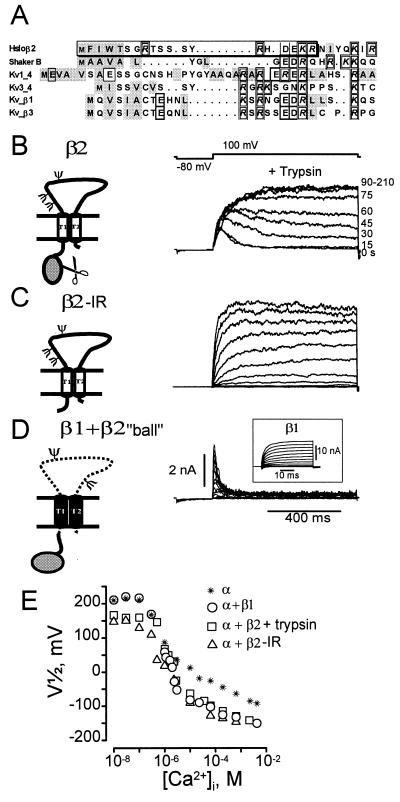
Figure 3.
Molecular determinant of MaxiK channel fast inactivation. (A) Sequence comparison of β2 N terminus with other N-terminal inactivating “balls.” Charged amino acids, individually boxed. Hydrophobic amino acids, shaded gray. Nineteen amino acid “ball” peptide, boxed. (B) Trypsin eliminates inactivation. Trypsin (0.2 mg/ml, [Ca2+]i = 1 μM) applied to the intracellular side of oocytes expressing α + β2 channels. Numbers at right, seconds after trypsin addition. (C) Inactivation is removed by deletion of residues 2–19 from the β2 subunit (β2-IR). β2-IR coexpressed with α subunit of MaxiK channels yields noninactivating currents. Voltages were −20 mV to 110 mV, 10 mV intervals; [Ca2+]i = 500 nM Ca2+. (D) Transfer of inactivation by fusion of 19 N-terminal amino acids of β2 subunit to the N terminus of noninactivating β1 subunit (β1 + β2 “ball”). Inactivating currents are from α coexpressed with β1 + β2 “ball.” Voltages: −20 mV to 110 mV in 10-mV increments, prepulse to −80 mV, [Ca2+]i = 1 μM. Inset: Noninactivating currents from α + β1 subunit, 1 μM Ca2+. Test potentials: −100 to 122 mV in steps of 12 mV. (E) Half activation potentials (V1/2) as a function of [Ca2+]i. Channels formed by α subunit alone (*), α + β1 subunits (○), α + β2 subunits after trypsin treatment (□), α+ β2-IR (Δ). β2 subunit increases Ca2+ sensitivity in the channel’s Ca2+ independent phase, e.g., V1/2 at 10 nM Ca2+ for α, α + β1, α + β2 (trypsin), and α + β2-IR is 209 ± 14, 211 ± 6, 159 ± 10, 146 ± 2, respectively (± SD, n ≥ 2). V1/2 values were obtained from voltage activation curves (2).