Abstract
Recent evidence suggests that mu opioid receptors (MOR) are key regulators of hippocampal structure and function. For example, exogenous MOR agonists morphine and heroin negatively impact hippocampal function and decrease adult hippocampal neurogenesis. Here we explored the role of MOR in the birth and survival of hippocampal progenitor cells by examining adult neurogenesis in mice that lack MOR. Adult male mice lacking exon 1 of MOR were injected with the S phase marker bromodeoxyuridine (BrdU) and sacrificed either two hours or four weeks later to evaluate proliferating and surviving BrdU-immunoreactive (IR) cells, respectively, in the adult hippocampal granule cell layer. WT, heterozygote, and homozygote mice did not differ in the number of BrdU-IR cells at a proliferation time point. However, four weeks after BrdU injection, heterozygote and homozygote mice had 57% and 54% more surviving BrdU-IR cells in the hippocampal granule cell layer as compared to WT mice. A decrease in apoptosis in the heterozygote and homozygote mice did not account for the difference in number of surviving BrdU-IR cells since there were no alterations in number of pyknotic, TUNEL-positive, or activated caspase 3-IR cells compared to WT. In concordance with the increased numbers of granule cells maturing into neurons, heterozygote and homozygote mice had larger hippocampal granule cell layers and increased numbers of granule cells. These findings indicate that MOR may play a role in regulating progenitor cell survival and more generally encourage further exploration of how MOR activation can influence hippocampal structure and function.
Keywords: mu, hippocampus, dentate gyrus, adult neurogenesis, BrdU, stereology
Introduction
The mu opioid receptor (MOR) is highly expressed in brain monoaminergic pathways, brainstem, and spinal cord, and has long been implicated in processes related to these brain regions, such as addiction, alertness, and nociception (Arvidsson et al., 1995, Mansour et al., 1995, Uhl et al., 1999, Kreek et al., 2005). However, recent evidence points to a more novel role for MOR: regulating hippocampal structure and function (Morris and Johnston, 1995, Martinez and Derrick, 1996, Terman et al., 2000, Kearns et al., 2001, Harrison et al., 2002, Guo et al., 2005, Vigano et al., 2005a, Vigano et al., 2005b). MOR is expressed on interneurons and primary cell types in all hippocampal regions (Meibach and Maayani, 1980, Arvidsson et al., 1995, Mansour et al., 1995, Svoboda et al., 1999, Drake and Milner, 2002), including in the granule cell layer (GCL) of the dentate gyrus. Hippocampal structure, physiology, and biochemistry are potently influenced by exogenous MOR agonists, like morphine and heroin, and by perturbation of the levels of endogenous agonists, like enkephalin and dynorphin (Morris and Johnston, 1995, Simmons and Chavkin, 1996, Chavkin, 2000). For example, chronic exposure to MOR agonists negatively impacts hippocampal function, defined here as hippocampal-dependent behaviors, while blockade of MOR and other opiate receptors enhances hippocampal function (Spain and Newsom, 1989, Spain and Newsom, 1991). Supporting the role for MOR in hippocampal function, transgenic mice that lack MOR or endogenous MOR ligands have altered behavior in hippocampal-dependent tasks (Jamot et al., 2003, Jang et al., 2003, Nguyen et al., 2005, Sanders et al., 2005). These studies encourage more detailed exploration of how MOR regulates hippocampal structure and function.
In this regard, an intriguing property of the adult hippocampal dentate gyrus is its capability to generate new neurons throughout life (Eisch, 2002, Abrous et al., 2005). Neural progenitors located in and adjacent to the GCL proliferate, differentiate, and mature to become functional granule cell neurons (van Praag et al., 2002, Kempermann et al., 2004). All stages of hippocampal progenitor development – proliferation, differentiation, and survival – can be influenced by environmental and physiological stimuli (Cameron et al., 1998, Eisch, 2002, Abrous et al., 2005). For example, adult in vivo exposure to MOR agonists decreases the proliferation and survival of new neurons in the hippocampus (Eisch et al., 2000, Mandyam et al., 2004, Kahn et al., 2005, Eisch and Harburg, 2006). In order to examine the impact of loss of MOR on adult hippocampal neurogenesis, we examine the proliferation, differentiation, and survival of new neurons in the hippocampus of adult transgenic mice lacking exon 1 of MOR (Sora et al., 1997). We find that MOR knockout (KO) mice have normal proliferation and differentiation, but enhanced survival of new neurons and more granule cells. These results suggest a previously unappreciated role for MOR in the normal development of hippocampal granule cells.
Experimental procedures
Animals
Adult male mice with a deletion of exon 1 of MOR were created on a C57BL/6 and 129Sv mixed background as previously described (Sora et al., 1997). MOR KO mice were generated from heterozygote crosses to produce wild-type (WT), heterozygote, and homozygote littermates. WT, heterozygote KO and homozygote KO mice were genotyped by PCR using two internal primers, one targeted at the NEO insertion in the KO construct and one targeted at the WT gene, and one external primer, which generated two products identifying the WT and KO genes. PCR using Tsg DNA polymerase (Lamda Biotech, St. Louis, MO) was performed on tail DNA eluted after digestion overnight in Protease K. The forward primer (5’ CTG GAT GAG CTG TAA GAA TAG G 3’) and the WT primer (5’ CAG CCA ACA CAA TAT CAC ATT C 3’) produced a 550 bp band, while the forward primer and the NEO primer (5’ CGG ACA GGT CGG TCT TGA C 3’) produced an 800 bp band. PCR amplification products were separated by electrophoresis on 4% agarose gels and bands were visualized under UV illumination. The mice were housed at 24°C with a 12:12 light/dark cycle and ad libitum access to food and water, and all procedures were performed according to AAALAC guidelines in a vivarium at NIDA-IRP in Baltimore, Maryland.
Bromodeoxyuridine (BrdU) injections and tissue preparation
In order to assess levels of cell proliferation, differentiation, and survival of new cells in the dentate gyrus, mice were given one i.p. injection of BrdU (150 mg/kg; Boehringer Mannheim, Mannheim, Germany) dissolved in 0.9% saline and 0.007 N NaOH at 10 mg/ml as previously described (Mandyam et al., 2004). A total of forty-four male mice were used for this study. To assess proliferation, 22 mice (WT=10, heterozygote=5, homozygote=7) were perfused two hours after BrdU injection. To assess differentiation and survival 22 mice (WT=8, heterozygote=5, homozygote=9) were perfused 28 days after BrdU injection to allow time for BrdU cells born four weeks earlier to achieve their mature phenotype. All mice were anesthetized with chloral hydrate and perfused transcardially with cold 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4) for 30 minutes at a rate of 7 ml/min. After perfusion, brains were removed and postfixed in 4% paraformaldehyde in 0.1 M PBS for at least 24 hours at 4°C. Brains were cryoprotected in 30% sucrose in 0.1M PBS with 0.1% NaN3 at 4°C until sectioning. Thirty µm coronal sections were taken on a freezing microtome (Leica, Wetzlar, Germany). Nine serial sets of sections were collected through the entire hippocampus. Sections were stored in 0.1% NaN3 in 1xPBS at 4°C until processed for immunohistochemistry (IHC).
IHC and histology
Every ninth section of the hippocampus was mounted on glass slides (Fisher Superfrost/Plus, Hampton, NH) and left to dry overnight prior to IHC. Slides were coded so the experimenter was blind to the genotype of the animal until completion of analysis. Sections used for IHC were pretreated as follows: DNA denaturation (0.01 M citric acid, pH 6.0, 95°C, 10 min), membrane permeabilization (0.1% trypsin in 0.1 M Tris and 0.1% CaCl2, 10 min), and acidification (2 M HCl in 1xPBS, 30 min). Primary antibody concentrations were as follows: rat anti-BrdU (Accurate, Westbury, NY; 1:100), rabbit anti-activated-caspase 3 (AC3; Cell Signaling Technology, Beverly, MA; 1:500), rabbit anti-glial fibrillary acidic protein (GFAP; Dako, Carpinteria, CA; 1:500), and mouse anti-NeuN (Chemicon, Temecula, CA; 1:50). Single-labeling IHC for BrdU or AC3 was completed using the avidin-biotin/diaminobenzidine visualization method (Vector Laboratories, Pierce, Rockford, IL) followed by counterstaining with Fast Red (Vector). Triple-labeling for BrdU, GFAP, and NeuN was performed by co-incubation in a primary cocktail overnight followed by co-incubation in a cocktail of the following fluorescent secondary antibodies (Jackson Immunoresearch, West Grove, PA; anti-rat CY2 (1:200), anti-mouse CY3 (1:200), and anti-rabbit CY5, (1:500)), and completed by counterstaining with DAPI (Roche, Basel, Switzerland; 1:5000). Apoptotic nuclei were detected using AC3-immunoreactivity as described above, presence of pyknotic cells in Fast Red-stained sections, or terminal transferase-mediated dUTP nick-end labeling (TUNEL). Before beginning the TUNEL procedure (DeadEnd Colorimetric TUNEL System; Promega, Madison, WI), slide-mounted sections were pretreated with 0.1 M sodium citrate buffer, pH 6.0 at 95°C for 5 min (Heine et al., 2004).
Quantification of immunoreactive (IR) and apoptotic cells
Cell birth, survival, and death were examined in the neurogenic region of the dentate gyrus. This region, referred to here as the GCL, was composed of the subgranular zone (SGZ; extending from the border of the GCL to three granule cell widths into the hilus) and the adjacent GCL (Kuhn et al., 1996, Kempermann et al., 1997, Donovan et al., 2006). Using modified stereology with the optical fractionator method (Eisch et al., 2000, Mouton, 2002), immunoreactive cells were quantified in the GCL along the longitudinal extent of the hippocampus (bregma −0.70 to −4.16, Franklin and Paxinos, 1997). Cell counts were performed at 400x and 1000x magnification with an Olympus BX-51 microscope while continually adjusting the focal plane through the depth of the section. Since counting of cells was conducted on every ninth section of the hippocampus, the number of counted cells in the region was multiplied by nine to obtain an estimate of the total number of cells per region. Cell birth and survival were determined by the number of BrdU-IR cells present in the GCL two hours or four weeks after BrdU injection, respectively. To control for bioavailability of BrdU between genotypes, BrdU-IR cells within the medial habenula, putatively dividing mast cells (Zhuang et al., 1999), were also quantified (Mandyam et al., 2004, Donovan et al., 2006, Lagace et al., 2006). The habenula is non-neurogenic, unlike more commonly used control regions (e.g. subventricular zone (SVZ) (Ming and Song, 2005), corpus callosum (Dayer et al., 2005)), and its small volume and relative homogeneity allows appropriate stereologic analysis throughout its longitudinal axis. Cell death was assessed in GCL and hilus by quantification of AC3-IR, TUNEL-positive, or pyknotic cells (Eisch et al., 2000, Donovan et al., 2006) as previously described (Gould et al., 1990, Geloso et al., 2002, Heine et al., 2004).
Phenotypic analysis
In order to determine the differentiation of adult-generated GCL cells into either neurons or glial, triple labeled sections from the four-week group (n=4 animals of each genotype) were examined for co-localization of BrdU with NeuN or GFAP. At least 45 BrdU-IR cells in the GCL of each animal (WT 48±3, heterozygote 74±9, homozygote 66±6) were analyzed. All cells were optically sectioned in the Z plane using a confocal microscope (Zeiss Axiovert 200 and LSM510-META, Carl Zeiss, Oberkochen, Germany) with three laser lines (emission wavelengths 488, 543, and 633), multi-track scanning, and a section thickness of 0.45 µm. Orthogonal and rotational analysis in a 3D reconstruction program (Volocity, Improvision Inc, Lexington, MA) confirmed co-localization.
Stereological Estimation of Structural Volume and Granule Cell Number
GCL volume and granule cell number were determined in 30 µm sections stained with Fast Red (Donovan et al., 2006). One section per 240 µm was analyzed spanning bregma −0.70 to −4.16 (Franklin and Paxinos, 1997) such that the entire longitudinal axis of the hippocampus was analyzed. Sections were coded so the experimenter was blind to the genotype of the animal until completion of analysis. All measurements were obtained using StereoInvestigator software (MBF Bioscience, Williston, VT) and an Olympus BX51 microscope. Volumes were measured according to the Cavalieri principle (Gundersen and Jensen, 1987, West and Gundersen, 1990) using a 40x objective. The total number of granule cells was determined using optical disectors along with the optical fractionator method (West et al., 1991) and a 100x/NA 1.30 oil objective. The right GCL of each animal was analyzed for neuron number since there was no statistical difference between the right and left GCL volumes. Data are presented as right GCL neuron numbers only. A grid size of 125 µm × 125 µm was superimposed over each section, and granule cells in fields within the GCL were counted in 15 × 15 × 6 µm sample volumes, with upper and lower guard volumes of 1 µm, resulting in an average of 160 sampling sites per GCL.
Statistical analyses and presentation
Data are represented as mean ± SEM. Statistical analyses were performed using a multiple variable analysis of variance (ANOVA) followed by a Bonferroni post-hoc test. For repeated measure variables (bregma), a repeated measures ANOVA was used to assess the effect of genotype on the number of BrdU-IR cells as specific bregma points, followed by a Bonferroni post-hoc test. All statistical analysis was performed on a Macintosh computer using SPSS version 11.0.2. Statistical significance was defined as p<0.05. Images were imported into Photoshop version 7.0.1 (Adobe Systems Inc., San Jose, California) and the only adjustments made were via gamma in the Levels function.
Results
Proliferation
Two hours after BrdU injection, BrdU-IR cells were evident in the SGZ and GCL of the hippocampus. As previously shown, proliferating cells were small, clustered, and irregularly shaped (Fig. 1). No differences were evident in the size, clustering, or shape of BrdU-IR cells across genotypes. Quantification of the total number of BrdU-IR cells showed no significant effect of genotype in the GCL (Fig. 1B; F(2,19)=1.430, p>0.05) or the habenula (Fig. 1B; F(2,19)=0.478, p>0.05), a region we use to assess bioavailability of BrdU (Mandyam et al., 2004, Donovan et al., 2006). The distribution of BrdU-IR cells in all groups differed significantly across the longitudinal axis of the GCL (Fig. 1C; F(14,266)=31.116, p<0.001), with two peaks of BrdU-IR cells in the GCL at bregma −1.66 and bregma −3.2. There was also a significant interaction of bregma and genotype (Fig. 1C, F(28,266)=1.843, p<0.01). However, post hoc analysis revealed no significant difference in cell number at any bregma value among the genotypes. Therefore, while a trend exists (Fig. 1B, 1C), the number of proliferating cells in the adult mouse GCL is unchanged in MOR KO mice.
Figure 1. MOR knockout (KO) mice have increased cell survival without changes in proliferation.
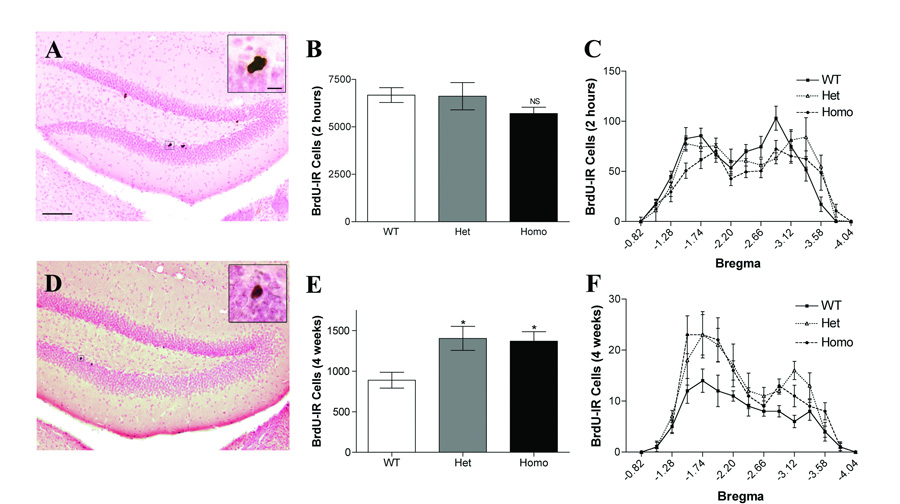
A–C: Cell proliferation in the adult hippocampal granule cell layer 2 hours after BrdU administration. A. BrdU-labeled cells in the subgranular zone of the dentate gyrus displayed a typical clustered appearance. Inset is a higher magnification image of a BrdU-IR cell cluster. Scale bar, 100 µm. Inset scale bar, 10 µm. B. Homozygote MOR KO mice show a non-significant trend to decrease in cell proliferation in comparison to wild-type (WT) mice (p=0.26). C. Distribution of BrdU-labeled cells along the anterior-posterior axis of the hippocampus. D–E: Survival of new cells in the adult hippocampal granule cell layer 4 weeks after BrdU administration. D. BrdU-labeled cells in the subgranular zone of the dentate gyrus displayed a mature neuron phenotype. Inset is a higher magnification of a BrdU-IR cell. E. Both heterozygote and homozygote animals show increased survival of newly born cells at 4 weeks in comparison to WT animals (* p<0.05). F. Distribution of BrdU-labeled cells along the anterior-posterior axis of the hippocampus. Data for B, C, E, and F are presented as mean ± SEM.
Survival
Surviving cells in the hippocampus were assessed in mice injected with BrdU and sacrificed four weeks later. Reflective of the maturation they undergo in the intervening four weeks, surviving BrdU-IR cells in the GCL were large, round, generally spotted or solid in staining, and rarely clustered (Fig. 1D). Quantification of BrdU-IR cells at this survival time point revealed a significant effect of genotype (Fig. 1E; F(2,19)=6.042; p<0.01). Heterozygous and homozygous mice had significantly more BrdU-IR GCL cells in comparison to wild-type mice, with increases of 57% and 54% respectively (p’s<0.05). There was no effect of genotype on the number of BrdU-IR cells in the habenula (F(2,19)=0.378, p>0.05), indicating that the increased BrdU-IR cell number was selective for the hippocampus. There was not an interaction of bregma and genotype (Fig. 1F; F(24,228)=1.281, p>0.05), suggesting that the increase in homozygous and heterozygous mice was spread across the hippocampus. These data show that while proliferation is unchanged in MOR KO mice, constitutive loss of or reduction in levels of MOR increases the number of surviving cells in the adult GCL.
Differentiation
Hippocampal progenitor cells can differentiate into several fates, including astrocytes or neurons (Reynolds and Weiss, 1992). To determine if loss or depletion of MOR influenced the fate of adult-generated hippocampal cells, we triple-labeled sections from mice given BrdU four weeks earlier with antibodies against glia (GFAP), neurons (NeuN), and BrdU. Confocal analysis showed that the majority of BrdU-IR cells in all three genotypes became neurons (Fig. 2; Table 1). There was no effect of genotype on the proportion of cells that became neurons (F(2,11)=1.349, p>0.05), glia (F(2,11)=0.919, p>0.05), or had an indeterminate fate (F(2,11)=0.616, p>0.05). These data show that the increase in surviving BrdU-IR cells seen after loss or reduction of MOR (Fig. 1D–F) reflects an increase in adult hippocampal neurogenesis.
Figure 2. Knockout of the µ opioid receptor does not alter cell fate during maturation.
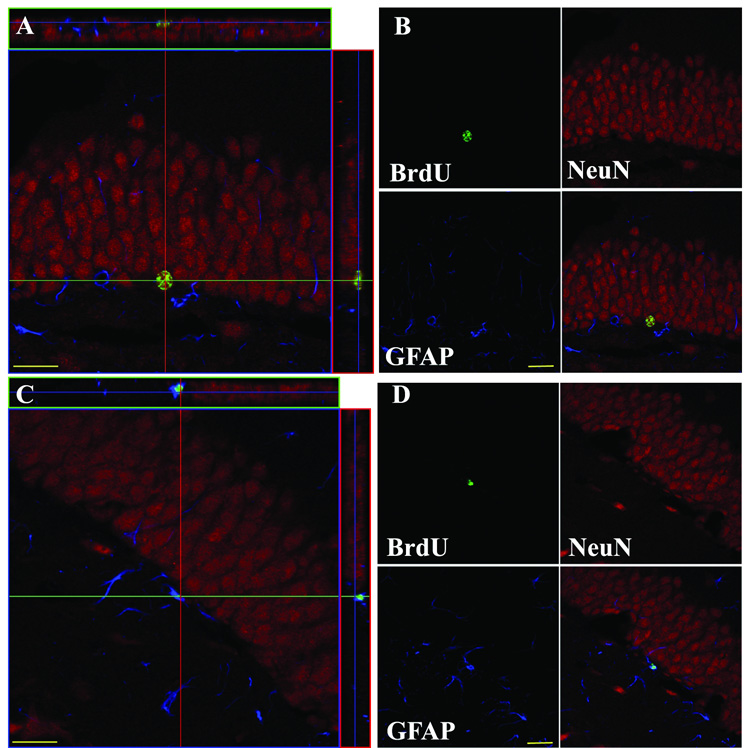
A, B: Example of a BrdU-labeled cell with a neuronal phenotype. BrdU labeling (green) colocalizes with the neuronal marker, NeuN (red), but not a glial marker, GFAP (blue). C, D: Example of a BrdU-labeled cell with a glial phenotype. BrdU labeling colocalizes with GFAP and not NeuN. Scale bars, 20 µm.
Table 1.
Phenotype of BrdU-IR GCL Cells
| % BrdU cells colocalizing with | |||
|---|---|---|---|
| NeuN | GFAP | Neither | |
| Wild-Type | 93.5(±2.4) | 1.7(±1.2) | 4.8(±2.7) |
| Heterozygote | 96.3(±2.9) | 0.6(±0.6) | 3.1(±3.1) |
| Homozygote | 98.6(±0.5) | 0.3(±0.3) | 1.1(±0.4) |
Cell Death
Many adult-generated hippocampal neurons will die prior to incorporation into hippocampal circuitry (Dayer et al., 2003). Since we found that loss or reduction of MOR increases the survival of adult-generated neurons (Fig. 1D–F), we hypothesized that the increased survival was due to decreased cell death. To assess apoptosis, adjacent sets of sections were examined for the number of AC3-IR, TUNEL-positive, and pyknotic cells (Fig. 3). The majority of apoptotic cells were located within the SGZ region of the GCL, underscoring the high turnover of cells in this region (Thomaidou et al., 1997, Gould et al., 1999, Dayer et al., 2003, Heine et al., 2004). As noted previously (Geloso et al., 2002), the majority of AC3-IR cells were large, round cells, and many extended processes through the GCL (Fig. 3D). This morphology reflects AC3’s early role in the apoptotic cascade (Faherty et al., 1999). TUNEL-positive (Fig. 3E) and pyknotic cells (Fig. 3F) were small and round, and processes were rare, emphasizing that these two methods of cell death detection capture cells in later stages of apoptosis (Faherty et al., 1999). Regardless of the method used to detect apoptosis, there was no significant effect of genotype (AC3, F(2,19)=0.984, p>0.05; TUNEL, F(2,12)=0.055, p>0.05, pyknosis, F(2,12)=1.166, p>0.05). These data show that GCL cell death is not different between WT and MOR KO mice.
Figure 3. Increased survival of neurons in MOR KO mice is not due to a detectable decrease in levels of apoptosis.

Quantitative analysis of (A) activated caspase-3-IR cells, (B) TUNEL-positive cells, and (C) pyknotic cells in the GCL. Representative images of (D) activated caspase-3 cells (indicated by white arrowhead), (E) TUNEL-positive cells (indicated by gray arrowheads), and (F) pyknotic cells (indicated by black arrows) in the GCL. Data for A–C are presented as mean ± SEM. Scale bar, 50 µm. Inset scale bar, 10 µm.
Stereological Estimation of Structural Volume and Granule Cell Number
Given that cell death was not different across genotypes (Fig. 3), the increased survival of newborn cells in the GCL of heterozygote and homozygote MOR KO mice (Fig. 1) would be expected to lead to an increased number of GCL cells. To address this possibility, we calculated the volume of the GCL and determined the number of granule cells using optical disectors along with the optical fractionator method (West and Andersen, 1980, Gundersen and Jensen, 1987, West and Gundersen, 1990). In regards to GCL volume, there was a significant effect of genotype (F(2,18)=5.833; p<0.05; Table 2) with the GCL of MOR heterozygote and homozygote KO mice being 22% and 17% larger than WT littermates (p<0.05 in each case). This increase in GCL volume was specific to the hippocampus since a main effect of genotype was also seen in the hilus (F(2,18)=5.071; p<0.05; heterozygote and homozygote 20% and 23% larger than WT), but no main effect of genotype was seen on the volume of the habenula, a region rich in MOR (F(2,17)=0.107; p>0.05; Fig. 4A, Table 2). There was also a main effect of genotype on the number of neurons in the GCL (F(2,17)=11.06; p<0.001) with heterozygote and homozygote possessing 30% (p<0.05) and 44% (p<0.001) more GCL neurons than WT (Fig. 4B, Table 3). Taken together these data show that enhanced survival of adult-generated neurons results in increased numbers of neurons in the GCL of adult mice lacking MOR.
Table 2.
Effect of Genotype on Size of Brain Regions
| GCL Volume (mm³) | % WT | Hilus Volume (mm³) | % WT | Habenula Volume (mm³) | % WT | |
|---|---|---|---|---|---|---|
| Wild-Type | 0.482(±0.024) | 100 | 0.494(±0.032) | 100 | 0.069(±0.002) | 100 |
| Heterozygote | 0.588(±0.026)* | 122 | 0.594(±0.021)* | 120 | 0.069(±0.003) | 100 |
| Homozygote | 0.565(±0.016)* | 117 | 0.608(±0.003)* | 123 | 0.067(±0.003) | 97 |
p<0.05
Figure 4. MOR KO mice have larger granule cell layers and increased numbers of granule cells.

A. Granule cell layer volumes. B. Number of granule cells per GCL as estimated by optical fractionator. (* p<0.05, *** p<0.001) Data is presented as mean ± SEM.
Table 3.
Effect of Genotype on GCL Neuron Number
| GCL Volume (mm³) | % WT | GCL Neurons (×105) | % WT | Neuron Density (×105/mm³) | |
|---|---|---|---|---|---|
| Wild-Type | 0.482(±0.024) | 100 | 5.254(±0.346) | 100 | 10.90(±0.490) |
| Heterozygote | 0.588(±0.026)* | 122 | 6.840(±0.798)* | 130 | 12.00(±1.222) |
| Homozygote | 0.565(±0.016)* | 117 | 7.590(±0.153)*** | 144 | 13.49(±0.386)* |
p<0.05
p<0.001
Discussion
These data show that heterozygote and homozygote MOR KO mice have enhanced adult hippocampal neurogenesis. This effect is due to an increase in the number of progenitor cells that survive, not to alteration in the number of proliferating GCL cells, and is independent of detectable changes in cell death.
There is no influence of the MOR KO on cell fate determination since WT, heterozygote and homozygote mice all had equivalent proportions of surviving cells maturing into neurons. The impact of increased neurogenesis on the hippocampus is emphasized by the increased volume and neuron number in the GCL of the dentate gyrus in heterozygote and homozygote mice. These results indicate that MOR plays a role in regulating the survival of maturing neurons in adult hippocampal neurogenesis.
The majority of factors currently known to influence neurogenesis do so via influence on proliferating progenitor cells. Thus, it is of particular interest that deletion of MOR appears to confer a survival advantage to maturing neurons without altering the numbers of proliferating progenitor cells. These data add MOR to the limited list of factors that have a stronger effect on survival versus proliferation of new hippocampal neurons (Ambrogini et al., 2005, Rizk et al., 2005, Galea et al., 2006, Lichtenwalner et al., 2006). The data presented here suggest that MOR is critical to normal maturation of adult-generated granule cells. Studies are in progress to explore whether other aspects of progenitor cell development, e.g. establishment of the granule cell dendritic tree, are altered in MOR KO mice.
The increase in survival of maturing neurons in MOR KO mice occurs without the expected concurrent decrease in cell death, as three measures (AC3, TUNEL, pyknosis) were similar between WT and KO mice. While it is difficult to reconcile increased cell survival in the face of no decrease in cell death, two things give us confidence that MOR KO mice indeed do have enhanced cell survival. First, despite the lack of detectable changes in cell death levels, our finding of increased cell survival (Fig. 1) is supported by a concomitant increase in the number of granule cell layer neurons (Fig. 4, Table 3). This emphasizes that more neurons appear to survive and likely integrate into the hippocampal circuitry in MOR KO mice. Second, available methods of cell death detection are hampered by the difficulty in catching the short window of time in which cells can be detected. Even those studies that show a correlation between cell birth and cell death show large differences between the number of proliferating cells and dying cells (Cameron and Gould, 1996, Cameron and McKay, 1999, Montaron et al., 1999, Biebl et al., 2000, Heine et al., 2004). This commonly-reported discrepancy between the number of proliferating progenitors and dying cells underscores the need for global technical advances in detection of cell death, as well as encourages more specific research into the relationship between these events as it relates to adult hippocampal neurogenesis.
It is interesting to note that heterozygote and homozygote MOR KO mice both have similar increases in cell survival in the GCL. While it is difficult to pinpoint the reason for the lack of a gene dosage effect, it is interesting to speculate if receptor density plays a role. MOR is expressed at low levels in the dentate gyrus (Arvidsson et al., 1995, Mansour et al., 1995, Drake and Milner, 2002), so a 50% reduction in MOR would have a significant impact on receptor density. In vivo studies have shown that MOR activity is highly dependent on receptor density (Pak et al., 1996, Law et al., 2000). Decreasing the number of functional MOR receptors by treatment with irreversible MOR antagonists (β-CAN, β-FNA) results in a non-linear decrease in receptor function. This reduction in functional efficacy of the receptor/effector unit necessitates a greater percentage occupancy of receptors in order to generate a functional response (Pak et al., 1996). This work also suggests that secondary cascades initiated by MOR may be uniquely sensitive to MOR receptor density (Pak et al., 1996). Thus, loss of one copy of MOR could translate into greater than expected decrease in receptor function and downstream activity in the dentate gyrus, resulting in a phenotype similar to the homozygote. Future studies, beyond the scope of this paper, will explore the mechanism behind our heterozygote phenotype by focusing on the kinetics of MOR specifically in dentate gyrus neurons.
Possible Mechanisms for MOR Regulation of Neurogenesis
Pharmacological evidence supports the hypothesis that MOR is a regulator of cell survival in the CNS. For example, pre- and postnatal exposure to the opioid antagonist naltrexone results in an increase in brain size (Zagon and McLaughlin, 1984, Zagon and McLaughlin, 1986a), while exposure to MOR agonists decreases brain size (Zagon and McLaughlin, 1977b, Zagon and McLaughlin, 1977a, Ford and Rhines, 1979). Full exploration of the mechanism for MOR regulation of adult neurogenesis is outside the scope of the present study. However, several recent findings provide clues to how MOR regulates the survival of new neurons, and these deserve discussion. The most pressing question to be addressed is whether MOR impacts maturing neurons directly, indirectly, or both. Here we propose two different mechanisms – one direct, and one indirect – via which MOR could regulate neurogenesis.
Indirectly, MOR could regulate neurogenesis by altering the levels of pro-survival factors in the dentate gyrus. MOR activity has been shown to be involved in the regulation of a number of neurotransmitters in the hippocampus including acetylcholine (ACh, Lapchak et al., 1989, Kaplan et al., 2004), GABA (Drake and Milner, 1999, Akaishi et al., 2000, Drake and Milner, 2002), and norepinephrine (NE, Matsumoto et al., 1994). GABA modulates neurogenesis, but appears to do so by altering cell differentiation, not survival (Tozuka et al., 2005, Karten et al., 2006). Manipulations of ACh levels can alter both proliferation and survival (Cooper-Kuhn et al., 2004, Mohapel et al., 2005). However, a recent paper showed that chronic acetylcholinesterase (AChE) antagonist treatment results in increased cell survival in the SGZ without affecting cell proliferation or fate (Kotani et al., 2006). While it is intriguing to consider whether perturbations in cholinergic signaling contribute to the enhanced survival reported here, cholinergic receptor binding as well as AChE activity are both normal in MOR KO mice (Tien et al., 2004). Thus, it seems unlikely that alterations in the cholinergic system are the cause of increased cell survival in MOR KO mice. NE is perhaps a more likely candidate. Antagonism of the alpha2-adrenoceptor, which increases NE levels, enhances neurogenesis by specifically increasing survival of new neurons in the hippocampus independent of changes in cell proliferation or fate (Rizk et al., 2005). MOR agonists inhibit release of NE (Matsumoto et al., 1994) and ACh (Lapchak et al., 1989) in the rat hippocampus. Perhaps deletion of MOR results in increased levels of these neurotransmitters in the hippocampus, leading to the increase in survival of new neurons reported here. The mechanisms by which these neurotransmitters promote cell survival have not yet been elucidated, however it has been proposed that NE, and possibly ACh, promote the survival of new neurons by increasing BDNF levels in the hippocampus (Rizk et al., 2005). Future experiments will address whether constitutive KO of MOR affects the levels of NE and BDNF in the hippocampus.
Alternatively, opioids could impact neurogenesis via direct activation of MOR. An in vitro study supports both the presence of MOR on hippocampal progenitors cells as well as the role of MOR in neuronal fate specification (Persson et al., 2003), and an in vivo study shows the presence of MOR on progenitors in another neurogenic region of the brain, the SVZ (Stiene-Martin et al., 2001). Furthermore, MOR may be expressed on a small population of cells of unknown identity in the dentate gyrus (Drake and Milner, 1999); these cells deserve further analysis for the possibility that they are progenitor cells. Studies are currently ongoing in our laboratory to establish if and when hippocampal progenitor cells and immature granule cells in vivo express MOR, and can thus directly respond to MOR agonists and antagonists. If these progenitor cells do express MOR, future studies could specifically knockout MOR in progenitor cells or immature neurons (e.g. via viral knockdown (van Praag et al., 2002) or specific transgenic KO) to determine if direct activation of MOR in these cells is necessary for its regulatory effects on neurogenesis.
As with all constitutive KO mice, the possibility that the findings reported here are due to compensatory changes in the brain must also be considered. Potential adaptive alterations in the brains of MOR KO mice have previously been described (Hall and Uhl, 2006), including changes in the cholinergic system (discussed above), as well as changes in the dopaminergic and glutamatergic systems. In the hippocampus, MOR KO mice have been shown to have alterations in non-opioid receptor activities, including reduced dopamine D2 receptor binding (Matthies et al., 2000) and enhanced metabotropic glutamate receptor and somatostatin binding (Grecksch et al., 2004). One way to address whether the reported increase in cell survival is MOR-specific or due to compensatory changes is to examine the effects of chronic administration of an MOR antagonist. In line with our findings in the MOR KO mice, chronic naltrexone treatment in early postnatal rats increases hippocampal size and granule cell number (Zagon and McLaughlin, 1984, Zagon and McLaughlin, 1986b). In vivo, chronic exposure of progenitor cells to another opioid antagonist, naloxone, results in increased neurogenesis (Persson et al., 2003). However, we have found that pharmacological blockade of MOR via chronic treatment with a high dose of the opioid antagonist naltrexone in adult mice does not alter cell proliferation or survival in the SGZ (Harburg and Eisch, unpublished data). This discrepancy with the MOR KO mice may be due to naltrexone’s non-specificity since it acts on the delta and kappa opioid receptors as well (Raynor et al., 1994). This multiple opioid receptor antagonism could mask MOR-specific effects. Contradictory results have also been found with naltrexone’s effects on cell proliferation in the SGZ (Persson et al., 2004, Galea et al., 2006), emphasizing the complexity of in vivo use of this compound, and the general challenges associated with attempts to mimic genetic manipulation with pharmacological blockade. Future studies will circumvent the natural confounds associated with constitutive KOs and the non-specificity of available MOR antagonists by studying the link between MOR and cell survival in a conditional MOR KO mouse.
Clearly, further studies are needed to elucidate whether the positive impact demonstrated here on adult neurogenesis is a result of the developmental expression of MOR on adult-generated neurons, an indirect effect such as via neurotransmitters, or perhaps a more global alteration in the hippocampal milieu that results in enhanced neurogenesis.
Implications of MOR Regulation of Neurogenesis on Behavior
Increased neurogenesis can positively impact hippocampal function, resulting in enhanced hippocampal-dependent learning. Neurons produced by adult hippocampal neurogenesis integrate into the structure of the hippocampus, extending axons along the mossy fiber tract to CA3 (Stanfield and Trice, 1988, Hastings and Gould, 1999, Markakis and Gage, 1999, Overstreet et al., 2004, Wadiche et al., 2005). The new neurons also gain the electrophysiological properties of mature granule cells and respond appropriately to stimulus of the perforant pathway (van Praag et al., 2002) strengthening the position that these cells are functionally integrated into the synaptic circuitry of the hippocampus. Correlative evidence suggests neurogenesis can enhance hippocampal-dependent learning in rodents (Shors et al., 2001, Shors et al., 2002, Drapeau et al., 2003, Snyder et al., 2005). In line with this theory, heterozygote and homozygote exon 1 MOR KO mice perform better than WT littermates in the Morris Water Maze, a task of hippocampal-dependent memory (Meilandt et al., 2004) and have enhanced adult hippocampal neurogenesis (present data). Further exploration of adult neurogenesis and hippocampal-dependent memory in MOR KO mice is warranted given differences in key physiological measures and behavior among distinct transgenic MOR KO mice (Matthes et al., 1996, Sora et al., 1997, Kitanaka et al., 1998, Loh et al., 1998, Schuller et al., 1999, Matthies et al., 2000, Jamot et al., 2003, Jang et al., 2003, Meilandt et al., 2004, Hall and Uhl, 2006). Identification of the similarities and differences among these mice will be central to determination of the mechanism that underlies the enhanced neurogenesis reported here in MOR KO mice.
In summary, these findings implicate MOR as necessary for the survival of newly mature neurons in the hippocampus. We extrapolate that endogenous opioids may act to regulate numbers of surviving newly born neurons by binding to MOR to cause this suppression. Thus, opioids and opiates have the potential to negatively impact hippocampal-dependent learning and memory by decreasing neurogenesis.
Acknowledgements
Funding for this research was generously provided by NIH/NIDA R01 DA016765 (AJE), NIDA T32 DA007290 (GCH), and the NIDA Intramural Research Program (FSH and GRU).
Abbreviations
- AC3
activated caspase 3
- ACh
achetylcholine
- AChE
acetylcholinesterase
- ANOVA
analysis of variance
- BrdU
bromodeoxyuridine
- CNS
central nervous system
- DAB
diaminobenzidine tetrahydrochloride
- DAPI
4’, 6-diamidino-2-phenylindole
- DNA
deoxyribonucleic acid
- GABA
gammaaminobutyrate
- GCL
granule cell layer
- GFAP
glial fibrillary acidic protein
- Het
heterozygote
- Homo
homozygote
- IHC
immunohistochemistry
- IR
immunoreactive
- KO
knockout
- LTP
long-term potentitation
- MOR
mu opioid receptor
- NE
norepinephrine
- PBS
phosphate-buffered saline
- PC
progenitor cell
- PCR
polymerase chain reaction
- SEM
standard error of the mean
- SGZ
subgranular zone
- SVZ
subventricular zone
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Akaishi T, Saito H, Ito Y, Ishige K, Ikegaya Y. Morphine augments excitatory synaptic transmission in the dentate gyrus through GABAergic disinhibition. Neurosci Res. 2000;38:357–363. doi: 10.1016/s0168-0102(00)00177-2. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Ferri P, Mancini C, Ciaroni S, Voci A, Gerdoni E, Gallo G. Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology. 2005;81:244–253. doi: 10.1159/000087648. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Distinct populations of cells in the adult dentate gyrus undergo mitosis or apoptosis in response to adrenalectomy. J Comp Neurol. 1996;369:56–63. doi: 10.1002/(SICI)1096-9861(19960520)369:1<56::AID-CNE4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Chavkin C. Dynorphins are endogenous opioid peptides released from granule cells to act neurohumorly and inhibit excitatory neurotransmission in the hippocampus. Prog Brain Res. 2000;125:363–367. doi: 10.1016/S0079-6123(00)25025-5. [DOI] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res. 2004;77:155–165. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res. 1999;849:203–215. doi: 10.1016/s0006-8993(99)01910-1. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–136. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ. Adult neurogenesis: implications for psychiatry. Prog Brain Res. 2002;138:315–342. doi: 10.1016/S0079-6123(02)38085-3. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Xanthoudakis S, Smeyne RJ. Caspase-3-dependent neuronal death in the hippocampus following kainic acid treatment. Brain Res Mol Brain Res. 1999;70:159–163. doi: 10.1016/s0169-328x(99)00143-6. [DOI] [PubMed] [Google Scholar]
- Ford DH, Rhines RK. Prenatal exposure to methadone HCL in relationship to body and brain growth in the rat. Acta Neurol Scand. 1979;59:248–262. doi: 10.1111/j.1600-0404.1979.tb02935.x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Geloso MC, Vercelli A, Corvino V, Repici M, Boca M, Haglid K, Zelano G, Michetti F. Cyclooxygenase-2 and caspase 3 expression in trimethyltin-induced apoptosis in the mouse hippocampus. Exp Neurol. 2002;175:152–160. doi: 10.1006/exnr.2002.7866. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37:367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Becker A, Schroeder H, Kraus J, Loh H, Hollt V. Accelerated kindling development in mu-opioid receptor deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:287–293. doi: 10.1007/s00210-004-0870-4. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Guo M, Xu NJ, Li YT, Yang JY, Wu CF, Pei G. Morphine modulates glutamate release in the hippocampal CA1 area in mice. Neurosci Lett. 2005;381:12–15. doi: 10.1016/j.neulet.2005.01.071. [DOI] [PubMed] [Google Scholar]
- Hall FS, Uhl GR. Transgenic mouse studies reveal substantial roles for opioid receptors in the rewarding effects of several classes of addictive drugs. Current Psychiatry Reviews. 2006;2:27–37. [Google Scholar]
- Harrison JM, Allen RG, Pellegrino MJ, Williams JT, Manzoni OJ. Chronic morphine treatment alters endogenous opioid control of hippocampal mossy fiber synaptic transmission. J Neurophysiol. 2002;87:2464–2470. doi: 10.1152/jn.2002.87.5.2464. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Jamot L, Matthes HW, Simonin F, Kieffer BL, Roder JC. Differential involvement of the mu and kappa opioid receptors in spatial learning. Genes Brain Behav. 2003;2:80–92. doi: 10.1034/j.1601-183x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Jang CG, Lee SY, Yoo JH, Yan JJ, Song DK, Loh HH, Ho IK. Impaired water maze learning performance in mu-opioid receptor knockout mice. Brain Res Mol Brain Res. 2003;117:68–72. doi: 10.1016/s0169-328x(03)00291-2. [DOI] [PubMed] [Google Scholar]
- Kahn L, Alonso G, Normand E, Manzoni OJ. Repeated morphine treatment alters polysialylated neural cell adhesion molecule, glutamate decarboxylase-67 expression and cell proliferation in the adult rat hippocampus. Eur J Neurosci. 2005;21:493–500. doi: 10.1111/j.1460-9568.2005.03883.x. [DOI] [PubMed] [Google Scholar]
- Kaplan TJ, Skyers PR, Tabori NE, Drake CT, Milner TA. Ultrastructural evidence for mu-opioid modulation of cholinergic pathways in rat dentate gyrus. Brain Res. 2004;1019:28–38. doi: 10.1016/j.brainres.2004.05.050. [DOI] [PubMed] [Google Scholar]
- Karten YJ, Jones MA, Jeurling SI, Cameron HA. GABAergic signaling in young granule cells in the adult rat and mouse dentate gyrus. Hippocampus. 2006;16:312–320. doi: 10.1002/hipo.20165. [DOI] [PubMed] [Google Scholar]
- Kearns IR, Morton RA, Bulters DO, Davies CH. Opioid receptor regulation of muscarinic acetylcholine receptor-mediated synaptic responses in the hippocampus. Neuropharmacology. 2001;41:565–573. doi: 10.1016/s0028-3908(01)00108-3. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kitanaka N, Sora I, Kinsey S, Zeng Z, Uhl GR. No heroin or morphine 6beta-glucuronide analgesia in mu-opioid receptor knockout mice. Eur J Pharmacol. 1998;355:R1–R3. doi: 10.1016/s0014-2999(98)00516-0. [DOI] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev. 2005;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Yee JK, Bolanos CA, Eisch AJ. Juvenile Administration of Methylphenidate Attenuates Adult Hippocampal Neurogenesis. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Collier B. Regulation of endogenous acetylcholine release from mammalian brain slices by opiate receptors: hippocampus, striatum and cerebral cortex of guinea-pig and rat. Neuroscience. 1989;31:313–325. doi: 10.1016/0306-4522(89)90376-x. [DOI] [PubMed] [Google Scholar]
- Law PY, Erickson LJ, El-Kouhen R, Dicker L, Solberg J, Wang W, Miller E, Burd AL, Loh HH. Receptor density and recycling affect the rate of agonist-induced desensitization of mu-opioid receptor. Mol Pharmacol. 2000;58:388–398. doi: 10.1124/mol.58.2.388. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I decreases survival of dentate granule neurons: Insights into the regulation of adult hippocampal neurogenesis. J Neurosci Res. 2006;83:199–210. doi: 10.1002/jnr.20719. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. mu Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res. 2004;76:783–794. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- Martinez JL, Jr, Derrick BE. Long-term potentiation and learning. Annu Rev Psychol. 1996;47:173–203. doi: 10.1146/annurev.psych.47.1.173. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Yoshioka M, Togashi H, Hirokami M, Tochihara M, Ikeda T, Smith CB, Saito H. mu-Opioid receptors modulate noradrenaline release from the rat hippocampus as measured by brain microdialysis. Brain Res. 1994;636:1–8. doi: 10.1016/0006-8993(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Matthies H, Schroeder H, Becker A, Loh H, Hollt V, Krug M. Lack of expression of long-term potentiation in the dentate gyrus but not in the CA1 region of the hippocampus of mu-opioid receptor-deficient mice. Neuropharmacology. 2000;39:952–960. doi: 10.1016/s0028-3908(99)00203-8. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Maayani S. Localization of naloxone-sensitive [3H]dihydromorphine binding sites within the hippocampus of the rat. Eur J Pharmacol. 1980;68:175–179. doi: 10.1016/0014-2999(80)90318-0. [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Villareal M, Hall FS, Sora I, Uhl GL, Martinez JL., Jr The effects of beta-funaltrexamine on spatial learning and novel object recognition memory in exon 1 mu opioid receptor knockout mice; Proceedings of the Society for Neuroscience; San Diego, CA. 2004. Abstract Viewer and Itinerary Planner, pp. Online. [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005;26:939–946. doi: 10.1016/j.neurobiolaging.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Petry KG, Rodriguez JJ, Marinelli M, Aurousseau C, Rougon G, Le Moal M, Abrous DN. Adrenalectomy increases neurogenesis but not PSA-NCAM expression in aged dentate gyrus. Eur J Neurosci. 1999;11:1479–1485. doi: 10.1046/j.1460-9568.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Johnston HM. A role for hippocampal opioids in long-term functional plasticity. Trends Neurosci. 1995;18:350–355. doi: 10.1016/0166-2236(95)93927-p. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology: An introduction for bioscientists. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- Nguyen XV, Masse J, Kumar A, Vijitruth R, Kulik C, Liu M, Choi DY, Foster TC, Usynin I, Bakalkin G, Bing G. Prodynorphin knockout mice demonstrate diminished age-associated impairment in spatial water maze performance. Behav Brain Res. 2005;161:254–262. doi: 10.1016/j.bbr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, Santangelo AM, Low MJ, Westbrook GL, Rubinstein M. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci. 2004;24:3251–3259. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak Y, Kouvelas A, Scheideler MA, Rasmussen J, O'Dowd BF, George SR. Agonist-induced functional desensitization of the mu-opioid receptor is mediated by loss of membrane receptors rather than uncoupling from G protein. Mol Pharmacol. 1996;50:1214–1222. [PubMed] [Google Scholar]
- Persson AI, Naylor AS, Jonsdottir IH, Nyberg F, Eriksson PS, Thorlin T. Differential regulation of hippocampal progenitor proliferation by opioid receptor antagonists in running and non-running spontaneously hypertensive rats. Eur J Neurosci. 2004;19:1847–1855. doi: 10.1111/j.1460-9568.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003;17:1159–1172. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rizk P, Salazar J, Raisman-Vozari R, Marien M, Ruberg M, Colpaert F, Debeir T. The Alpha2-Adrenoceptor Antagonist Dexefaroxan Enhances Hippocampal Neurogenesis by Increasing the Survival and Differentiation of New Granule Cells. Neuropsychopharmacology. 2005 doi: 10.1038/sj.npp.1300954. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Kieffer BL, Fanselow MS. Deletion of the mu opioid receptor results in impaired acquisition of Pavlovian context fear. Neurobiol Learn Mem. 2005;84:33–41. doi: 10.1016/j.nlm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. Endogenous opioid regulation of hippocampal function. Int Rev Neurobiol. 1996;39:145–196. doi: 10.1016/s0074-7742(08)60666-2. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain JW, Newsom GC. Chronic naltrexone enhances acquisition of the radial maze task in rats; Proc West Pharmacol Soc; 1989. pp. 141–142. [PubMed] [Google Scholar]
- Spain JW, Newsom GC. Chronic opioids impair acquisition of both radial maze and Y-maze choice escape. Psychopharmacology (Berl) 1991;105:101–106. doi: 10.1007/BF02316870. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, Trice JE. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Knapp PE, Martin K, Gurwell JA, Ryan S, Thornton SR, Smith FL, Hauser KF. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001;36:78–88. [PMC free article] [PubMed] [Google Scholar]
- Svoboda KR, Adams CE, Lupica CR. Opioid receptor subtype expression defines morphologically distinct classes of hippocampal interneurons. J Neurosci. 1999;19:85–95. doi: 10.1523/JNEUROSCI.19-01-00085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman GW, Drake CT, Simmons ML, Milner TA, Chavkin C. Opioid modulation of recurrent excitation in the hippocampal dentate gyrus. J Neurosci. 2000;20:4379–4388. doi: 10.1523/JNEUROSCI.20-12-04379.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaidou D, Mione MC, Cavanagh JF, Parnavelas JG. Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J Neurosci. 1997;17:1075–1085. doi: 10.1523/JNEUROSCI.17-03-01075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien LT, Fan LW, Sogawa C, Ma T, Loh HH, Ho IK. Changes in acetylcholinesterase activity and muscarinic receptor bindings in mu-opioid receptor knockout mice. Brain Res Mol Brain Res. 2004;126:38–44. doi: 10.1016/j.molbrainres.2004.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Sora I, Wang Z. The mu opiate receptor as a candidate gene for pain: polymorphisms, variations in expression, nociception, and opiate responses. Proc Natl Acad Sci U S A. 1999;96:7752–7755. doi: 10.1073/pnas.96.14.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005a;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Vaccani A, Bianchessi S, Marmorato P, Castiglioni C, Parolaro D. Molecular mechanisms involved in the asymmetric interaction between cannabinoid and opioid systems. Psychopharmacology (Berl) 2005b;182:527–536. doi: 10.1007/s00213-005-0114-4. [DOI] [PubMed] [Google Scholar]
- Wadiche LO, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- West MJ, Andersen AH. An allometric study of the area dentata in the rat and mouse. Brain Res. 1980;2:317–348. doi: 10.1016/0165-0173(80)90012-0. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Effect of chronic maternal methadone exposure on perinatal development. Biol Neonate. 1977a;31:271–282. doi: 10.1159/000240975. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Morphine and brain growth retardation in the rat. Pharmacology. 1977b;15:276–282. doi: 10.1159/000136699. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Naltrexone modulates body and brain development in rats: a role for endogenous opioid systems in growth. Life Sci. 1984;35:2057–2064. doi: 10.1016/0024-3205(84)90563-0. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Opioid antagonist (naltrexone) modulation of cerebellar development: histological and morphometric studies. J Neurosci. 1986a;6:1424–1432. doi: 10.1523/JNEUROSCI.06-05-01424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Opioid antagonist-induced modulation of cerebral and hippocampal development: histological and morphometric studies. Brain Res. 1986b;393:233–246. doi: 10.1016/0165-3806(86)90025-8. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Distribution and local differentiation of mast cells in the parenchyma of the forebrain. J Comp Neurol. 1999;408:477–488. doi: 10.1002/(sici)1096-9861(19990614)408:4<477::aid-cne3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]


