Abstract
The β-adrenergic receptor/cyclic AMP/protein kinase A (PKA) signalling pathway regulates heart rate and contractility. Here, we identified a supramolecular complex consisting of the sarcoplasmic reticulum Ca2+-ATPase (SERCA2), its negative regulator phospholamban (PLN), the A-kinase anchoring protein AKAP18δ and PKA. We show that AKAP18δ acts as a scaffold that coordinates PKA phosphorylation of PLN and the adrenergic effect on Ca2+ re-uptake. Inhibition of the compartmentalization of this cAMP signalling complex by specific molecular disruptors interferes with the phosphorylation of PLN. This prevents the subsequent release of PLN from SERCA2, thereby affecting the Ca2+ re-uptake into the sarcoplasmic reticulum induced by adrenergic stimuli.
Keywords: cAMP, calcium, heart, phospholamban, AKAP
Introduction
Coordinated handling of Ca2+ in cardiac myocytes is essential for the efficient contraction, and relaxation of the heart. Sympathetic control of the heart through β-adrenergic stimulation increases both the rate and force of contraction, and relaxation of the cardiac muscle by regulating Ca2+ handling at the level of the L-type Ca2+ channel, the ryanodine receptor (RYR), a Ca2+-activated Ca2+ release channel, and the cardiac sarcoplasmic reticulum Ca2+-ATPase (SERCA2; Simmerman & Jones, 1998; Bers, 2002). SERCA2 has a crucial role in Ca2+ homoeostasis by controlling Ca2+ re-uptake into the sarcoplasmic reticulum, a rate-limiting step for the relaxation and filling of the heart before the next contraction (Szentesi et al, 2004). Phospholamban (PLN), a 52-amino-acid sarcoplasmic reticulum phosphoprotein, is a crucial regulator of SERCA2 (MacLennan & Kranias, 2003). In its dephosphorylated state, PLN binds to SERCA2 and suppresses its ATPase activity, whereas phosphorylation of PLN on Ser 16 by protein kinase A (PKA) dissociates PLN from SERCA2, releasing the Ca2+ pump from inhibition. Alterations in the levels and function of PLN and SERCA2 have been linked to post-infarction heart failure, and PLN mutations have been shown to cause heritable dilated cardiomyopathy both in mice and humans (Haghighi et al, 2003; MacLennan & Kranias, 2003; Schmitt et al, 2003).
Stimulation of β-adrenergic receptors generates many discrete microdomains of cyclic AMP in the region of the transverse tubules and the sarcoplasmic reticulum, which leads to specific activation of anchored pools of PKA (Zaccolo & Pozzan, 2002). This specificity in cAMP signalling is conferred by the binding of PKA to A-kinase anchoring proteins (AKAPs), which target specific intracellular locations and provide spatial and temporal control of cAMP signalling events (Tasken & Aandahl, 2004; Wong & Scott, 2004). Several AKAPs have been identified in adult cardiac myocytes, including AKAP-LBC, AKAP15/18α, muscle-selective AKAP, AKAP79, yotiao, gravin, D-AKAP1, D-AKAP2, ezrin, AKAP95, BIG2, AKAP220 and the recently described AKAPs sphingosine kinase-interacting protein 1 and synemin (Ruehr et al, 2004; Russell et al, 2006; Scholten et al, 2006). AKAP18α in complex with the L-type Ca2+ channel and muscle-selective AKAP complexed with RYR have been implicated in β-adrenergic regulation of Ca2+ handling, however, no AKAP has yet been reported to target a pool of PKA to the PLN–SERCA2 complex to provide accurate controlled PLN phosphorylation and thereby Ca2+ re-uptake into the sarcoplasmic reticulum. Here, we show that AKAP18δ, a large splice variant derived from the AKAP18 gene (Henn et al, 2004), forms a supramolecular complex with PKA, PLN and SERCA2 in cardiac myocytes. We show that this AKAP18δ-anchored pool of PKA phosphorylates PLN in response to adrenergic stimuli and thereby regulates SERCA2-mediated Ca2+ re-uptake into the sarcoplasmic reticulum.
Results And Discussion
AKAP18δ is present in the heart sarcoplasmic reticulum
In a search for the heart sarcoplasmic reticulum AKAP associated with PLN, we analysed sarcoplasmic reticulum fractions for the presence of PKA-RII-binding proteins by using an RII overlay assay. Rat hearts were homogenized, subjected to discontinuous sucrose density gradient fractionation and overlaid with 32P-labelled RII in the absence or presence of the anchoring disruptor peptide Ht31 (Fig 1A). RII-binding proteins with mobilities of more than 200 and approximately 110, 90, 60 and 50 kDa were detected in the fractions containing sarcoplasmic reticulum, in addition to PKA-RII itself, owing to dimerization with low levels of monomer R in the solution. Immunoblotting of the heart fractions showed that AKAP18δ was present in fractions enriched in sarcoplasmic reticulum (Fig 1B), together with PKA subunits (Fig 1C). Furthermore, the distribution of AKAP18δ differed from that of the sarcolemmal marker, Na+/Ca2+ exchanger (NCX), which peaked in fractions 8–10, indicating that AKAP18δ is not a sarcolemmal protein (Fig 1B). We confirmed the presence of AKAP18δ in the heart by immunoprecipitation of AKAP18δ from total rat heart homogenate and analysis of immunoprecipitates by using an RII overlay (Fig 1D). The detection of AKAP18δ in the heart is also in agreement with our previous northern blot analysis (Henn et al, 2004).
Figure 1.
AKAP18δ is present in the heart sarcoplasmic reticulum. (A) Fractions of rat heart sarcoplasmic reticulum (SR) were subjected to a solid-phase binding assay using 32P-labelled RIIα (RII overlay) as a probe in the absence (upper panel) or presence (middle panel) of the Ht31 anchoring disruptor peptide (500 nM). The same fractions were analysed by immunoblot for the presence of SR proteins ryanodine receptor 2 (RYR2), Ins(1,4,5)P3 receptor II (IP3RII) and calsequestrin, a major Ca2+-binding protein of SR (lower panels). Calsequestrin was routinely used in the following as an SR marker and indicator for the quality of SR enrichment. Fraction numbers refer to a discontinuous sucrose density gradient fractionation. (B) Detection of AKAP18δ in rat heart SR fractions by immunoblotting (IB). Pep: AKAP18δ antibody was preincubated with the peptide used for immunization as specificity control. NCX was used as a sarcolemmal marker. (C) Levels of immunoreactive PKA regulatory (RIα, RIIα and RIIβ) and catalytic (C) subunits in SR fractions. (D) Rat heart homogenate was subjected to immunoprecipitation (IP) with AKAP18δ antibody or pre-immune IgG. Total extract and immunoprecipitates were analysed by using RII overlay. Recombinant AKAP18δ protein was used as a positive control. AKAP, A-kinase anchoring protein; NCX, Na+/Ca2+ exchanger; PKA, protein kinase A.
Subcellular localization of AKAP18δ
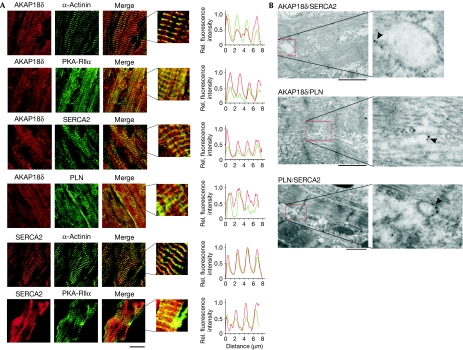
The subcellular localization of AKAP18δ in rat heart tissue was examined by co-immunostaining of AKAP18δ and α-actinin, PKA-RIIα, SERCA2 and PLN (Fig 2A). The α-actinin staining identifies z-lines, allows visualization of the myofibrils and acts as a reference for the position of the sarcoplasmic reticulum (Vangheluwe et al, 2003). AKAP18δ immunofluorescence produced a striated pattern overlapping that of α-actinin, PLN and SERCA2. Furthermore, AKAP18δ colocalized with PKA-RIIα. In addition, SERCA2 colocalized with α-actinin, in agreement with earlier studies (Vangheluwe et al, 2003), and with PKA-RIIα (Fig 2A). AKAP18δ immunostaining was specific, as evident from controls with pre-immune serum and secondary antibody only (see supplementary Fig S1 online). Immunogold staining of neonatal heart tissue using specific antibodies labelled with two different sizes of gold particles allowed colocalization of AKAP18δ, PLN and SERCA2 by electron microscopy (Fig 2B). As evident from the ultrastructure, all three proteins (85–95% of grains) localized on stacks of sarcoplasmic reticulum that were interspersed with the contractile machinery. Approximately 10% of AKAP18δ and SERCA2, 12% of AKAP18δ and PLN, and 20% of PLN and SERCA2 colocalized within distances of less than 60 nm, indicating significant colocalization using this technique. Similar data were obtained by examination of adult heart tissue. The presence of AKAP18δ, PLN and SERCA2 in such close proximity suggests the possibility of a SERCA2–PLN–AKAP18δ–PKA supramolecular complex in heart tissue.
Figure 2.
AKAP18δ and PKA colocalize with SERCA2 and PLN in heart tissue. (A) Rat heart tissue sections were immunostained for AKAP18δ (red) in combination with α-actinin (green), PKA-RIIα (green), SERCA2 (green) and PLN (green), and for SERCA2 (red) in combination with α-actinin (green) and PKA-RIIα (green). The relative fluorescence intensities along an axis perpendicular to the orientation of the sarcomeres are shown (right panels). Scale bar, 20 μm. (B) Immunogold staining was carried out using secondary antibodies labelled with gold particles of different sizes to allow dual staining. Co-staining of AKAP18δ (15 nm) and PLN (10 nm), AKAP18δ (18 nm) and SERCA2 (12 nm), and PLN (15 nm) and SERCA2 (10 nm). Scale bars, 1 μm. The magnified views show representative areas where the indicated proteins colocalize (arrowheads). AKAP, A-kinase anchoring protein; PKA, protein kinase A; PLN, phospholamban; SERCA2, sarcoplasmic reticulum Ca2+-ATPase.
AKAP18δ interacts with PLN
To examine whether AKAP18δ forms a complex with PKA in the sarcoplasmic reticulum, a cAMP pull-down experiment from sarcoplasmic reticulum fractions using Rp-8-AHA-cAMP-agarose beads was carried out. An approximately 50-kDa protein was recognized by a specific AKAP18δ antibody in the eluate, which also contained PKA-RIIα, PKA-C and SERCA2 (Fig 3A). As SERCA2 co-purified on cAMP-agarose, we tested next whether AKAP18δ forms a complex with the PKA substrate PLN. Immunoprecipitation of AKAP18δ from sarcoplasmic reticulum fractions showed the presence of PLN in the precipitate (Fig 3B). PLN also co-precipitated with AKAP18δ from the left ventricles of adult rat hearts (data not shown). Interestingly, in the opposite immunoprecipitation experiment using anti-PLN, AKAP18δ was not detected, presumably because the epitope for the PLN antibody overlapped the AKAP18δ-binding site (Fig 3D; data not shown), but it could also be due to AKAP18δ, like SERCA2, interacting only with the monomer PLN but not the pentamer population of PLN. However, immunoprecipitation of green fluorescent protein (GFP)–PLN, but not GFP, from HEK293 cells co-transfected with AKAP18δ in the absence of SERCA2 pulled down AKAP18δ, as detected by immunoblotting (Fig 3C). Conversely, immunoprecipitation of AKAP18δ co-precipitated GFP–PLN but not GFP (Fig 3C). Collectively, these experiments indicate that AKAP18δ forms a complex with PLN and SERCA2 in situ through interaction with PLN.
Figure 3.
AKAP18δ interacts with PLN in the heart sarcoplasmic reticulum. (A) Pooled rat heart sarcoplasmic reticulum (SR) fractions were subjected to affinity chromatography on Rp-8-AHA-cAMP-agarose in the absence (lane 1) or presence (lane 2) of excess cAMP. Eluates were analysed by immunoblot (IB) for the presence of PKA-RIIα, PKA-C, AKAP18δ and SERCA2 (Rp-8-AHA-cAMP is a PKA antagonist that does not dissociate PKA-C from holoenzyme). (B) Pooled rat heart SR fractions were subjected to immunoprecipitation (IP) with AKAP18δ or control rabbit IgG antibodies. Lysates and immunoprecipitates were analysed by immunoblot for the presence of PLN pentamer (upper band) and monomer (lower band; lysate not boiled; note the relative abundance of monomer). (C) HEK293 cells were transfected with expression vectors encoding AKAP18δ and PLN fused to GFP. Lysates were subjected to immunoprecipitation with anti-GFP (left panel) or anti-AKAP18δ (right panel), and lysates and precipitates were analysed for the presence of AKAP18δ and GFP–PLN by immunoblot. GFP-transfected cells were used as negative controls. (D) PLN residues important for AKAP18δ binding were identified by overlaying an array of immobilized PLN 20-mer peptides (2-amino-acid offset) with GST–AKAP18δ (top panel). PLN peptides with or without phosphorylated Ser 16 (pS) were subjected to GST–AKAP18δ overlay experiments (bottom panel). GST alone and GST–AKAP18δ preincubated with the AKAP18δ–PLN disruptor peptide were used as negative controls. Underscoring indicates residues relevant for AKAP18δ–PLN binding. AKAP, A-kinase anchoring protein; GFP, green fluorescent protein; GST, glutathione-S-transferase; PKA, protein kinase A; PLN, phospholamban; SERCA2, sarcoplasmic reticulum Ca2+-ATPase.
AKAP18δ binds to the cytoplasmic domain of PLN
As AKAP18δ does not have any transmembrane domains or lipid anchors, we examined next the cytoplasmic domain of PLN to identify the AKAP18δ-binding site. The PLN (1–36) sequence was synthesized as 20-mer peptides with 2-amino-acid offset on cellulose membranes and analysed for AKAP18δ binding by overlay with purified, recombinant glutathione-S-transferase (GST)–AKAP18δ protein, followed by anti-GST immunoblotting (Fig 3D). GST alone was used as a negative control. To evaluate the specificity of the assay, an AKAP18δ–PLN disruptor peptide (described below) was included in the overlay, which inhibited binding. The AKAP18δ core binding sequence was mapped to amino acids 13–20 in PLN (Fig 3D, underlined). This sequence is positioned at the end of domain IA (amino acids 1–16) and in the loop domain (amino acids 17–21), which is part of the hinge region between the two helical domains of PLN (Metcalfe et al, 2004). The identified AKAP18δ-binding site overlaps with the PKA phosphorylation site (RRAS) in PLN. To analyse whether PKA phosphorylation affected the AKAP18δ–PLN interaction, PLN was synthesized with a phosphorylated Ser 16 (pS) to mimic PKA-phosphorylated PLN and spots were overlaid with GST–AKAP18δ (Fig 3D). Introduction of the phosphorylated Ser 16 abolished AKAP18δ binding. Thus, the AKAP18δ–PLN interaction seems to be direct and possibly dynamically regulated by PKA phosphorylation of Ser 16 providing an on/off mechanism.
A two-dimensional peptide array, in which each residue in the PLN sequence from 13 to 23 was replaced with all natural amino acids, was analysed for AKAP18δ binding (see supplementary Fig S2 online). Substitutions of Arg 13, Arg 14 and Pro 21 almost totally abolished AKAP18δ binding, indicating the relevance of these amino acids for binding. The importance of Arg 13 and Arg 14 was also shown in a proline scanning array of PLN (data not shown). Interestingly, deletion of Arg 14 is associated with inherited human dilated cardiomyopathy and premature death (Haghighi et al, 2006). Conversely, the PLN-binding site in AKAP18δ was delineated by deletional mapping and interaction analysis by overlaying arrays of the cytoplasmic domain of PLN with truncated GST–AKAP18δ proteins, and by coexpression and co-immunoprecipitation analysis (see supplementary Fig S3 online).
Effect of disrupting PLN–AKAP18δ interaction
To evaluate the significance of AKAP18δ in the coordination of the PKA-mediated phosphorylation of PLN, we made a short peptide from PLN covering the AKAP18δ-binding domain to compete with and displace the AKAP18δ–PLN interaction. Plasma membrane-permeable PLN derivatives were generated by the coupling of 9 or 11 arginine residues to the amino or carboxyl terminus of the peptide (Arg 9–11-PLN or PLN-Arg 9–11). N- or C-terminal coupling, or length of the poly-arginine sequence between 9 and 11 residues did not affect biological activity, as evident from in vitro and in situ testing (data not shown). We then used rat neonatal cardiac myocytes, which have been shown to contain AKAP18δ (Fig 4A), for further functional experiments. The active peptide abolished the striated distribution pattern of AKAP18δ detected by immunofluorescence microscopy, indicating that the peptide disrupts the interaction of the two binding partners, whereas the control peptide did not seem to influence the distribution of AKAP18δ (Fig 4B). Neither peptide affected the distribution of PLN or α-actinin (data not shown). The isoproterenol-induced phosphorylation of PLN-Ser 16 was analysed in the presence or absence of the disruptor peptide (Fig 4C). Neonatal cardiac myocytes were incubated with or without the Arg 9-PLN peptide (Arg 9-RRASTIEMPQQ) for 30 min and then stimulated with isoproterenol. The phosphorylation of PLN at Ser 16 increased fivefold by β-adrenergic stimulation. The presence of the PLN peptide inhibited the increase in phosphorylation by almost 50%, indicating that AKAP18δ is necessary for the recruitment of PKA to its target, PLN. As Ser 16-phosphorylated PLN does not seem to bind to AKAP18δ, we used Arg 9-pSer 16-PLN as a negative control. This had no influence on the phosphorylation level of PLN-Ser 16 after stimulation with isoproterenol (Fig 4C). By contrast, a peptide in which Ser 16 was substituted with cysteine to control for substrate competition was equally effective as the PLN-derived peptide (data not shown). Furthermore, we examined the consequence of disrupting the AKAP18δ–PLN complex on Ca2+ re-uptake into the sarcoplasmic reticulum. Neonatal cardiac myocytes were transfected with the fluorescence resonance energy transfer (FRET)-based Ca2+ sensor Cameleon D1ER (Palmer et al, 2004) targeted to the sarcoplasmic reticulum, and the response to a 10 mM caffeine pulse was recorded in the presence or absence of 10 μM norepinephrine in control cells or cells pretreated with 25 μM PLN-Arg 11 for 40 min (Fig 4D). Ca2+ re-uptake showed a recovery time of approximately 1 min, which is consistent with the kinetics by the Kasai et al (2004) using the same sensor, and reflects simultaneous re-uptake of Ca2+ through SERCA2, the release of Ca2+ through RYR owing to elevated cytosolic Ca2+ and reassociation with Ca2+ buffers in the sarcoplasmic reticulum after the depletion. Neonatal cardiac myocytes treated with PLN-Arg 11 showed significantly reduced Ca2+ re-uptake into the sarcoplasmic reticulum, both at the basal level and after treatment with norepinephrine (Fig 4D).
Figure 4.
Disruption of the AKAP18δ–PLN complex influences PLN-Ser 16 phosphorylation and Ca2+ re-uptake in the sarcoplasmic reticulum. (A) Immunofluorescent labelling of AKAP18δ (red) and α-actinin (green) in rat neonatal cardiac myocytes. Scale bar, 20 μm. (B) Disruption of the AKAP18δ–PLN interaction with peptide PLN-Arg 11. Adult cardiac myocytes were attached to laminin-coated glass coverslips and incubated with the AKAP18–PLN disruptor peptide PLN-Arg 11 or the corresponding control peptide pSer 16-PLN. AKAP18δ was detected by immunofluorescence microscopy. Scale bar, 20 μm. (C) Rat neonatal cardiac myocytes were treated with or without Arg 9-PLN; peptide (50 μM, 30 min) before stimulation with isoproterenol (iso; 0.1 μM, 5 min) as indicated and analysed for immunoreactive pSer 16-PLN (IB; immunoblots; top panel). Phosphorylated PLN peptide (Arg 9-pSer 16-PLN; right lane) was used as a negative control. Dotted lines indicate lanes excised/combined from a single gel. The histogram shows levels of phosphorylated Ser 16-PLN quantified by densiometry relative to calsequestrin levels (bottom panel). Bars represent the mean±s.e.m. from 3–6 independent experiments (*P<0.005, Student's t-test; NS, not significant). (D) Rat neonatal cardiac myocytes were transfected with the D1ER sensor. Responses to a 10 mM caffeine pulse (1 s, arrow) in control cells (red curves) or cells pretreated with PLN-Arg 11 peptide (25 μM, 40 min, green curves) with (open symbols) or without (filled symbols) treatment with norepinephrine (NE; 10 μM, 20 min) as indicated were recorded. Time constant averages (τ, mean±s.e.m.) were calculated (right). For each sample, more than 20 independent cells were examined (*P<0.025, by Student's t-test and one-way ANOVA for paired and independent samples, respectively). AKAP, A-kinase anchoring protein; PLN, phospholamban; SERCA2, sarcoplasmic reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum.
To confirm the involvement of AKAP18δ, we knocked down AKAP18δ using short interfering RNA (siRNA; see blot in Fig 5 (right) for siRNA efficacy tested in HaCaT cells) and measured Ca2+ re-uptake. Cy3-labelled siRNA was transfected into cardiomyocytes together with the FRET-based Ca2+ sensor D1ER. Sarcoplasmic reticulum was Ca2+-depleted by blocking SERCA2 with 2,5-di-tert-butylhydroquinone (BHQ, a reversible inhibitor) in a Ca2+-free solution, BHQ was washed out, Ca2+ was added and Ca2+ re-uptake was measured in Cy3-positive cells with the Ca2+ sensor in the absence or presence of norepinephrine. As shown in Fig 5, AKAP18δ siRNA oligonucleotides abolished the effect of norepinephrine on Ca2+ re-uptake in the sarcoplasmic reticulum, whereas control siRNA did not interfere with the action of norepinephrine.
Figure 5.
Knockdown of AKAP18δ affects Ca2+ re-uptake in the sarcoplasmic reticulum. Kinetics of Ca2+ release and re-uptake in the sarcoplasmic reticulum of depolarized cardiac myocytes transfected with the D1ER sensor alone (red curves), together with control siRNA (blue curves) or AKAP18δ siRNA (green curves) in the presence (open symbols) or absence (filled symbols) of 10 μM norepinephrine (NE; the arrow indicates the time of NE addition). SR Ca2+ was depleted by 50 μM BHQ, the cells were washed and extracellular Ca2+ was added. siRNA was Cy3-labelled and transfected cells were thus identified. For clarity, only every second data point is shown. Note: timescale differs at breakpoint. The time constant averages (τ, mean±s.e.m.) were calculated excluding outlier values outside mean±2 s.d. (right). For each sample, 10–17 independent cells were analysed (*P<0.025, by Student's t-test and one-way ANOVA for paired and independent samples, respectively; NS, not significant). Immunoblot (IB, right): siRNA efficacy tested in easily transfectable HaCaT cells expressing GFP–AKAP18δ. Tx. ctr.: transfection control, an unrelated Flag-tagged construct (β-arrestin) was co-transfected and detected by anti-Flag to control for transfection and loading. AKAP, A-kinase anchoring protein; BHQ, 2,5-di-tert-butylhydroquinone; GFP, green fluorescent protein; siRNA, short interfering RNA; SR, sarcoplasmic reticulum.
Our results indicate that AKAP18δ recruitment of PKA to a supramolecular complex containing PLN and SERCA2 is important to discretely regulate PKA phosphorylation of PLN at Ser 16, and thereby the PLN inhibitory effect on SERCA2 and Ca2+ re-uptake into heart sarcoplasmic reticulum. Moreover, our data indicate that the β-adrenoceptor/PKA-dependent phosphorylation of PLN requires the interaction of AKAP18δ with PLN. Our results provide a mechanism for the precise spatiotemporal control of PLN phosphorylation through the interaction with AKAP18δ and a pharmacologic tool that can specifically target PKA phosphorylation of a particular substrate and determine its role in the response of failing myocardium to catecholamines and to catecholamine antagonists. Further work will be necessary to determine how and to what extent the manipulation of the SERCA2–PLN–AKAP18δ–PKA complex can be targeted to regulate PLN/SERCA2 function under pathophysiological conditions.
Methods
Experimental procedures are provided in detail in the supplementary information online. Briefly, rat hearts were homogenized or fractionated according to protocols described in the supplementary information online. Neonatal and adult cardiac myocytes were isolated from the ventricles of 1- to 3-day-old or 8- to 12-week-old Wistar rat hearts, as described in the supplementary information online. RII overlays were carried out as described previously using 32P-labelled recombinant murine RIIα with some modifications (see the supplementary information online). Immunodetection and immunostainings were carried out using standard methods, with details on protocols and antibodies provided in the supplementary information online. Peptides (PLN-Arg 9–11: RRASTIEMPQQ-Arg 9–11; Arg 9-PLN: Arg 9-RRASTIEMPQQ; Arg 9-pSer 16-PLN: Arg 9-RRApSTIEMPQQ) were synthesized as described previously. Calcium re-uptake was assessed by transfection of the FRET-based Ca2+ sensor Cameleon D1ER targeted to the sarcoplasmic reticulum, with details provided in the supplementary information online.
Disclosures. Some of the authors have filed a pending patent application on drug targeting of the above-described signal complex.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Figures and Information
Acknowledgments
We are grateful to G. Opsahl, J. Solheim, G. Tjørhom and J. Eichorst for technical assistance, and to M. Amarzguioui for the help in designing siRNAs. We thank R. Tsien for kindly providing the D1ER construct. This work was supported by grants from the Norwegian Functional Genomics Programme (FUGE), The Research Council of Norway, The Norwegian Cancer Society, Novo Nordic Foundation Committee, Deutsche Forschungsgemeinschaft (Kl 1415/2-2, 4-1), Telethon (TCP00089 and GGP05113), the Italian Cystic Fibrosis Research Foundation, the Fondazione Compagnia di San Paolo, the Human Frontier Science Programme Organization (HFSPO) (RGP0001/2005-C) and the European Union (RTD grant no. QLK3-CT-2002-02149 and STREP grant no. 037189).
References
- Bers DM (2002) Cardiac excitation–contraction coupling. Nature 415: 198–205 [DOI] [PubMed] [Google Scholar]
- Haghighi K et al. (2003) Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 111: 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K et al. (2006) A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci USA 103: 1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn V et al. (2004) Identification of a novel A-kinase anchoring protein 18 isoform and evidence for its role in the vasopressin-induced aquaporin-2 shuttle in renal principal cells. J Biol Chem 279: 26654–26665 [DOI] [PubMed] [Google Scholar]
- Kasai H et al. (2004) Direct measurement of Ca2+ concentration in the SR of living cardiac myocytes. Biochem Biophys Res Commun 314: 1014–1020 [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG (2003) Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577 [DOI] [PubMed] [Google Scholar]
- Metcalfe EE, Zamoon J, Thomas DD, Veglia G (2004) (1)H/(15)N heteronuclear NMR spectroscopy shows four dynamic domains for phospholamban reconstituted in dodecylphosphocholine micelles. Biophys J 87: 1205–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AE, Jin C, Reed JC, Tsien RY (2004) Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA 101: 17404–17409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehr ML, Russell MA, Bond M (2004) A-kinase anchoring protein targeting of protein kinase A in the heart. J Mol Cell Cardiol 37: 653–665 [DOI] [PubMed] [Google Scholar]
- Russell MA, Lund LM, Haber R, McKeegan K, Cianciola N, Bond M (2006) The intermediate filament protein, synemin, is an AKAP in the heart. Arch Biochem Biophys 456: 204–215 [DOI] [PubMed] [Google Scholar]
- Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE (2003) Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299: 1410–1413 [DOI] [PubMed] [Google Scholar]
- Scholten A, Poh MK, van Veen TA, van BB, Vos MA, Heck AJ (2006) Analysis of the cGMP/cAMP interactome using a chemical proteomics approach in mammalian heart tissue validates sphingosine kinase type 1-interacting protein as a genuine and highly abundant AKAP. J Proteome Res 5: 1435–1447 [DOI] [PubMed] [Google Scholar]
- Simmerman HK, Jones LR (1998) Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev 78: 921–947 [DOI] [PubMed] [Google Scholar]
- Szentesi P, Pignier C, Egger M, Kranias EG, Niggli E (2004) Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ Res 95: 807–813 [DOI] [PubMed] [Google Scholar]
- Tasken K, Aandahl EM (2004) Localised effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84: 137–167 [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Louch WE, Ver HM, Sipido K, Raeymaekers L, Wuytack F (2003) Ca2+ transport ATPase isoforms SERCA2a and SERCA2b are targeted to the same sites in the murine heart. Cell Calcium 34: 457–464 [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD (2004) AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol 5: 959–970 [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T (2002) Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295: 1711–1715 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Figures and Information