Abstract
Prevention of acid is important in gastroesophageal reflex disease (GERD)-related asthma therapy. Proton pump inhibitors (PPI) and H2-receptor blockers have been reported as useful therapies for improving asthma symptoms. GERD prevalence is high in asthma; however, methods for validating GERD existence based on questionnaire, endoscopic examination and 24h-pH monitoring do not directly determine GERD influence on the airway. Exhaled breath condensate analysis is a novel and non-invasive tool for assessing information directly from the airway. Breath collected by cooling can be applied to pH, 8-isoprostane and cytokine analysis in patients with GERD-related asthma, and the pH and 8-isoprostane levels have been shown to reflect the effects of PPI therapy in these patients. Although the analysis of cooled breath has not yet been established in a clinical setting, this method is expected to provide a novel tool for monitoring airway acidification associated with GERD.
Keywords: exhaled breath condensate, asthma, gastroesophageal reflux disease (GERD), isoprostanes, proton pump inhibitor
Introduction
The prevalence of gastroesophageal reflex disease (GERD) in asthma patients has been reported to be as high as ~30–80% as compared with non-asthmatic subjects [1]. GERD is generally assessed by 24h-pH monitor, questionnaire or endoscopy. The prevalence of GERD among asthma patients evaluated by 24h-pH monitoring has been reported variously as 32% [2] and 82% [3]. By the questionnaire for the diagnosis of reflux disease (QUEST) method [4], the prevalence of GERD in asthma patients was found to vary from 42% [5] to 69.2% [6], and by endoscopic examination, hiatal hernia was found to be present in about 40% [7] and esophagitis in 47% [6] of asthmatic patients. Theoretical mechanisms to explain the prevalence of GERD-related asthma are based on aspiration theory or reflux theory. Airway inflammation seems to be caused either by vagal reflux leading to acid exposure of the esophagus [8] or by microaspiration of acid to the airway [9].
Anti-acid therapy has been reported to be effective for treating GERD-related asthma [10–12]. Proton pump inhibitors (PPIs) and H2-receptor blockers (H2-blockers) are beneficial in GERD-related asthma; however, the duration of anti-acid therapy and the dosage of these drugs have not been established, and to date no suitable methods have been developed for directly and non-invasively evaluating airway acidification by GERD. Although there are established methods for assessing GERD, including 24h-pH monitor, questionnaire and endoscopic examination, these methods do not directly evaluate airway acid stress due to GERD.
Exhaled breath condensate (EBC) has been recently reported as a new tool for monitoring airway condition in various pulmonary diseases and has the advantage of non-invasive, reproducible and objective evaluation [13, 14]. The exhaled breath is collected through a mouthpiece attached to cooling equipment, and the collected fluid can be applied to cytokine assay or pH measurement. In this article, we review the adaptation of EBC analysis to monitoring airway acid stress and anti-acid therapy in GERD-related asthma.
Collection of Exhaled Breath Condensate
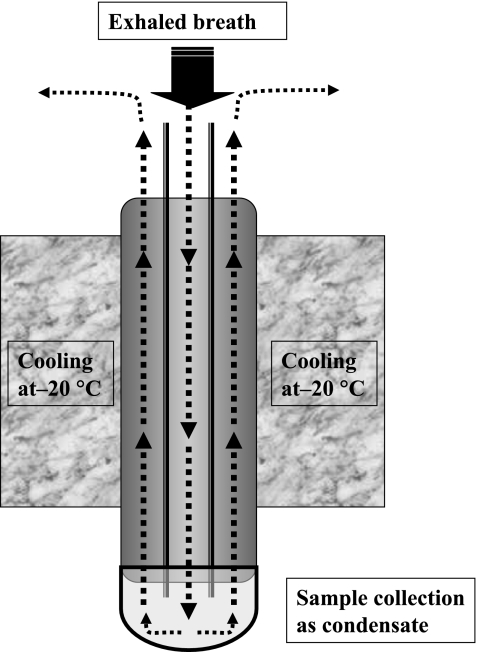
The surface of the lower airway is covered with airway lining fluid (ELF), and exhaled breath includes various nonvolatile substances as an ELF aerosol [15]. The substance of ELF is obtained by cooling breath at −20°C, and the principle of the cooling equipment is shown in Fig. 1. EcoScreen® (Jaeger, Berlin, Germany) and RTubeTM (Respiratory Research, Inc, Virginia) are systems that are commercially available at present. Patients breathe tidally through a mouthpiece attached to the EBC equipment for 15 min, while wearing a nose clip. By this method, approximately 1 to 2 ml of breath condensate is collected, depending on the patients’ breathing tidal volume. The samples collected should be frozen immediately and stored at −80°C. Storage for more than several months is not recommended. The sample can be used to measure various cytokines, H2O2, pH or other biomarkers.
Fig. 1.
Attachment of exhaled breath condensate. Exhaled air is cooled through the attachment at −20°C. Sample is collected at the bottom of attachment as liquid or ice.
Contamination of EBC by saliva should be examined by measuring the amylase concentration of samples, and the reproducibility of sample measurements should be confirmed. For pH measurements, in order to avoid contamination with ambient CO2, it is recommended that the EBC sample should be treated with gentle nitrogen bubbling. The pH levels of EBC differ by the two commercially available collection devices described above, and storage of samples for 8 weeks without deaeration can significantly influence measurements [16].
Analysis of EBC in GERD-Related Asthma
Analysis of EBC in asthma patients has been intensively performed. LeukotrienB4, interleukin(IL)-4, IL-8, IL-17, tumor necrosis factor (TNF)-α, regulated on activation normal T-cell expressed and presumably secreted (RANTES), interferon-γ-inducible protein 10, transforming growth factor(TGF)-β, macrophage inflammatory protein1a, and 1b in EBC are increased in asthma patients [17, 18]. Evaluation of the pH of EBC has also been reported to reflect airway acidity in asthma patients (Table 1). The pH of healthy subjects is in the range of ~7.5 to 7.7; however, with asthma exacerbation the pH decreases markedly to about 5.5 to 6.0. Severity of asthma affects the decrease in airway pH, and airway pH in patients with moderate asthma is lower than that in patients with mild asthma (Table 1) [19–21].
Table 1.
pH and 8-isoprostane levels in healthy subjects and asthma patients measured by exhaled breath condensate
| Disease condition | pH | 8-iso pg/ml | Reference |
|---|---|---|---|
| Healthy | 7.65 ± 0.20 | [19] | |
| Asthma exacebration | 5.23 ± 0.21 | [19] | |
| Stable asthma | 7.8 ± 0.1 | [19] | |
| Healthy | 7.57(CI, 7.51–7.64) | 20 ± 7.0 | [20] |
| Mild asthma | 7.6 (CI, 7.55–7.65) | 25 ± 7.0 | [20] |
| Moderate | 7.27 (CI, 7.15–7.39) | 40 ± 9.0 | [20] |
| Healthy | 7.70 (CI 7.62–7.74) | 3.5 (CI 2.6–7.9) | [21] |
| Mild asthma | 7.53 (CI 7.41–7.68) | 16.2 (CI11.7–19.1) | [21] |
| Healthy | 7.5 ± 0.2 | 6.6 ± 1.2 | [22] |
| Moderate asthma without GERD | 7.3 ± 0.6 | 24.6 ± 3.8 | [22] |
| Moderate asthma with GERD | 7.2 ± 0.1 | 32.7 ± 3.4 | [22] |
| Healthy | 6.9 (CI 5.6–9.7) | [23] | |
| Mild asthma without GERD | 17.9 (CI 8.9–23.8) | [23] | |
| Mild asthma with GERD | 26.5 (CI 15.1–36.5) | [23] |
Recently, measurement of EBC pH has been used to evaluate the influence of GERD on airway acidity [22]. The pH levels of EBC in asthma patients with GERD are lower than those in asthma patients without GERD (Table 1). In terms of possible mechanisms underlying the effects of GERD on EBC pH in asthma, studies of induced sputum have shown that GERD evaluated 24h-pH monitoring causes neutrophilic inflammation accompanied by an increase in IL-6 levels in the airway, and also that eosinophilic inflammation is exaggerated in GERD-related asthma, as compared with patients with GERD alone [23]. These inflammatory mechanisms might cause the changes in EBC pH in GERD-related asthma. Changes in pH are also observed in various pulmonary diseases. Chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) have been found to decrease the pH of EBC, and unstable disease conditions in asthma were associated with decreased EBC pH in our study (Fig. 2). In addition, in cystic fibrosis the pH of EBC has also been reported to be decreased [24]. In terms of the mechanisms underlying the changes in airway pH, the Na+-H+ exchanger, the H+-K+ ATPase, the vacuolar K+ ATPase, H+ channels and the glutaminase pathway have been proposed to contribute to deviations in the intracellular and extracellular pH of the airway [25, 26]. Although pH measurement seems to be useful to evaluate lower airway acidification [15, 20, 26], it is still debated whether pH levels determined by orally collected EBC reflect airway acidity due to contamination by NH4 and saliva [27–30]. To determine the efficacy of EBC pH measurement for assessing GERD, further studies are needed on a larger number of GERD patients and on other GERD-related pulmonary diseases; in addition, more suitable markers of GERD should be developed.
Fig. 2.
pH levels of various pulmonary diseases in healthy subjects, stable asthma, unstable asthma, COPD and IPF. Unstable asthma is defined as follows: 1. daytime symptoms is more than twice/week, 2. Presence of limitation of activity and nocturnal symptoms/awakened, 3. Needed for rescue treatment is more than twice/week.
The oxidative stress markers isoprostanes are eicosanoids that are non-enzymatically produced by the oxidation of cell membrane and plasma phospholipids. Among the isoprostanes, 8-isoprostane (8-iso) has been reported as a tissue marker of oxidative stress [31]. Oxidative stress is reported to be increased in asthmatic subjects, as reflected by the 8-iso levels in EBC [32]. In healthy subjects, the levels of 8-iso present in EBC are in the range of 3.5 to 20 pg/ml; in asthma patients, however, these 8-iso levels are markedly higher. Furthermore, if asthma severity is divided into mild, moderate and severe categories, then the severity is correlated with the increase in airway 8-iso; in other words, the 8-iso level is higher in patients with moderate asthma (24 to 40 pg/ml) than in those with mild asthma (16 to 25 pg/ml) (Table 1) [20–22]. Furthermore, the levels of 8-iso are higher in asthma patients with GERD than in those without GERD (Table 1), and 8-iso levels are higher in patients with moderate asthma with GERD than those with moderate asthma without GERD. The levels of 8-iso in asthma patients with GERD are probably higher than those in patients with the same severity of asthma but without GERD. In patients with GERD alone, the 8-iso levels are also higher than in healthy subjects [23]. The mechanisms underlying the GERD-induced production of 8-iso in the airway remain unresolved.
Reflux mechanisms or microaspiration mechanisms due to GERD [8, 9] might increase the oxidative stress-related production of 8-iso in the airway epithelium. The action of 8-iso on asthma is considered to be mediated via the Rho G-protein in bronchial smooth muscle. 8-Iso acts on Rho and Rho-kinase signaling via the thromboxane A2 receptor [33]. The Rho/Rho-kinase pathway plays an important role in bronchial smooth muscle constriction and inflammation; therefore, elevated 8-iso levels possibly stimulate the bronchial smooth muscle in asthma pathogenesis [34–36]. Thus, measuring the levels of 8-iso in EBC would seem to be useful for the evaluation for GERD-related asthma, and targeting this pathway might be a novel strategy in the treatment of GERD-related asthma.
Changes of Breath Marker in PPI Treatment for GERD-Related Asthma
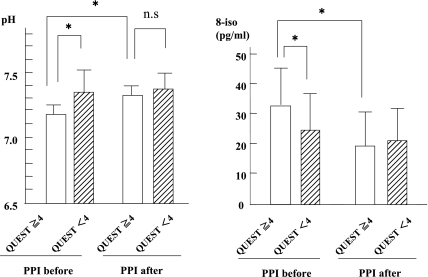
PPIs and H2-blockers have been reported to be beneficial in GERD-related asthma [10]; however, the duration of anti-acid therapy and the suitable dosage of these drugs have not been established. In addition, to date there are no non-invasive monitoring methods to determine the on-off anti-acid therapy under directly and subjectively observation on airway acidification. EBC analysis is a considered as a novel approach to monitor airway condition that is non-invasive, direct and objective. The pH and 8-iso levels in EBC in patients with moderate asthma and GERD were found to be improved by lansoprazole at a dosage of 30 mg/day, along with improvements of GERD symptoms; by contrast, in patients with moderate asthma but no GERD, the levels of these breath markers were not changed by this therapy (Fig. 3) [22]. This finding indicates that the pH and 8-iso levels measured in EBC would be useful markers for the diagnosis of GERD-related asthma, and that the these breath markers might be direct and objective markers for determining the intervention timing of anti-acid therapy.
Fig. 3.
Changes of pH and 8-isoprostane levels by PPI therapy in QUEST≥4 group and QUEST<4 group in moderate asthmatic patients. QUEST≥4 is indicates existence of GERD. Statistically significant differences between groups are expressed by *p<0.05. Reprinted with permission [22].
In another study, the prevalence of abnormal acid reflux was found to be high in IPF patients. PPIs were used to treat IPF at a dose of omeprazole 20–40 mg/day; however, this therapy did not suppress the abnormal acid reflux, as evaluated by 24h-pH monitor [37]. The author suggested that further studies are needed to assess whether the abnormal acid represents an important risk factor for IPF exacerbation and to assess the optimal dose for suppression of acid [37]. In COPD patients, the prevalence of GERD was reported to be high and the exacerbation of it appeared to be associated with GERD [38]. Although measurement of EBC pH was not performed in those studies, and not all of deviation in the EBC pH reflects the effects of GERD on the airway, GERD appears to be a risk factor of exacerbation and progression in pulmonary disease.
Screening of GERD-Related Asthma
A proposal for screening of GERD-related asthma is shown in Table 2. Asthma severity should be divided into mild, moderate and severe categories [39]. When asthma patients show either difficult-to-control asthma or non-typical symptoms relative to the estimated severity, it is important to suspect the GERD affects from one of the differential diagnosis. Lung cancer should be ruled out. GERD is known to cause persistent cough and asthma-like symptoms [40], and questions concerning GERD-related symptoms provide diagnostic clues to GERD-related asthma. To screen for GERD existence, the QUEST score [4] and F-scale [41] are useful; in addition, doctors should ask patients about the timing of asthma exacerbations in relation to food intake or excessive intake. Because questionnaires are subjective evaluation methods for GERD and are not necessarily suitable for detecting asymptomatic GERD [42], measurement of pH and 8-iso levels in EBC has the potential to provide another validation method for GERD-related asthma. In terms of anti-acid therapy, PPIs seem to be more useful than H2-blockers in GERD-related asthma [12]. The proposed PPI therapy is recommended for two to three months. Furthermore, it is important to educate patients about improving their lifestyle and diet. If asthma does not improve, and GERD-related asthma is still suspected, endoscopic examination, 24h-pH monitoring and consultation with a gastroenterologist are needed.
Table 2.
Screening and treatment of GERD-related asthma
 |
Conclusion
Measurement of pH and 8-iso levels in EBC seems to be useful for evaluating GERD influence on airway condition and the efficacy of PPI therapy in GERD-related asthma; however, one study has reported that an improvement in GERD-related cough by PPI therapy is not associated with changes in EBC pH or capsaicin cough response [43]. A study comparing biomarkers in EBC and bronchoalveolar lavage fluid (BAL) found no correlation in pH, 8-iso, H2O2 and NO. For example, 8-iso levels were much higher in EBC than in BAL, and pH was also higher in EBC than in BAL [44]. Thus, EBC was not necessarily found to be a reflection of BAL fluid for the following reasons. EBC is collected from a larger area than BAL, contamination with blood could occur during BAL collection, and there are differences in dilution between the two methods [44]. Thus, although observation of pH and 8-iso in EBC is relatively easy, the levels of both breath markers have not been yet established as a reflection of airway condition affected by GERD. Factors including differential severity of the pulmonary disease itself, receiving medication, body mass index, meals and sample storage conditions could affect the values measured in EBC [45]. In addition, further methodological improvement is needed to reproducibly obtain reliable data [46].
To determine the efficacy of EBC measurement in GERD-related asthma in a clinical setting, further studies are needed on larger numbers of both patients with GERD alone and patients with GERD-related pulmonary diseases. In addition to pH and 8-iso measurements, other markers of airway acidification by GERD expected to be developed.
References
- 1.Harding S.M. Gastroesophageal reflux and asthma: insight into the association. J. Allergy Clin. Immunol. 1999;104:251–259. doi: 10.1016/s0091-6749(99)70360-x. [DOI] [PubMed] [Google Scholar]
- 2.Vincent D., Cohen-Jonathan A.M., Leport J., Merrouche M., Geronimi A., Pradalier A., Soule J.C. Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur. Respir. J. 1997;10:2255–2259. doi: 10.1183/09031936.97.10102255. [DOI] [PubMed] [Google Scholar]
- 3.Sontag S.J., O’Connell S., Khandelwal S., Miller T., Nemchausky B., Schnell T.G., Serlovsky R. Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology. 1990;99:613–620. doi: 10.1016/0016-5085(90)90945-w. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson R., Dent J., Bolling-Sternevald F., Johnsson F., Johnsson F., Junghard O., Lauritsen K., Riley S., Lundell L. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand. J. Gastroenterol. 1998;33:1023–1029. doi: 10.1080/003655298750026697. [DOI] [PubMed] [Google Scholar]
- 5.Tsugeno H., Mizuno M., Fujiki H., Okada H., Okamoto M., Hosaki Y., Ashida S., Mitsunobu F., Tanizaki Y., Shiratori Y. A proton-pump inhibitor, rabeprazole, improves ventilatory function in patients with asthma associated with gastroesophageal reflux. Scand. J. Gastroenterol. 2003;38:456–461. doi: 10.1080/00365520310002490. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu Y., Dobashi K., Kobayashi S., Ohki I., Tokushima M., Kusano M., Kawamura O., Shimoyama Y., Utsugi M., Mori M. High prevalence of gastroesophageal reflux disease with minimal mucosal change in asthmatic patients. Tohoku J. Exp. Med. 2006;209:329–336. doi: 10.1620/tjem.209.329. [DOI] [PubMed] [Google Scholar]
- 7.Sontag S.J., O’Connell S., Khandelwal S., Greenlee H. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am. J. Gastroenterol. 2003;98:987–999. doi: 10.1111/j.1572-0241.2003.07503.x. [DOI] [PubMed] [Google Scholar]
- 8.Altschuler S.M. Laryngeal and respiratory protective reflexes. Am. J. Med. 2001;111:90S–94S. doi: 10.1016/s0002-9343(01)00862-2. [DOI] [PubMed] [Google Scholar]
- 9.Tuchman D.N., Boyle J.T., Pack A.L., Scwartz J., Kokonos M., Spitzer A.R., Cohen S. Comparison of airway responses following tracheal or esophageal acidification in the cat. . Gastroenterology. 1984;87:872–881. [PubMed] [Google Scholar]
- 10.Field S.K., Sutherland L.R. Does medical antireflux therapy improve asthma in asthmatics with gastroesophageal reflux?: a critical review of the literature. Chest. 1998;114:275–283. doi: 10.1378/chest.114.1.275. [DOI] [PubMed] [Google Scholar]
- 11.Kiljander T.O., Harding S.M., Field S.K., Stein M.R., Nelson H.S., Ekelund J., Illueca M., Beckman O., Sostek M.B. Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2006; 173:1091–1097. doi: 10.1164/rccm.200507-1167OC. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu Y., Dobashi K., Kobayashi S., Ohki I., Tokushima M., Kusano M., Kawamura O., Shimoyama Y., Utsugi M., Sunaga N., Ishizuka T., Mori M. A proton pump inhibitor, lansoprazole, ameliorates asthma symptoms in asthmatic patients with gastroesophageal reflux disease. Tohoku J. Exp. Med. 2006;209:181–189. doi: 10.1620/tjem.209.181. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan J., Ngamtrakulpanit L., Pajewski T.N., Turner R., Nguyen T.A., Smith A., Arban P., Hom S., Gaston B., Hunt J. Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur. Respir. J. 2003;22:889–894. doi: 10.1183/09031936.03.00038803. [DOI] [PubMed] [Google Scholar]
- 14.Mutlu G.M., Garey K.W., Robbins R.A., Danziger L.H., Rubinstein I. Collection and analysis of exhaled breath condensate in humans. Am. J. Respir. Crit. Care Med. 2001;164:731–737. doi: 10.1164/ajrccm.164.5.2101032. [DOI] [PubMed] [Google Scholar]
- 15.Horvath I., Hunt J., Barnes P.J., Alving K., Antczak A., Baraldi E., Becher G., van Beurden W.J., Corradi M., Dekhuijzen R., Dweik R.A., Dwyer T., Effros R., Erzurum S., Gaston B., Gessner C., Greening A., Ho L.P., Hohlfeld J., Jobsis Q., Laskowski D., Loukides S., Marlin D., Montuschi P., Olin A.C., Redington A.E., Reinhold P., van Rensen E.L., Rubinstein I., Silkoff P., Toren K., Vass G., Vogelberg C., Wirtz H. ATS/ERS task force on exhaled breath condensate. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur. Respir J. 2005;26:523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 16.Prieto L., Ferrer A., Palop J., Domenech J., Llusar R., Rojas R. Differences in exhaled breath condensate pH measurements between samples obtained with two commercial devices. Respir. Med. 2007;101:1715–1720. doi: 10.1016/j.rmed.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Kostikas K., Gaga M., Papatheodorou G., Karamanis T., Orphanidou D., Loukides S. Leukotriene B4 in exhaled breath condensate and sputum supernatant in patients with COPD and asthma. Chest. 2005;127:1553–1559. doi: 10.1378/chest.127.5.1553. [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga K., Yanagisawa S., Ichikawa T., Ueshima K., Akamatsu K., Hirano T., Nakanishi M., Yamagata T., Minakata Y., Ichinose M. Airway cytokine expression measured by means of protein array in exhaled breath condensate: correlation with physiologic properties in asthmatic patients. J. Allergy Clin. Immunol. 2006;118:84–90. doi: 10.1016/j.jaci.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Hunt J.F., Fang K., Malik R., Snyder A., Malhotra N., Platts-Mills T.A., Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am. J. Respir. Crit. Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 20.Kostikas K., Papatheodorou G., Ganas K., Psathakis K., Panagou P., Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am. J. Respir. Crit. Care. Med. 2002;165:1364–1370. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J.J., Shimizu Y., Kawata T., Yanagitani N., Hisada T., Kaira K., Sunaga N., Utsugi M., Ishizuka T., Dobashi K., Mori M. The relationship between oxidative stress and acid stress in mild adult asthma patients. Journal Invest. Allerg. Clin. 2008;18 In press. [PubMed] [Google Scholar]
- 22.Shimizu Y., Dobashi K., Zhao J.J., Kawata T., Ono A., Yanagitani N., Kaira K., Utsugi M., Hisada T., Ishizuka T., Mori M. Proton pump inhibitor improves breath marker in moderate asthma with gastroesophageal reflux disease. Respiration. 2007;74:558–564. doi: 10.1159/000101437. [DOI] [PubMed] [Google Scholar]
- 23.Carpagnano G.E., Resta O., Ventura M.T., Amoruso A.C., Di Gioia G., Giliberti T., Refolo L., Foschino-Barbaro M.P. Airway inflammation in subjects with gastro-oesophageal reflux and gastro-oesophageal reflux-related asthma. J. Intern. Med. 2006;259:323–331. doi: 10.1111/j.1365-2796.2005.01611.x. [DOI] [PubMed] [Google Scholar]
- 24.Ojoo J.C., Mulrennan S.A., Kastelik J.A., Morice A.H., Redington A.E. Exhaled breath condensate pH and exhaled nitric oxide in allergic asthma and in cystic fibrosis. Thorax. 2005;60:22–26. doi: 10.1136/thx.2003.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer H., Widdicombe J.H. Mechanisms of acid and base secretion by the airway epithelium. J. Membr. Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricciardolo F.L., Gaston B., Hunt J. Acid stress in the pathology of asthma. J. Allergy Clin. Immunol. 2004;113:610–619. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Effros R.M., Bosbous M., Foss B., Shaker R., Biller J. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am. J. Respir. Crit. Care Med. 2003; 168:1500–1505. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 28.Effros R.M., Casaburi R., Su J., Dunning M., Torday J., Biller J., Shaker R. The effects of volatile salivary acids and bases on exhaled breath condensate pH. Am. J. Respir. Crit. Care. Med. 2006;173:386–392. doi: 10.1164/rccm.200507-1059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells K., Vaughan J., Pajewski T.N., Hom S., Ngamtrakulpanit L., Smith A., Nguye A., Turner R., Hunt J. Exhaled breath condensate pH assays are not influenced by oral ammonia. Thorax. 2005; 60:27–31. doi: 10.1136/thx.2003.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt J., Yu Y., Burns J., Gaston B., Ngamtrakulpanit L., Bunyan D., Walsh B.K., Smith A., Hom S. Identification of acid reflux cough using serial assays of exhaled breath condensate pH. Cough. 2006;2:1–8. doi: 10.1186/1745-9974-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrono C., FitzGerald G.A. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. Arterioscler. Thromb. Vasc. Biol. 1997;17:2309–2315. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- 32.Montuschi P., Corradi M., Ciabattoni G., Nightingale J., Kharitonov S.A., Barnes P.J. Increased 8-isoprostane, a marker of oxidative stress, in exhaled air condensate of asthma patients. Am. J. Respir. Crit. Care Med. 1999;60:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- 33.Janssen L.J. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L1067–L1082. doi: 10.1152/ajplung.2001.280.6.L1067. [DOI] [PubMed] [Google Scholar]
- 34.Iizuka K., Shimizu Y., Tsukagoshi H., Yoshii A., Harada T., Dobashi K., Murozono T., Nakazawa T., Mori M. Evaluation of Y-27632, a rho-kinase inhibitor, as a bronchodilator in guinea pigs. Eur. J. Clin. Pharmacol. 2000;13:273–279. doi: 10.1016/s0014-2999(00)00504-5. [DOI] [PubMed] [Google Scholar]
- 35.Yoshii A., Iizuka K., Dobashi K., Horie T., Harada T., Nakazawa T., Mori M. Relaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+ sensitization. Am. J. Respir. Cell Mol. Biol. 1999;20:1190–1200. doi: 10.1165/ajrcmb.20.6.3441. [DOI] [PubMed] [Google Scholar]
- 36.Montuschi P., Barnes P.J., Roberts L.J., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004; 18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 37.Raghu G., Freudenberger T.D., Yang S., Curtis J.R., Spada C., Hayes J., Sillery J.K., Pope C.E., 2nd, Pellegrini C.A. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur. Respir. J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 38.Rascon-Aguilar I.E., Pamer M., Wludyka P., Cury J., Coultas D., Lambiase L.R., Nahman N.S., Vega K.J. Role of gastroesophageal reflux symptoms in exacerbations of COPD. Chest. 2006;130:1096–1101. doi: 10.1378/chest.130.4.1096. [DOI] [PubMed] [Google Scholar]
- 39.National Institute of Health, National Heart, Lung, and Blood institute. Medical Communications Resources, Inc.; Washington: 2006. Global strategy for asthma treatment and prevention (GINA) pp. 1–92. [Google Scholar]
- 40.Niimi A., Nguyen L.T., Usmani O., Mann B., Chung K.F. Reduced pH and chloride levels in exhaled breath condensate of patients with chronic cough. Thorax. 2004;59:608–612. doi: 10.1136/thx.2003.012906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimoyama Y., Kusano M., Sugimoto S., Kawamura O., Maeda M., Minashi K., Kuribayashi S., Higuchi T., Zai H., Ino K., Horikoshi T., Moki F., Sugiyama T., Toki M., Ohwada T., Mori M. Diagnosis of gastroesophageal reflux disease using a new questionnaire. J. Gastroenterol. Hepatol. 2005; 20:643–647. doi: 10.1111/j.1440-1746.2005.03776.x. [DOI] [PubMed] [Google Scholar]
- 42.Harding S.M., Guzzo M.R., Richter J.E. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am. J. Respir. Crit. Care Med. 2000;162:34–39. doi: 10.1164/ajrccm.162.1.9907072. [DOI] [PubMed] [Google Scholar]
- 43.Torrego A., Cimbollek S., Hew M., Chung K.F. No effect of omeprazole on pH of exhaled breath condensate in cough associated with gastro-oesophageal reflux. Cough. 2005;1:1–4. doi: 10.1186/1745-9974-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson A.S., Sandrini A., Campbell C., Chow S., Thomas P.S., Yates D.H. Comparison of biomarkers in exhaled breath condensate and bronchoalveolar lavage. Am. J. Respir. Crit. Care Med. 2007;175:222–227. doi: 10.1164/rccm.200601-107OC. [DOI] [PubMed] [Google Scholar]
- 45.Komakula S., Khatri S., Mermis J., Savill S., Haque S., Rojas M., Brown L., Teague G.W., Holguin F. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir. Res. 2007;8:1–10. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tufvesson E., Bjermer L. Methodological improvements for measuring eicosanoids and cytokines in exhaled breath condensate. Respir. Med. 2006;100:34–38. doi: 10.1016/j.rmed.2005.04.007. [DOI] [PubMed] [Google Scholar]