Abstract
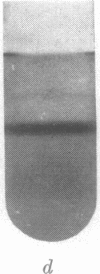
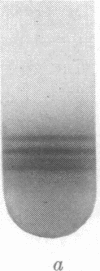
The separation between open and closed circular DNA in buoyant CsCl gradients containing intercalating dyes depends on the superhelix density of the closed form. These separations are about 1.8 times larger with propidium iodide than with ethidium bromide. The superhelix densities of mitochondrial DNA from HeLa cells and Lytechinus pictus eggs appear to be about two thirds that of mitochondrial DNA from rat and rabbit liver.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Bujard H. Studies on circular DNA. II. Number of tertiary turns in papilloma DNA. J Mol Biol. 1968 Apr 28;33(2):503–505. doi: 10.1016/0022-2836(68)90207-6. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Waring M. J. Supercoiling of polyoma virus DNA measured by its interaction with ethidium bromide. J Mol Biol. 1967 Apr 14;25(1):23–30. doi: 10.1016/0022-2836(67)90276-8. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Waring M. J. The supercoiling of papilloma virus DNA. J Gen Virol. 1967 Jul;1(3):387–390. doi: 10.1099/0022-1317-1-3-387. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Becker A., Hurwitz J. The enzymatic repair of DNA. I. Formation of circular lambda-DNA. Proc Natl Acad Sci U S A. 1967 Jul;58(1):240–247. doi: 10.1073/pnas.58.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Little J. W., Zimmerman S. B., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands, II. An enzyme-adenylate intermediate in the dpn-dependent DNA ligase reaction. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2004–2011. doi: 10.1073/pnas.58.5.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Blair D. G., Tyler A., Vinograd J. Cytoplasmic DNA in the unfertilized sea urchin egg: physical properties of circular mitochondrial DNA and the occurrence of catenated forms. Proc Natl Acad Sci U S A. 1968 Mar;59(3):838–845. doi: 10.1073/pnas.59.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttenberg G. J., Smit E. M., Borst P., van Bruggen E. F. The number of superhelical turns in mitochondrial DNA. Biochim Biophys Acta. 1968 Apr 22;157(2):429–432. doi: 10.1016/0005-2787(68)90101-9. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Watson R. Early and late helix-coil transitions in closed circular DNA. The number of superhelical turns in polyoma DNA. J Mol Biol. 1968 Apr 14;33(1):173–197. doi: 10.1016/0022-2836(68)90287-8. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Baumgarten D., Olivera B. M. On the origin of tertiary turns in covalently closed double-stranded cyclic DNA. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1852–1858. doi: 10.1073/pnas.58.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Little J. W., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands: a novel reaction of diphosphopyridine nucleotide. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1841–1848. doi: 10.1073/pnas.57.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]