Abstract
Background & Aims
The UNC5H netrin-1 receptors (UNC5H1-3, or UNC5A-C) belong to the functional dependence receptors family that share the ability to induce apoptosis in the absence of their ligands. Such a trait has been hypothesized to confer a tumor suppressor activity. Indeed, cells harboring these receptors are thought to be dependent on ligand availability for their survival, thereby inhibiting uncontrolled tumor cell proliferation. We investigate here whether UNC5C acts as a tumor suppressor in colorectal malignancies.
Methods
The level of UNC5C was analyzed in a panel of 86 primary sporadic colorectal carcinomas. Loss of heterozygosity in the UNC5C locus and epigenetic alterations in the UNC5C promoter were also analyzed. Intestinal tumor progression was monitored in mice bearing both UNC5C and APC1638N mutations and apoptosis was measured in intestinal tumors developed in UNC5C/APC1638N mutant mice.
Results
We show here that UNC5C expression is down-regulated in a large fraction of human colorectal cancers, mainly through promoter methylation. Moreover, in mice, inactivation of UNC5C is associated with increased intestinal tumor progression and a decrease in tumor cell apoptosis.
Conclusion
We demonstrate here that the loss of UNC5C expression observed in human colorectal cancer is a selective advantage for tumor progression, in agreement with the dependence receptor hypothesis. Thus, the UNC5C dependence receptor is a tumor suppressor that regulates sporadic colorectal cancer.
Background & Aims
Netrin-1, a diffusible laminin-related protein, plays a major role in the control of neuronal navigation during the development of the nervous system by interacting with its main receptors, DCC (Deleted in Colorectal Cancer) 1 2 3 and UNC5H 4, 5. However, more recently, netrin-1 has emerged as a completely different molecule regulating cell survival: indeed, DCC and UNC5H, (UNC5H1, UNC5H2, and UNC5H3 also called UNC5A, UNC5B and UNC5C) belong to the so-called dependence receptor family 6 7.
Dependence receptors, which also include RET 8, Patched 9, some integrins 10, neogenin 11 and p75NTR 12, share the functional property of inducing cell death when disengaged from their ligands, whereas their pro-apoptotic activity is blocked in the presence of their ligand. These receptors thus create cellular states of dependence on their respective ligands 13, 14. The molecular mechanisms used by these unbound receptors to trigger apoptosis are in large part unknown, yet it appears that their cleavage by caspases is required for cell death induction, as it allows the exposure/release of a pro-apoptotic domain that in turn induces apoptosis 6 15. Other mechanisms, including interaction with pro-apoptotic proteins like DAPK or NRAGE have also been proposed 16, 17.
The pro-apoptotic activity of unbound dependence receptors has so far mainly been observed in vitro, and has been suggested to act as a means of eliminating tumor cells that would develop in settings of ligand unavailability, such as proliferation of tumor cells in a cell environment with constant and limited ligand presence or migration of metastatic tumor cells in tissues where the ligand is absent. This hypothesis fits with the observation that both the DCC and UNC5H genes are down-regulated in tumors, thereby suggesting that their loss represents a selective advantage for tumor development 18, 19 20. However, the only in vivo evidence so far to confirm this model is that netrin-1 overexpression in the mouse gastrointestinal tract leads to tumor initiation and progression 21. Whereas this observation suggests that one or several netrin-1 receptors act(s) as tumor suppressor(s), it falls short of (i) mimicking the human disease, as netrin-1 rarely seems to be over-expressed in human colorectal tumors 21 and (ii) demonstrating per se that one of these dependence receptors acts as a true tumor suppressor.
DCC was proposed in the early 1990s to be a tumor suppressor gene mainly associated with the later stages of intestinal tumor development 19. A large number of subsequent reports support the implication of DCC in colorectal tumor suppression (for a review see 22). However, very few mutations in DCC have been detected in cancer and especially in hereditary cancer diseases, and both heterozygous and homozygous inactivations of DCC in mice have failed to show increased tumor initiation or progression 23, therefore, DCC's role as a tumor suppressor is still unclear.
Here we investigate whether UNC5H3/UNC5C acts as a tumor suppressor in colorectal malignancies. We first demonstrate that expression of the UNC5C gene is down-regulated in colorectal cancers, mainly because of tumor-associated specific promoter methylation. We also show that inactivation of UNC5C in mice is associated with increased intestinal tumor progression and decreased tumor cell apoptosis. These data, taken together with human data on loss of UNC5C pro-apoptotic activity in colorectal cancers, are strongly in support of the UNC5C dependence receptor being a tumor suppressor.
Methods
A detailed methods section is provided in supplementary materials
Cell line and reagents
Colorectal cancer cell lines HCT116, SW480, HCT-15, SW48, LoVo, LS174T, Caco-2, TC7, and Co115 were previously described. The HCT116 double knock-out for DNMT1 and DNMT3b were described previously and were kindly provided by B. Vogelstein 24. HCT116 or SW480 colorectal tumor cells were treated with 5µM 5-Aza-2′-deoxycytidine (Sigma –Aldrich) 24h after plating, treatment was replenished every 24h during 48h. 0.3µM Trichostatin A (ICN) was then added during 24h.
Quantitative real-time RT-PCR and Laser Capture Microdissection
To assay UNC5C expression in human colorectal tumors, total RNA was extracted from biopsies of patients undergoing surgery for colorectal cancer, as described in the supplementary materials. The ubiquitously expressed RxRα, PPIA and GAPDH genes, showing the less variability in expression between normal and colorectal tumoral tissues, were used as internal controls. Moreover, 8 other ordinarily housekeeping genes were also used to strengthen the results: β-actin, Phosphoglycerokinase 1(PGK), β2-microglobulin, Hypoxanthine ribosyltransferase (HPRT), TATA-box-binding protein (TBP), Porphobilinogen deaminase (PBGD), Transferin receptor (TfR), ribosomal protein large P0 (RPLP0). Laser capture was performed under direct microscopic visualization using a PixCell II LCM system (Arcturus, Mountain View, CA) on 16 µm-thick tissue frozen sections prepared from colon tumor and normal biopsies stained with KIT0415 HistoGene staining solution (Arcturus) as described in supplementary materials.
LOH analysis
Genomic DNA (50ng) from matched tumor and corresponding normal tissue was amplified by PCR and analysed as described 18, using fluorescently labeled primers for the indicated polymorphic microsatellite markers on chromosomes 4q21-23 for UNC5C locus. At the difference of Thiebault et al., markers D4S470, D4S1174, D4S1218 were excluded from the study because they were not adequate. Two closer (one inside the gene) and more significant markers designed in our laboratory called C1 and C2inside were selected and respectively amplified using primers: C1For5′CTCCTCCTCCATGCTTTCAG3′ and C1Rev5′GTTTCCATTATGTTTTGTTGGAAAG3′, C2insideFor5′TTCCAGCAAAGGACACATCA3′ and C2insideRev5′ GTTTGGAAAAGAGATCTGCTCA3′.
UNC5C promoter methylation analysis
Methylation-specific PCR (MSP) analysis of sodium-bisulfite-treated DNA from human colorectal biopsies of normal and tumoral tissues was performed as described in supplementary materials. The UNC5C promoter region, amplified from the bisulfite-modified DNA by PCR was also sequenced as described in supplementary materials.
Mice breeding, genotyping and tumor analysis
UNC5CRCM and Apc +/1638N mice were described previously 4 21 and mice genotyping is described in supplementary materials. All animals were used in hybrid B6/C3H genetic background and specific attention was paid to using animals issued of similar crosses for the studies on tumor progression. Indeed, the distribution in low grade/high grade adenoma or adenocarcinoma varies significantly, depending on the genetic background studied and breeding conditions. UNC5Crcm/rcm and wild type animals were killed at 20 months of age. Apc +/1638N UNC5C+/+, Apc +/1638N UNC5C+/rcm, Apc +/1638N UNC5Crcm/rcm mice were killed at a similar age: 6 months. Histological classification and grading of neoplastic lesions was performed in a blinded fashion similarly to 21 and as described in supplementary materials.
Apoptosis, Proliferation and differentiation scoring
Apoptosis, proliferation and differentiation scoring was performed essentially as before 21 and as described in supplementary materials.
In situ hybridization
Specific DIG-labelled sense and antisense RNA probes corresponding to human UNC5C cDNA were generated according to the methods described in the Roche “DIG RNA labelling kit” and as described in the supplementary materials.
Results
Epithelial expression of UNC5C is down-regulated in human colorectal cancers
In initial studies, we analyzed whether UNC5C is expressed in normal colonic epithelium. In situ hybridizations performed on human colon show that UNC5C is expressed in the colonic epithelium (Fig.1A, panels abc). We next investigated whether UNC5C shows a decreased expression in human colorectal cancers. UNC5C expression was analyzed by real-time Q-RT-PCR in a panel of 86 colorectal tumors versus corresponding normal tissues. As shown in Fig.1BCD, a reduction in UNC5C expression was detected in more than 74% of colorectal tumors, with 45% of tumors showing more than a 10 fold reduction. UNC5C expression reduction was also detected by in situ hybridization (Fig.1A, panel d). To discard a potential bias of heterogeneity in tumor/normal tissue samples, epithelial cells from tumor/normal tissue were laser micro-dissected and assessed for UNC5C expression by real-time Q-RT-PCR. In the 4 pairs of samples assessed, an important decrease in UNC5C expression was detected in tumor cells (in 3 sample pairs the loss of UNC5C was actually amplified compared to crude samples) (Fig.1E), demonstrating per se that human colorectal cancer is associated with a loss of UNC5C expression specifically in tumor cells. Interestingly, by comparing the reduction in UNC5C in a fraction of the tested tumors with available clinical charts (43 on 86) of corresponding patients, we observed that loss of UNC5C is associated with tumor progression and aggressiveness (Fig.2 and Supplementary Fig.1). However, even though this correlation is statistically significant, a larger scale study remains to be performed to further demonstrate that loss of UNC5C is a marker for colorectal tumor progression.
Figure 1. Loss of UNC5C expression in colorectal tumors.
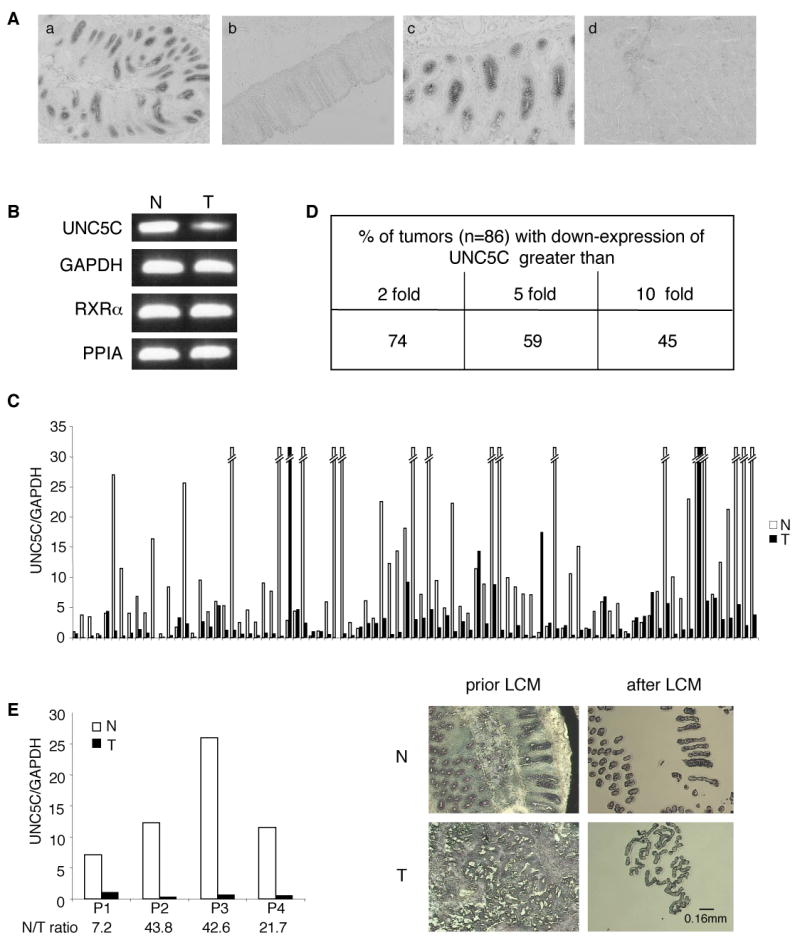
A, In situ hybridization performed in human colon on UNC5C. a: antisense probe on normal colon, b: sense probe, c: enlargement of a, d: antisense probe on adjacent tumor. Original magnifications: X50 (a,b,d), X100 (c). B,C,D, Quantitative real-time RT-PCR was performed using total RNA extracted from normal (N) and tumoral (T) tissues with specific human UNC5C primers 34 and eleven couples of specific human genes primers showing the less variability in their expression between normal and colorectal tumoral tissues, as described in 35, 36 37 38 (see Experimental procedures section). B, A PCR in the exponential phase of a representative pair tumor/normal tissue is shown. C, The expression levels in 86 colorectal tumors (T) and corresponding normal tissue (N) are given only as the ratio between UNC5C and GAPDH. Similar results were obtained with 10 other housekeeping genes described in Experimental procedures D, The percentage of patients showing a loss of UNC5C expression in tumor compared to normal tissue is indicated. E, Laser capture microdissection (LCM) was performed on 4 pairs of tumor/normal tissues and UNC5C expression was determined as in (B). Left: The expression levels in 4 colorectal tumors (T) and corresponding normal tissue (N) are given only as the ratio between UNC5C and GAPDH. Similar results were obtained with the other housekeeping RXRα, PPIA genes. Right: typical microscopic visualization of LCM on colon section from human normal (N) or tumoral biopsies (T). Sections are shown prior to LCM, and after LCM, as the captured material is confirmed under microscopic visualization prior to processing for RNA extraction.
Figure 2. Correlation between patient clinical features and UNC5C expression N/T ratio in a limited panel of patient.
Correlation between UNC5C expression N/T ratio and TNM39 status classified in 4 stages from good to poor prognosis i.e. stage I (T1 or T2, N0M0), stage II (T3 or T4, N0M0), stage III (any T, N+M0) and stage IV (any T, any N, M+) 39. A Kruskall-Wallis test was used comparing the overall panel, p=0.001. A Mann-Whitney test was also used to compare stage I to stage IV (p=0.001), stage II to stage IV (p=0.004). The difference between stage III and stage IV (p=0.120) fails to be significant.
UNC5C expression is inhibited via tumor associated UNC5C promoter methylation
Searching for the mechanisms that may explain such down-regulation, we analyzed whether loss of expression was associated with loss of heterozygosity (LOH) at the UNC5C locus. As previously described, LOH in UNC5C was found in some tumors with reduced UNC5H levels (18 and not shown). However, the relatively low occurrence of LOH at the UNC5C locus (25-35% depending on the microsatellite markers used –see Experimental procedures-) suggested an alternative mechanism for UNC5C loss of expression in colorectal tumors. This prompted our search for UNC5C promoter methylation. While UNC5C expression is hardly detectable in numerous colorectal cancer cell lines (not shown, list in the Methods section), treatment with the methylation inhibitor 5-aza-2′-deoxycytidine (5aza2dC) led to a robust increase of UNC5C expression in both colorectal HCT116 or SW480 cells. Moreover, co-treatment with 5aza2dC and trichostatin A (TSA), a specific inhibitor of the histone deacetylases that interact with methylated CpGs 25, massively turns on UNC5C transcription (Fig.3A). To discard drug toxicity, expression of UNC5C was analyzed in HCT116 cells with a double knock-out for both DNMT1 and DNMT3b, two crucial DNA methyltransferases 24. As shown in Fig.3B, UNC5C expression was more than 130 times higher in the double knock-out HCT-116 cells than in wild-type HCT116 cells, demonstrating the role of DNA methylation in UNC5C regulation. To further determine whether this methylation process is specifically associated with tumor development, we analyzed UNC5C promoter methylation in a panel of 18 colorectal tumors versus adjacent normal tissues. Putative CpG islands found in the UNC5C promoter region were investigated for methylation by Methylation Specific PCR (MSP). As shown in Fig.3C, 78% of the tumor samples showed methylation in the UNC5C promoter, as compared to no methylation in the corresponding normal tissue. The MSP results were confirmed by direct sequencing of CpG islands in the UNC5C promoter region, including that of the first exon in 3 pairs of tumor/normal tissues in which down-regulation of UNC5C had been previously observed. Indeed, we observed that the UNC5C promoter region is highly methylated in the tumor samples, while no methylation occurs in normal tissues (Fig.3D and not shown). Thus, UNC5C expression is lost or highly decreased in colorectal tumors through specific tumor-associated promoter methylation.
Figure 3. Epigenetic inactivation of UNC5C in human colorectal tumors.
A, HCT116 and SW480 cells were treated with 5µM 5-Aza-2′-deoxycytidine (5aza2dc) for 48H and then with 0.3µM TSA for 24H. Quantitative real-time RT-PCR was performed with UNC5C primers relatively to GAPDH, RXRα and PPIA, as explained in Fig.1. The expression level is presented as a ratio between UNC5C and GAPDH. B, UNC5C expression was compared in HCT116 versus HCT116 DKO for DNMT1 and 3b 24 by quantitative real-time RT-PCR, as described previously. C, Methylation-specific PCR (MSP) analysis of sodium-bisulfite-treated DNA from human colorectal biopsies of Normal tissues (N) and Tumoral tissues (T). Amplification using primers that hybridize specifically on sequences of the UNC5C promoter that are Unmethylated and/or Methylated (data shown only for 4 different biopsies, biopsy 4 showing both the methylated and unmethylated states of the UNC5C promoter in tumoral tissue, whereas in the three others, UNC5C promoter is completely methylated in tumoral tissue). Compiled results on 18 human colorectal biopsies are presented in the table. D, Change in MeCpG content in the region of the UNC5C promoter in three different tumors (T1,T2,T3). Upper: Representation of the DNA region surrounding the first exon (in grey) with transcriptional start site arrowed in black (Accession n°NM_003728). The locus includes a CpG island of 2298bp in length (%G+C=59; ObsCpG/ExpCpG=0.729; CG island searcher software). The region amplified by PCR primers is enlarged and the position of each CpG is indicated from the UNC5C transcription start site. Bottom: Ten PCR clones were sequenced from three different tumors (T1,T2,T3) and from the corresponding normal tissues (N1,N2,N3) (not shown as no methylation is detected) to determine the percentage of methylation at the CpG sites in the analysed region.
Inactivation of UNC5C is associated with tumor progression in mice
Loss of UNC5C expression and the known inhibitory effect of UNC5C on hallmarks of cell transformation in vitro –anchorage-independent growth and invasion- 18 were suggestive of a putative UNC5C tumor suppressor activity. To further investigate this in vivo, we studied whether mutations in UNC5C were associated with tumor predisposition in mice. RCM (Rostral Cerebellum Malformation) mice have been shown to display a natural loss-of-function mutation in UNC5C, mainly associated with developmental problems in the cerebellum 4. Interestingly, while inactivation of the other netrin-1 receptors encoding genes –DCC, UNC5HA/1, UNC5HB/2- in mice is associated with embryonic lethality, UNC5Crcm mutant mice go through adulthood. We first analyzed spontaneous tumor development. However, we failed to detect any obvious intestinal tumor development when compared to control mice, hence suggesting that UNC5C inactivation in mice is not a sufficient genetic event to trigger tumor initiation (not shown). We then investigated whether UNC5C inactivation affects tumor progression. UNC5Crcm mice were backcrossed to an Apc+/1638N genetic background 26. APC mutations exist in the vast majority of human colorectal tumors, and in mice, specific Apc mutations trigger the formation of tumors in the gastrointestinal tract –mainly low-grade adenomas but also, albeit at a smaller extent, more aggressive tumors, depending on the genetic background and animal breeding 23, 26 27-. In the genetic background studied here, the number of neoplastic lesions per control (Apc +/1638NUNC5C+/+) mouse was 1.13, with a predominance of low-grade adenomas (Fig.4A). A striking difference was observed, however, in Apc+/1638NUNC5C+/rcm and Apc +/1638NUNC5Crcm/rcm mice (Fig. 4). Although these mice displayed an overall frequency of neoplastic lesions similar to Apc +/1638N mice –a slight increase that was not statistically significant-, they showed a dramatic shift of low-grade adenomas towards higher grade tumors (low grade tumors: 71% (12 tumors out of 17) (UNC5C+/+) vs. 43% (36/83) (UNC5C+/rcm) vs. 17% (5/30) (UNC5Crcm/rcm), significance of the overall differences between UNC5C+/+ and UNC5Crcm/rcm: p<0.001). The shift appears to occur both at the level of high grade adenoma (6% (1/17) (UNC5C+/+) vs 40% (12/30) (UNC5Crcm/rcm)) and at the level of higher grade lesions, fulfilling all the histological criteria for an adenocarcinoma diagnosis (Fig.4F), including stroma formation and signs of local invasion (involving the submucosa in most cases and the muscularis propria in some, 28) (23% (4/17) (UNC5C+/+) vs 43% (13/30) (UNC5Crcm/rcm)). Thus, UNC5C inactivation is associated with enhanced tumor stage in the presence of an Apc mutation, demonstrating per se that UNC5C loss-of-function is a selective advantage for tumor progression. Interestingly, a gradual higher grade tumor development occurs from UNC5C+/+ to UNC5C+/rcm and to UNC5Crcm/rcm mice, suggesting an inverse correlation between the level of functional UNC5C and tumor progression.
Figure 4. Increased intestinal tumor progression in UNC5C mutant mice.
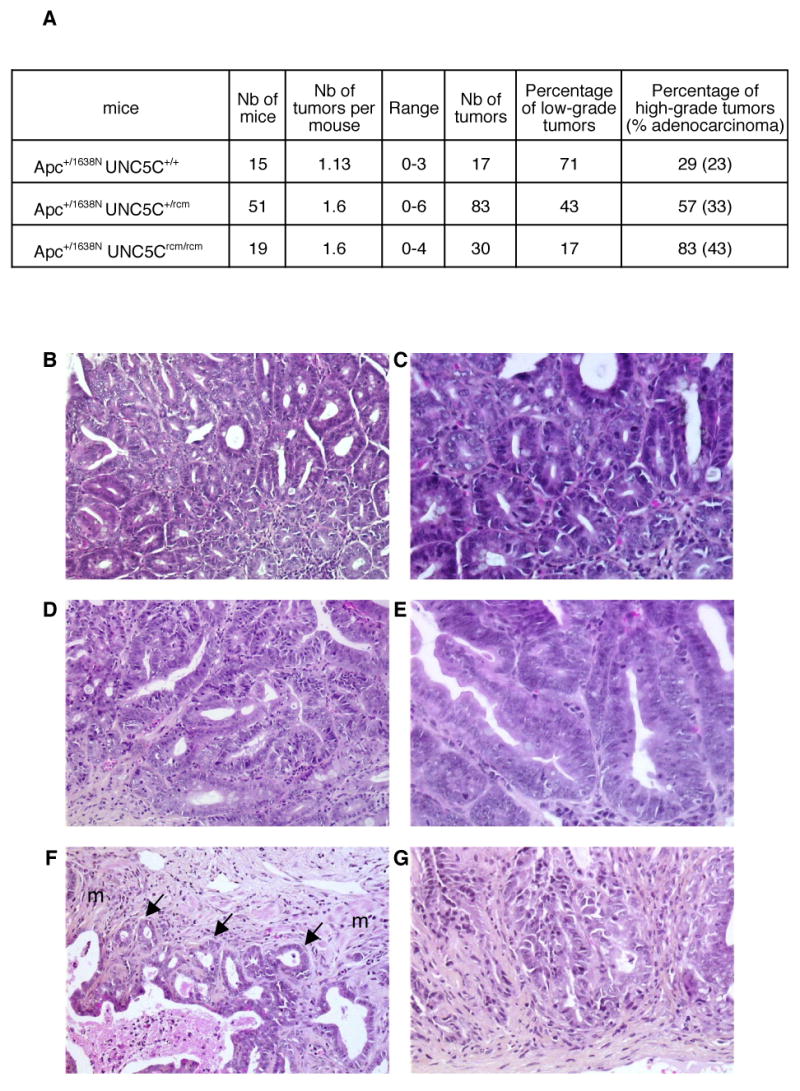
UNC5Crcm mice were backcrossed into an Apc1638N background and analysed for tumor development in 6 months old mice. A, Table showing the number (Nb) of Apc+/1638N UNC5Crcm/rcm, Apc+/1638N UNC5C+/rcm or Apc+/1638N UNC5C+/+ mice studied, the average number of tumors per mouse, the range, the number of tumor studied and the proportion of low-grade tumors and high-grade tumors including adenocarcinomas. Significance: χ2 test, p<0.001. B-G: representative lesions from Apc +/1638N UNC5Crcm/rcm mice: low grade adenoma (B,C), high grade adenoma (D,E) and adenocarcinoma (F, note the presence of muscularis (m) invasion indicated by arrows, G). C,E,G enlargement of respectively B,D,F, Original magnifications: B,D,F, X80; C,E,G, X250
UNC5C inactivation is associated with a reduction in apoptosis in mice tumors
In an attempt to identify whether this increased tumor progression susceptibility was due to increased proliferation or to decreased apoptosis, we analyzed proliferation/differentiation/cell death in the intestinal tract of UNC5C+/+ or UNC5Crcm/rcm mice. No obvious change was observed, neither in differentiation, as determined by numbering goblet cells (Suppl Fig.2AB), nor in proliferation, as measured by BrdU incorporation (Suppl Fig.2CD), nor in cell death, as determined by counting cells with apoptotic morphology (not shown). In contrast, when analyzing the proliferation/apoptosis status in tumors from either Apc+/1638N UNC5C+/+, Apc+/1638N UNC5C+/rcm or Apc+/1638N UNC5Crcm/rcm mice, an interesting observation was made. While the number of proliferative cells was not affected when comparing same grade tumors (Suppl Fig.2E), a significant reduction in the number of apoptotic cells was detected in adenomas and adenocarcinomas from UNC5C mutant animals (Fig.5). In particular, whereas in adenocarcinomas from Apc+/1638N UNC5C+/+ mice, islets of cells undergoing apoptosis were frequently detected, very few were observed in adenocarcinomas from Apc+/1638N UNC5C+/rcm and Apc+/1638N UNC5Crcm/rcm mice (Fig.5). It is therefore tempting to speculate that progression from low-grade adenoma towards high grade adenoma or adenocarcinoma associated with UNC5C loss-of-function occurs through loss of apoptosis induction, as would be expected following the dependence receptor hypothesis.
Figure 5. Tumors from UNC5C mutant mice are less prone to apoptosis.

A, Table showing the number of apoptotic cells per section of tumors (sample) issued from Apc+/1638N UNC5C+/+ mice, Apc+/1638N UNC5C+/rcm or Apc+/1638N UNC5Crcm/rcm. B,C, Representative microscopic images from adenocarcinoma from either Apc+/1638N UNC5C+/+ (B) or Apc+/1638N UNC5Crcm/rcm (C) mice. Note in adenocarcinomas from Apc+/1638N UNC5C+/+ mice, the presence of several (more than 3) apoptotic cells side by side that form islets of apoptotic cells. Apoptotic cells are shown by arrows. Original magnifications: X450. D,E, similar as in B, C except that TUNEL staining was performed as described in the Experimental procedures section.
Conclusion
Here we present evidence that UNC5C is a tumor suppressor gene lost in the vast majority of human colorectal tumors. UNC5C loss-of-function in mice is associated with tumor progression. In agreement with the dependence receptor model, we have shown here that losing UNC5C in order to survive in settings of netrin-1 limitation would be a selective advantage for a tumor cell. In the human pathology, this selective advantage appears to be achieved at low frequency by LOH or at a much larger extent by promoter methylation.
Together with the fact that overexpression of netrin-1 in mouse gastrointestinal tract is associated with tumor initiation and progression 21, these results give support to the view that the UNC5C/netrin-1 pair acts as a rheostat, regulating colorectal tumorigenesis. Receptor loss-of-function or gain of its ligand have a similar effect on tumor development, strongly supporting the notion that dependence receptors regulate tumorigenesis by eliminating tumor cells through apoptosis.
It is however interesting to note that while netrin-1 overexpression in the gut was associated with a reduction of apoptosis in the intestinal epithelium 21, no such effect was observed in the intestinal epithelium of UNC5C mutant mice. One can speculate that this difference is related to an alternative role of UNC5C, independent of its dependence receptor activity. UNC5H receptors were indeed initially proposed to be involved in cell migration during embryonic development, as shown with neuronal migration especially 29. However, such a migratory activity remains to be shown in adult tissue. Recently, UNC5B/H2 was also shown to be a negative regulator of embryonic vascularisation 30. However, UNC5C is not expressed in endothelial cells, and neither netrin-1 nor UNC5C mutant mice showed obvious defects in vasculature formation. Thus, it is tempting to speculate that the increased tumor progression detected here is related to the pro-apoptotic function of UNC5C. Along this line, the observed increased frequency of adenocarcinomas associated with UNC5C loss-of-function and decreased apoptosis in these surnumerary adenocarcinomas strongly suggest that the effect seen on tumor progression is due to loss of UNC5C pro-apoptotic activity. The difference in the apoptotic status of transgenic netrin-1 mice normal intestinal epithelium and that of UNC5C mutant mice could rather be explained by the fact that, while overexpression of netrin-1 blocks apoptosis of all the netrin-1 dependence receptors –DCC and UNC5A,B,C-, loss of UNC5C should only affect UNC5C-induced cell death. Along this line, while netrin-1 overexpression affects both tumor initiation (i.e., spontaneous adenoma formation) and progression (i.e., shift toward high-grade adenoma and adenocarcinoma when associated with the Apc mutation), loss of UNC5C appears to affect only tumor progression. This would also suggest that not only UNC5C, but other netrin-1 dependence receptors, such as DCC, UNC5A/H1 or UNC5B/H2, also play an important role as tumor suppressors in colorectal cancer.
Almost nothing is known on UNC5A/H1 and UNC5B/H2 regarding their possible implications in cancer, except that the UNC5B gene has been shown to be a direct transcriptional target of p53 and, as such, able to mediate part of p53 pro-apoptotic activity 31. On the opposite, DCC was proposed in the early 1990s to be a tumor suppressor gene. Loss of DCC expression was mainly associated with human colorectal tumor development 19, and a large number of subsequent reports support the implication of DCC in colorectal tumor suppression (for a review see 22). Yet, in large part because Fazeli and colleagues showed that DCC heterozygous mutant mice only display a modest, non significant increase in intestinal tumors and that homozygous inactivation of DCC in mice in an APCmin background does not lead to increased tumor progression 23, serious doubts about DCC's role have been raised. If the controversy on DCC is still open, and most probably requires the analysis of additional mice models, it is interesting to note that both DCC and UNC5C appear to be down-regulated in later stages of colorectal malignancy. Indeed, decreased DCC expression was mainly associated with later stages of intestinal tumor development 19 and in most studies, DCC has been considered as a marker of poor prognosis 32 33. Here, even though a larger scale study remains to be performed, we observe that decreased UNC5C expression is associated with stage III-IV tumors. Thus, UNC5C may turn out to be an interesting marker for colorectal malignancies.
Supplementary Material
Acknowledgments
We wish to thank C Rey for the help on laser microdissection, B. Vogelstein, R. Fodde and S. Robine for materials, S. Tavtigian for LOH studies, P.H Romeo, A. Chedotal, L. Pays and R. Dante for precious advice and H. Bilak for text correction.
Grant support: This work was supported by the Ligue Contre le Cancer (to PM), the NIH (NS45093, to PM), the FRM (PM), the ARC (PM), the Region Rhone-Alpes and the Canceropole CLARA (to PM, JYS) and the Institut National du Cancer (INCA) (to PM and JYS).
Footnotes
No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–14. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 2.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–85. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 3.Forcet C, Stein E, Pays L, Corset V, Llambi F, Tessier-Lavigne M, Mehlen P. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417:443–7. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–42. doi: 10.1038/386838a0. [DOI] [PubMed] [Google Scholar]
- 5.Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–41. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 6.Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395:801–4. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 7.Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. Embo J. 2001;20:2715–22. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen DE, Edery P, Mehlen P. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. Embo J. 2000;19:4056–63. doi: 10.1093/emboj/19.15.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–6. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 10.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:459–70. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsunaga E, Tauszig-Delamasure S, Monnier PP, Mueller BK, Strittmatter SM, Mehlen P, Chedotal A. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004;6:749–55. doi: 10.1038/ncb1157. [DOI] [PubMed] [Google Scholar]
- 12.Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–8. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 13.Mehlen P, Thibert C. Dependence receptors: between life and death. Cell Mol Life Sci. 2004;61:1854–66. doi: 10.1007/s00018-004-3467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredesen DE, Mehlen P, Rabizadeh S. Receptors that mediate cellular dependence. Cell Death Differ. 2005;12:1031–43. doi: 10.1038/sj.cdd.4401680. [DOI] [PubMed] [Google Scholar]
- 15.Mehlen P, Bredesen DE. The dependence receptor hypothesis. Apoptosis. 2004;9:37–49. doi: 10.1023/B:APPT.0000012120.66221.b2. [DOI] [PubMed] [Google Scholar]
- 16.Williams ME, Strickland P, Watanabe K, Hinck L. UNC5H1 induces apoptosis via its juxtamembrane domain through an interaction with NRAGE. J Biol Chem. 2003;278:17483–17490. doi: 10.1074/jbc.M300415200. [DOI] [PubMed] [Google Scholar]
- 17.Llambi F, Lourenco FC, Gozuacik D, Guix C, Pays L, Del Rio G, Kimchi A, Mehlen P. The dependence receptor UNC5H2 mediates apoptosis through DAP-kinase. Embo J. 2005;24:1192–201. doi: 10.1038/sj.emboj.7600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiebault K, Mazelin L, Pays L, Llambi F, Joly MO, Saurin JC, Scoazec JY, Romeo G, Mehlen P. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc Natl Acad Sci U S A. 2003;100:4173–4178. doi: 10.1073/pnas.0738063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- 20.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 21.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, Bredesen DE, Scoazec JY, Mehlen P. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–4. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 22.Mehlen P, Fearon ER. Role of the dependence receptor DCC in colorectal cancer pathogenesis. J Clin Oncol. 2004;22:3420–8. doi: 10.1200/JCO.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 24.Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW, Baylin SB, Vogelstein B. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–6. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 25.Bird AP, Wolffe AP. Methylation-induced repression--belts, braces, and chromatin. Cell. 1999;99:451–4. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 26.Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, Breukel C, Alt E, Lipkin M, Khan PM, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci U S A. 1994;91:8969–73. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–56. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 28.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 29.Mehlen P, Furne C. Netrin-1: when a neuronal guidance cue turns out to be a regulator of tumorigenesis. Cell Mol Life Sci. 2005;62:2599–2616. doi: 10.1007/s00018-005-5191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–86. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 31.Tanikawa C, Matsuda K, Fukuda S, Nakamura Y, Arakawa H. p53RDL1 regulates p53-dependent apoptosis. Nat Cell Biol. 2003;5:216–23. doi: 10.1038/ncb943. [DOI] [PubMed] [Google Scholar]
- 32.Shibata D, Reale MA, Lavin P, Silverman M, Fearon ER, Steele G, Jr, Jessup JM, Loda M, Summerhayes IC. The DCC protein and prognosis in colorectal cancer. N Engl J Med. 1996;335:1727–32. doi: 10.1056/NEJM199612053352303. [DOI] [PubMed] [Google Scholar]
- 33.Sun XF, Rutten S, Zhang H, Nordenskjold B. Expression of the deleted in colorectal cancer gene is related to prognosis in DNA diploid and low proliferative colorectal adenocarcinoma. J Clin Oncol. 1999;17:1745–50. doi: 10.1200/JCO.1999.17.6.1745. [DOI] [PubMed] [Google Scholar]
- 34.Latil A, Chene L, Cochant-Priollet B, Mangin P, Fournier G, Berthon P, Cussenot O. Quantification of expression of netrins, slits and their receptors in human prostate tumors. Int J Cancer. 2003;103:306–15. doi: 10.1002/ijc.10821. [DOI] [PubMed] [Google Scholar]
- 35.Feroze-Merzoug F, Berquin IM, Dey J, Chen YQ. Peptidylprolyl isomerase A (PPIA) as a preferred internal control over GAPDH and beta-actin in quantitative RNA analyses. Biotechniques. 2002;32:776–8. 780, 782. doi: 10.2144/02324st03. [DOI] [PubMed] [Google Scholar]
- 36.Blanquicett C, Johnson MR, Heslin M, Diasio RB. Housekeeping gene variability in normal and carcinomatous colorectal and liver tissues: applications in pharmacogenomic gene expression studies. Anal Biochem. 2002;303:209–14. doi: 10.1006/abio.2001.5570. [DOI] [PubMed] [Google Scholar]
- 37.Feilchenfeldt J, Brundler MA, Soravia C, Totsch M, Meier CA. Peroxisome proliferator-activated receptors (PPARs) and associated transcription factors in colon cancer: reduced expression of PPARgamma-coactivator 1 (PGC-1) Cancer Lett. 2004;203:25–33. doi: 10.1016/j.canlet.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 38.de Kok JB, Roelofs RW, Giesendorf BA, Pennings JL, Waas ET, Feuth T, Swinkels DW, Span PN. Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab Invest. 2005;85:154–9. doi: 10.1038/labinvest.3700208. [DOI] [PubMed] [Google Scholar]
- 39.American Joint Committee on Cancer: AJCC Cancer Staging Manual. 5th. Philadelphia: Pa: Lippincott-Raven Publishers; 1997. Colon and rectum; pp. 83–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




