioactivity in pulse-labeled RNA in Bacillus brevis 47-5, a high-protein-producing bacterium, was found in the polyadenylated fraction [termed poly(A)-RNA] isolated by adsorption to oligodeoxythymidylic acid-cellulose. Labeled RNA was bound to the cellulose regardless of whether the radioactive precursor was [3H]adenosine or [3H]uridine, showing that the adsorbed material was poly(A)-RNA rather than free poly(A). Poly(A) tracts, isolated after digestion of pulse-labeled RNA with pancreatic and T1 RNases, were homogeneous, with a length of about 95 nucleotides. Susceptibility of the isolated poly(A) tracts to degradation by snake venom phosphodiesterase and polynucleotide phosphorylase indicated that the poly(A) sequences were located directly at the 3'-terminal of the RNA molecules. Comparison of the poly(A)-RNA content in high-protein-producing and nonprotein-producing cells of B. brevis 47 showed much higher levels in the former. Electrophoretic analysis in both denaturing and denaturing polyacrylamide gels of the poly(A)-RNAs showed a heterogeneous population of molecules ranging in size from 23S to 4S. Comparison of the molecular-weight distribution patterns revealed that a significantly greater amount of high-molecular-weight poly(A)-RNA (comigrating with 23S RNA) was present under conditions in which extracellular protein production was high. The possibility that a substantial fraction of the poly(A)-RNA might be involved in the synthesis of extracellular proteins in B. brevis 47 is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust C. H., Jr, Diggelmann H., Mach B. Isolation of poly(adenylic acid)-rich ribonucleic acid from mouse myeloma and synthesis of complementary deoxyribonucleic acid. Biochemistry. 1973 Feb 27;12(5):925–931. doi: 10.1021/bi00729a021. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna Y., Langley D., Sarkar N. Detection of high levels of polyadenylate-containing RNA in bacteria by the use of a single-step RNA isolation procedure. Nucleic Acids Res. 1981 Jul 24;9(14):3545–3554. doi: 10.1093/nar/9.14.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. Messenger RNA metabolism of animal cells. Possible involvement of untranslated sequences and mRNA-associated proteins. J Cell Biol. 1975 Feb;64(2):269–288. doi: 10.1083/jcb.64.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N., Phillips S. Length heterogeneity in the poly (adenylic acid) region of yeast messenger ribonucleic acid. Biochemistry. 1974 Dec 17;13(26):5378–5383. doi: 10.1021/bi00723a020. [DOI] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Hubert E., Leclercq M., Nudel U., Soreq H., Salomon R., Lebleu B., Revel M., Littauer U. Z. Role of the polyadenylate segment in the translation of globin messenger RNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3143–3146. doi: 10.1073/pnas.71.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Jayaraman K. Appearance of polyadenylated RNA species during sporulation in Bacillus polymyxa. Biochem Biophys Res Commun. 1979 Jan 30;86(2):331–339. doi: 10.1016/0006-291x(79)90870-2. [DOI] [PubMed] [Google Scholar]
- Kerjan P., Szulmajster J. Isolation and characterization of polyadenylated RNA species from sporulating cells of Bacillus subtilis. Biochem Biophys Res Commun. 1980 Mar 13;93(1):201–208. doi: 10.1016/s0006-291x(80)80266-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato H., Venkatesan S., Edmonds M. Polyadenylic acid sequences in E. coli messenger RNA. Nature. 1975 Jul 10;256(5513):144–146. doi: 10.1038/256144a0. [DOI] [PubMed] [Google Scholar]
- Ohta N., Sanders M., Newton A. Poly(adenylic acid) sequences in the RNA of Caulobacter crescenus. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2343–2346. doi: 10.1073/pnas.72.6.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Lack of polyadenylic acid sequences in the messenger RNA of E. coli. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1593–1600. doi: 10.1016/0006-291x(72)90896-0. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Staynov D. Z., Gratzer W. B. Electrophoresis of RNA in formamide. Biochemistry. 1974 Dec 17;13(26):5373–5378. doi: 10.1021/bi00723a019. [DOI] [PubMed] [Google Scholar]
- Sarkar N., Langley D., Paulus H. Isolation and characterization of polyadenylate-containing RNA from Bacillus brevis. Biochemistry. 1978 Aug 22;17(17):3468–3474. doi: 10.1021/bi00610a007. [DOI] [PubMed] [Google Scholar]
- Schultz G. A., Chaconas G., Moore R. L. Polyadenylic acid sequences in the RNA of Hyphomicrobium. J Bacteriol. 1978 Feb;133(2):569–575. doi: 10.1128/jb.133.2.569-575.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R., Kates J., Kelley D. E., Perry R. P. Polyadenylic acid sequences on 3' termini of vaccinia messenger ribonucleic acid and mammalian nuclear and messenger ribonucleic acid. Biochemistry. 1972 Sep 26;11(20):3829–3834. doi: 10.1021/bi00770a023. [DOI] [PubMed] [Google Scholar]
- Sogin S. J., Saunders C. A. Fluctuation in polyadenylate size and content in exponential- and stationary-phase cells of Saccharomyces cerevisiae. J Bacteriol. 1980 Oct;144(1):74–81. doi: 10.1128/jb.144.1.74-81.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P. R., Ramanarayanan M., Rabbani E. Presence of polyriboadenylate sequences in pulse-labeled RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2910–2914. doi: 10.1073/pnas.72.8.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Tsukagoshi N., Udaka S. Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1981 Oct;148(1):322–332. doi: 10.1128/jb.148.1.322-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


